Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is associated with a long-term risk of progression to multiple myeloma (MM) or related malignancy. To prevent serious myeloma-related complications, lifelong annual follow-up has been recommended, but its value is unknown. We reviewed all patients from southeastern Minnesota seen at Mayo Clinic between 1973 and 2004 with MGUS who subsequently progressed to MM. Of 116 patients, 69% had optimal follow-up of MGUS. Among these, abnormalities on serial follow-up laboratory testing led to the diagnosis of MM in 16%, whereas MM was diagnosed only after serious MM-related complications in 45%. In the remaining, workup of less serious symptoms (25%), incidental finding during workup of unrelated medical conditions (11%), and unknown (3%) were the mechanisms leading to MM diagnosis. High-risk MGUS patients (≥ 1.5 g/dL and/or non-IgG MGUS) were more likely to be optimally followed (81% vs 64%), and be diagnosed with MM secondary to serial follow-up testing (21% vs 7%). This retrospective study suggests that routine annual follow-up of MGUS may not be required in low-risk MGUS. Future studies are needed to replicate and expand our findings and to determine the optimal frequency of monitoring in higher-risk MGUS patients.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 2197.
Disclosures
The authors, the Associate Editor Martin S. Tallman, and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the epidemiology and prognosis of MGUS
Compare outcomes of excellent follow-up monitoring with suboptimal follow-up monitoring among patients with MGUS
Design monitoring strategies for patients with low-risk MGUS
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a frequent finding in the older adult population, affecting 3% of whites age 50 or older and with prevalence increasing with age.1-4 Prevalence varies among different races, being 2-fold higher in black populations and less frequent in Asians compared with whites.5-9 MGUS is a premalignant plasma cell dyscrasia, carrying a lifelong risk of transformation to hematologic malignancy, mainly multiple myeloma (MM), at a fixed but unremitting rate of approximately 1% per year.10-13 Because MM remains an incurable disease with significant associated morbidity from skeletal and renal events, close monitoring of MGUS patients has been recommended to diagnose malignant transformation before the onset of serious complications. Current guidelines recommend that newly diagnosed MGUS patients should undergo a general physical examination and routine laboratory screen (complete blood count, creatinine, and calcium) with a repeated serum protein electrophoresis in 6 months and, if stable, yearly thereafter.10,14-16 Follow-up is particularly important for MGUS patients presenting with high-risk features (non-IgG type M protein, higher concentrations of monoclonal component, and/or abnormal free light chain ratio).17-19 The rationale for these recommendations is based on the possibility that such follow-up can result in the timely diagnosis of malignancy (myeloma) before the onset of serious complications, such as pathologic fracture or acute renal failure.
Although MGUS carries a lifelong risk of progression to MM, it is not clear whether routine annual follow-up for life is of benefit.20 It is also not clear whether such an approach prolongs survival. On the other hand, routine annual follow-up may have adverse psychologic and economic implications. The purpose of this study was to explore the extent to which routine annual follow-up of MGUS is of clinical value in terms of preventing MM-related complications, need for hospitalization, time to progression, and overall survival. In addition, recent studies indicate that low-risk MGUS patients who constitute approximately half of all MGUS patients may not need annual follow-up.8 The second goal of this study was to assess whether the impact of annual follow-up varied in low-risk MGUS compared with higher-risk subsets, to justify a risk-adapted approach to follow-up.
Methods
Study cohort
We searched both paper and electronic medical records of all patients from southeastern Minnesota seen at Mayo Clinic between 1973 and 2004 who were diagnosed with MGUS and then subsequently progressed to symptomatic MM. According to the International Myeloma Working Group diagnostic criteria, and consistent with prior studies by our group in Southeastern Minnesota and in Olmsted County, MGUS was defined as a serum monoclonal immunoglobulin level of less than 3 g/dL, less than 10% plasma cells in the bone marrow if done, and absence of plasma cell dyscrasia-related end organ damage (hypercalcemia, renal insufficiency, anemia, or lytic bone lesions).1,17,21 Patients who met criteria for smoldering multiple myeloma (SMM), primary amyloidosis, or symptomatic MM at the time of first assessment were excluded. This retrospective study was approved by the Mayo Clinic's Institutional Review Board, and only those patients with research authorization for review of medical records were included.
Patient categories
To assess the impact of monitoring, we first divided patients into 6 categories according to the frequency of follow-up. The excellent category included patients who were followed every 6 months (± 6 months); satisfactory corresponded to follow-up occurring every 24 months (± 12 months). Patients in the excellent and satisfactory groups were considered to be optimally followed. Follow-up occurring at a frequency greater than 36 months was considered sporadic; patients who were diagnosed with symptomatic MM more than 5 years after the last follow-up were assigned to the category of inadequate follow-up. A category of no follow-up included those patients who were not followed after the initial detection of MGUS. Patients in the sporadic, inadequate follow-up, and no follow-up categories were defined as suboptimally followed. Finally, for a minority of patients, the pattern of follow-up could not be established as the diagnosis of symptomatic MM occurred within 1 year from the initial detection of MGUS. Patients in this last category (n = 8) were not included in any analysis looking at the effect of optimal follow-up on outcome because the progression to symptomatic MM within 1 year from monoclonal peak detection made it impossible to establish the appropriateness of follow-up. Patients with a baseline M spike less than 1.5 g/dL of the IgG subtype were considered low-risk MGUS.8 Conversely, patients with an M protein of 1.5 g/dL or more or of the non-IgG type were considered to have high-risk features.
Assessment of quality of follow-up
We defined worrisome laboratory values as a 50% increase in M spike, an unexplained 50% increase in creatinine, an unexplained abnormal calcium level (or 25% above the initial value), or a decrease in hemoglobin of 20% or 2 g/dL (whichever is more) between 2 consecutive values. Complaints of concern for progression of disease included new, severe, unremitting bone pain; extreme fatigue; malaise; or recurrent infections. Worrisome x-ray findings were the presence of compression fractures or bone fractures with minimal trauma. Failure to order a closer follow-up of abnormal values or further diagnostic evaluations in the presence of one of the aforementioned situations was considered a “missed workup.”
Data collection and endpoints
By records relating to each follow-up visit or hospitalization, we obtained pertinent patient symptoms, symptoms, signs, and clinical findings. Relevant laboratory values (hemoglobin, total protein, M spike, immunoglobulin fraction quantification, creatinine, calcium, phosphate, proteinuria, and β2-microglobulin) were abstracted. Bone surveys, bone x-rays, and bone marrow studies were also reviewed.
Statistical analysis
The primary study endpoint was impact of close follow-up on preventing MM-related complications and hospitalizations. The secondary endpoints were to estimate proportion of patients in whom changes in follow-up laboratory studies led to early diagnosis of MM and to determine the impact of follow-up in low-risk versus higher-risk subsets of MGUS. Other endpoints studied were effect of follow-up on time to progression to symptomatic MM and overall survival and level of adherence to recommended guidelines for follow-up of MGUS patients.
Two-sided Fisher exact tests were used to test for differences between categorical variables. Two-sided Wilcoxon rank-sum tests were used to compare continuous variables. Survival analysis was done using the Kaplan-Meier method. Differences between survival curves were tested for statistical significance using the 2-sided log-rank test.
Results
Patient characteristics
We identified 116 patients with MGUS between 1973 and 2004 who subsequently progressed to symptomatic MM. The median age at MGUS diagnosis was 67 years (range, 35-87 years). Men represented 54% of the sample (63 patients). Seventy percent (n = 81) of patients had a monoclonal IgG, 24% (n = 28) had IgA, 4% (n = 5) had a biclonal M protein, and 2% (n = 2) had a light chain only disease. The median M spike at the time of MGUS diagnosis was 1.5 g/dL, ranging between undetectable and 2.9 g/dL (data not shown).
Baseline anemia, kidney disease, and hypercalcemia, if present, were verified as being unrelated to plasma cell dyscrasia. Anemia was in most cases iron-deficient secondary to chronic blood loss from the upper or lower gastrointestinal tract. In one patient, anemia was secondary to myelodysplastic syndrome. In patients in whom a clear etiology could not be established, other laboratory testing, including a bone marrow aspiration and biopsy within normal limits, excluded plasma cell dyscrasia as the cause. Two patients had a diagnosis of hyperparathyroidism with baseline calcium greater than 10.5 mg/dL. Two patients had baseline chronic kidney disease (baseline creatinine > 2 mg/dL), whose etiology could not be established. Again, the absence of other laboratory abnormalities, including bone marrow plasmacytosis, and the longstanding history of kidney disease excluded plasma cell dyscrasia as the cause. All 4 patients belonged to the optimal follow-up group.
Effect of routine follow-up on timely diagnosis of myeloma
We were able to obtain laboratory values and diagnostic imaging reports, as well as information regarding the circumstances that led to the diagnosis of symptomatic MM, for most of the 116 patients. The most frequently missed test was a metastatic bone survey at the time of diagnosis of symptomatic MM (11 patients). Of the 116 patients, 69% received optimal follow-up, 24% received suboptimal follow-up, and in 7% (8 patients) no assessment of adequacy of follow-up was possible because MM was diagnosed within 1 year of the recognition of MGUS. For the purpose of this study, the 8 patients whose MGUS progressed to MM within 1 year were excluded from further analysis, as stated earlier. The baseline characteristics of the optimal versus suboptimal follow-up groups are delineated in Table 1. The 2 populations were similar at baseline, with the exception of a statistically significant difference in the calcium level at the time of MGUS diagnosis.
In reviewing the patients' records, we established the course of events that led to the diagnosis of MM in 113 of the 116 patients. Among the 80 optimally followed patients, the routine laboratory follow-up of MGUS led to the diagnosis of MM in only 16% of patients (n = 13). Serious myeloma-related complications, often requiring hospitalization, led to the diagnosis of MM in 45% of optimally followed patients (n = 36) and included pathologic fractures (often related to plasmacytoma, n = 24, 67%); symptomatic anemia or pancytopenia with recurrent infections (n = 6, 17%); severe hypercalcemia with altered mental status (n = 4, 11%); and acute renal failure (n = 2, 6%). In 25% of optimally followed patients (n = 20), the diagnosis of MM was by diagnostic workup of other less serious symptoms reported by the patient, including bone pain, mostly localized to the back and accompanied at times by radiculopathy, fatigue, anorexia, weight loss, and weakness. In 11% of patients (n = 9), the diagnosis of MM was incidental in the context of workup or hospitalization for other conditions, whereas the mechanism was unknown in 3% of patients (n = 2). Among optimally followed patients, those receiving excellent follow-up (every 6 months ± 6 months, n = 56) had a lower incidence of MM diagnosis secondary to onset of serious complications compared with patients followed less frequently (41% vs 54%; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Suboptimal follow-up resulted in a higher proportion of patients being diagnosed with symptomatic MM incidentally (25% vs 11%, Table 2) and routine follow-up contributed to the MM diagnosis in only 1 of 28 patients (3.5%). Interestingly, more than 40% of those patients who did not receive medical follow-up for many years returned to their primary care physician of concern for symptoms that subsequently led to the diagnosis of MM. For both optimal and suboptimal follow-up groups, serious MM-related complications and MM-related symptoms together represented the mechanisms of MM diagnosis in more than two-thirds of patients.
No statistically significant differences in tumor burden at bone marrow biopsy, extent of skeletal involvement, stage III Durie-Salmon, or need for hospitalization at the time of diagnosis were noted between the 2 subgroups (Table 2). As this lack of differences might be the result of inadequate workup of worrisome laboratory values, patients' symptoms, or x-ray findings, we reviewed the charts to assess the frequency of such possibly missed warning events. We found that 6 patients (6%) had a “missed workup” of a worrisome laboratory value, represented in all instances by a significant increase in M protein. In 2% of the cases, the primary care physician failed to further investigate worrisome symptoms (severe back pain in one case and unremitting chest pain in the other); for another 6% of patients, the presence of a compression fracture or a fracture arising with minimal trauma was not investigated in depth. Of note, one of the patients with compression fracture of the spine had severe osteoporosis.
Effect of routine follow-up on timely diagnosis of myeloma by baseline MGUS risk stratification
Patients with high-risk MGUS features of M spike of 1.5 g/dL or more and/or non-IgG M spike were more likely to be optimally followed compared with low-risk patients (81% vs 64%, P = .07; Table 3). Among 52 patients with one or both high-risk features who were optimally followed, routine laboratory testing led to the diagnosis of symptomatic MM in 21% (n = 11) of patients, whereas the diagnosis of MM was made after the onset of serious MM-related complications in 40% (n = 21) of patients (Table 4). In the remaining patients, the diagnosis of MM was from diagnostic workup of less serious symptoms reported by the patients (27%, n = 14), during the workup evaluation or hospitalization for other conditions (8%, n = 4), and unknown (4%, n = 2). In contrast, among optimally followed patients with no risk factors for MGUS progression, the proportion of patients identified after the onset of serious MM-related complication was higher (52%), whereas the fraction of patients diagnosed merely on the base of laboratory abnormalities was substantially lower (7%; Table 4).
Effect of optimal follow-up on diagnosis of SMM
As expected, adequate follow-up resulted in an increased rate of diagnosing SMM. Thirty percent of patients in optimal and 11% in suboptimal follow-up were diagnosed with SMM (Table 2). The difference in the probability of diagnosing SMM between the 2 groups is even more substantial considering that 2 of 3 SMM diagnoses in the suboptimal group were incidental. In the optimal follow-up group, the diagnosis of SMM was secondary to an increased M spike at follow-up examination in the majority of cases. However, in 4 of 24 cases (17%), it was the persistent symptom of back pain that prompted the bone marrow studies subsequently leading to the diagnosis of SMM. Among optimally followed patients, the diagnosis of SMM was achieved in a significantly higher proportion of MGUS patients with high-risk features (M spike ≥ 1.5 g/dL and/or non-IgG MGUS) compared with low-risk MGUS (38% vs 15%, P = .04, Table 4). Optimally followed patients carrying a diagnosis of SMM were slightly more likely to be diagnosed secondary to follow-up of abnormal laboratory values (21% vs 14%) and less likely to be diagnosed after onset of serious MM-related complications (38% vs 48%), although these differences were not statistically significant (supplemental Table 2).
Effect of follow-up on time to progression and survival
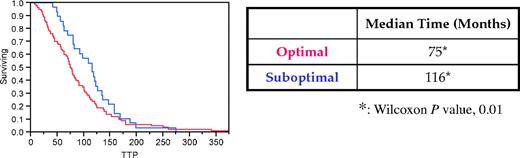
The time from initial diagnosis of MGUS to diagnosis of MM was significantly shorter in optimally followed patients compared with patients who had a suboptimal follow-up (75 months vs 116 months, respectively, P = .01; Figure 1).
Effect of optimal follow-up on time to progression from MGUS to symptomatic MM. Optimal follow-up resulted in a statistically significant shorter interval time from diagnosis of MGUS to diagnosis of symptomatic MM for patients in the optimally followed category (red line) compared with the suboptimally followed patients (blue line).
Effect of optimal follow-up on time to progression from MGUS to symptomatic MM. Optimal follow-up resulted in a statistically significant shorter interval time from diagnosis of MGUS to diagnosis of symptomatic MM for patients in the optimally followed category (red line) compared with the suboptimally followed patients (blue line).
Survival from the time of MM diagnosis was not significantly different between optimal and suboptimal groups (P = .4). Overall survival was similar after adjusting for access to novel agents on multivariate analysis (P = .6) and when analysis was restricted to patients diagnosed with MGUS before 1999 (P = .5). As medical treatment plays a major role in determining overall survival in MM, we reviewed patient records to establish whether the lack of significantly improved survival in the optimal subgroup could be the result of inferior treatment options (such as lack of availability of novel agents) compared with the suboptimal group. These findings are summarized in supplemental Table 3 and show that the optimal follow-up group did not have a lower access to novel agents.
Discussion
MGUS is a common premalignant plasma cell disorder characterized by a lifelong risk of transformation to hematologic malignancy, mainly MM.15,21 Yearly follow-up of MGUS patients (careful history, physical examination, and laboratory studies, including quantification of M component, complete blood count, creatinine, electrolytes, and calcium) has been recommended as a means to timely diagnose malignant transformation, thus avoiding complications, hospitalizations, and costs.16 Although this approach appears reasonable, evidence supporting a positive impact on patients' quality of life, a decreased risk of MM-related complications or hospitalization, or improved overall survival is lacking. Moreover, MM is still an incurable disease, and there are no data to prove that early intervention prolongs survival. On the other hand, more sensitive laboratory tests for MGUS, increased testing for monoclonal proteins in patients presenting with a wide variety of clinical problems, and a greater awareness of MM are leading to a continuing increase in the proportion of the general population with a clinical diagnosis of MGUS. With a prevalence of more than 3% among white and twofold higher among black persons age 50 and older, MGUS affects millions of persons worldwide, and the overall costs associated with annual follow-up need to be justified. Finally, most of the MGUS patients diagnosed today (≥ 90%) will never develop MM in their lifetime. Consequently, it is important that the value of routine annual laboratory monitoring of MGUS be critically examined, especially in consideration of relevant medical, social, epidemiologic, and economic connotations. Recent studies indicate that low-risk MGUS patients, who constitute approximately half of all MGUS patients, may not need annual follow-up.8 It is important to determine whether follow-up recommendations can be individualized based on estimated risk of progression to malignancy.
In this retrospective study, we show that the majority of patients (69%) received optimal follow-up after the diagnosis of MGUS, suggesting a reasonable adherence to the current consensus guidelines for clinical MGUS management. Although we limited referral bias by including only patients from southeastern Minnesota, the study was conducted in a large, center specializing in MGUS, and adherence to this guideline is probably lower in other areas. It is noteworthy that all (9 of 9) patients diagnosed with MM after 1999 had received optimal follow-up, suggesting a behavioral change among practicing clinicians in the follow-up of MGUS, perhaps based on a revised perception of MM as a treatable malignancy concomitantly with the introduction of highly effective, less toxic novel agents.22
We show that in the optimally followed patients, a diagnosis of MM on the basis of abnormal laboratory findings, in the absence of symptoms or complications, is achieved in 16% of patients. Almost all of the other patients would have been diagnosed with MM at the same time, and with approximately the same degree of clinical effort, as they would, had they never been followed for MGUS. In particular, optimal follow-up did not result in reduced hospitalization or decreased MM-related complication rate. In 45% of optimally followed patients, a serious myeloma-related complication was the first disease-defining event, indicating that interval progression (between screening visits) may be a contributing factor to the inadequacy of follow-up. This suggests that progression from MGUS to symptomatic myeloma and the doubling time of plasma cells after malignant transformation are more rapid than previously thought. More frequent monitoring (or a preceding diagnosis of SMM; supplemental Tables 1-2) appears to reduce the number of patients diagnosed after onset of serious complications and needs further study. One other contributing factor in retrospect may be that in 6% of optimally followed patients, worrisome laboratory values or symptoms were not fully investigated, thus providing the potential for a missed early diagnosis.
Although a survival benefit was not seen with optimal follow-up, it must be emphasized that our study was not powered to study the impact of optimal follow-up on survival, which requires a much larger sample size. Second, it is not appropriate to compare effect of optimal follow-up versus suboptimal follow-up on survival in this study because the 2 groups were not randomly assigned, and high-risk patients were more probable to be optimally followed. Finally, the rationale for the recommended annual follow-up in MGUS has always been to diagnose progression to MM early before the onset of serious skeletal and renal complications, not to improve overall survival. Overall survival improvements with annual follow-up of MGUS are unrealistic because there are no data to suggest that early treatment of MM prolongs survival.
Recent studies support a risk-adapted approach to follow-up of MGUS.13,16 Risk stratification can be accomplished by assessing the presence of 3 established risk factors: non-IgG type M protein, higher concentrations of monoclonal component, and abnormal free light chain ratio.13,16 As most of the patients in this study were diagnosed with MGUS before the free light chain assay was commercially available, it was not possible to assess patients for this relevant risk factor for MGUS progression. Nevertheless, when analyzed for the other 2 known high-risk features (non-IgG monoclonal protein and M protein > 1.5 g/dL), we found that the probability of optimal follow-up is significantly higher among patients presenting with 1 or 2 risk factors (Table 3), suggesting that clinicians appropriately provide closer follow-up for high-risk patients. We also found a trend suggesting a lower probability of diagnosing MM after serious complications in the high-risk MGUS subset (Table 4) and a greater benefit from serial follow-up laboratory testing.
As expected, optimal follow-up did lead more frequently to a diagnosis of SMM, and this was more often in the high-risk MGUS subset (38% of patients vs 15%). At present, the clinical benefit of an early diagnosis of SMM is not clear, and current guidelines recommend observation only (every 2-3 months after the initial diagnosis, and periodically thereafter).23 The increasing availability of new agents in myeloma with less toxicity and greater efficacy has raised the question of whether to use such agents in SMM to delay progression, and clinical trials are currently testing the impact of these drugs on progression-free and overall survival in SMM.24-26 An increased rate of SMM diagnosis among MGUS patients, particularly those with high-risk MGUS, may therefore be a consideration in the future. However, we would like to emphasize that therapy for SMM is not recommended outside of clinical trials.23 Although not statistically significant, patients with a preceding diagnosis of SMM were less probable to have MM-related complications at the time of MM diagnosis (supplemental Table 2).
There are some important limitations to our study. As mentioned earlier, the study is not randomized. However, prospective data regarding the efficacy of annual follow-up of MGUS are at present not available; and because MGUS progresses to MM only at a rate of 1% per year, it will take decades for a randomized trial to be completed. Second, to study preventable complications, we restricted the study to progression to MM. MGUS does carry a risk of progression to other related plasma cell disorders, such as amyloidosis, and the potential benefits of follow-up on such disorders cannot be discerned from this study. Finally, we may have underestimated the current benefits of annual follow-up in this study as most patients were diagnosed decades ago, and knowledge of myeloma and its complications among primary care providers and oncologists has probably increased in recent years.
In a time of change, frequent laboratory screening for epidemiologically relevant conditions assumes a crucial value from an ethical, social, and economic viewpoint.27,28 Our findings suggest that follow-up of patients diagnosed with MGUS could be individualized according to risk of progression. In low-risk MGUS patients who represent almost 50% of all MGUS cases and have only a 2% lifetime risk of progression to myeloma,8 it would appear reasonable to investigate disease progression only if symptoms suggestive of myeloma or related malignancy occur. However, further studies are needed to replicate and expand our findings and to determine the optimal frequency of monitoring in higher-risk MGUS patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by NIH grants CA62242 and CA107476 from the National Cancer Institute, and the Rochester Epidemiology Project, grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
National Institutes of Health
Authorship
Contribution: G.B. and S.V.R. analyzed data and wrote the manuscript; and R.A.K., C.L.C., D.R.L., S.K., J.A.K., A.D., T.M.T., J.R.C., and L.J.M. provided critical review, helpful insights, and edits to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Vincent Rajkumar, Department of Internal Medicine, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: rajks@mayo.edu.