Abstract
Drug-induced immune thrombocytopenia (DITP) is often suspected in patients with acute thrombocytopenia unexplained by other causes, but documenting that a drug is the cause of thrombocytopenia can be challenging. To provide a resource for diagnosis of DITP and for drug safety surveillance, we analyzed 3 distinct methods for identifying drugs that may cause thrombocytopenia. (1) Published case reports of DITP have described 253 drugs suspected of causing thrombocytopenia; using defined clinical criteria, 87 (34%) were identified with evidence that the drug caused thrombocytopenia. (2) Serum samples from patients with suspected DITP were tested for 202 drugs; drug-dependent, platelet-reactive antibodies were identified for 67 drugs (33%). (3) The Food and Drug Administration's Adverse Event Reporting System database was searched for drugs associated with thrombocytopenia by use of data mining algorithms; 1444 drugs had at least 1 report associated with thrombocytopenia, and 573 (40%) drugs demonstrated a statistically distinctive reporting association with thrombocytopenia. Among 1468 drugs suspected of causing thrombocytopenia, 102 were evaluated by all 3 methods, and 23 of these 102 drugs had evidence for an association with thrombocytopenia by all 3 methods. Multiple methods, each with a distinct perspective, can contribute to the identification of drugs that can cause thrombocytopenia.
Introduction
Acute immune-mediated thrombocytopenia is a potentially serious adverse reaction to many drugs.1,2 Therefore, drug-induced immune thrombocytopenia (DITP) should be considered in all patients with acute thrombocytopenia not explained by other causes. It is not uncommon for patients with DITP to be initially diagnosed as having autoimmune thrombocytopenic purpura.3 The estimated population incidence of DITP (1-2 cases per 100 000 per year4-6 ) is similar to the estimated incidence of autoimmune thrombocytopenic purpura in adults (3.3 cases per 100 000 per year7 ); however, the frequency of DITP is much greater in users of specific medications.8 For example, the estimated incidence of thrombocytopenia among users of trimethoprim-sulfamethoxazole and quinine has been estimated to be 198 and 135 per 100 000 users per year, respectively.6 The frequency of DITP may be even greater than these estimates, because identification of a drug as the cause of thrombocytopenia is a difficult problem for clinicians, especially in patients taking multiple medications. The appropriate first step in the evaluation and management of patients with suspected DITP is to discontinue drugs that may be the most likely causes of thrombocytopenia; however, current information provides only limited evidence for determining which among a patient's drugs are most likely to cause thrombocytopenia.
To address this common clinical problem, we used 3 distinct analytical methods and their corresponding datasets to identify drugs that have evidence for an association with thrombocytopenia: (1) We performed serial systematic reviews of published case reports of DITP and analyzed the clinical evidence for a causal association of the drug with thrombocytopenia.9,10 (2) We analyzed data from diagnostic laboratory testing at the BloodCenter of Wisconsin for drug-dependent, platelet-reactive antibodies.11,12 (3) We performed data mining analyses of the Adverse Event Reporting System (AERS) database of the US Food and Drug Administration (FDA) to identify drugs that had been suspected of causing thrombocytopenia and to determine which among these drugs had a statistically distinctive reporting association with thrombocytopenia.13 We used the results of these 3 analyses to develop a comprehensive, current database of drugs associated with thrombocytopenia that we hope will be helpful to clinicians in their evaluation of patients suspected of having DITP and to health professionals involved in drug safety surveillance.
Methods
Inclusion criteria for drugs
Agents described in case reports, laboratory data, and data mining of the AERS database were checked to determine whether they were currently approved drugs in worldwide markets by use of the Lexi-Comp electronic database,14 Micromedex electronic medical database,15 and the Martindale Pharmacopoeia.16 We used Lexi-Comp as the primary reference database. If an agent was not identified as a currently approved drug with Lexi-Comp, then the Micromedex database was used to determine whether the drug was currently approved. If an agent was not identified by either Lexi-Comp or Micromedex, the Martindale Pharmacopoeia was then searched. Drugs not currently approved by regulatory agencies in any country, including investigational drugs, herbal compounds, illegal drugs, and agents that are not used as drugs (for example, pesticides), were excluded. Marrow-suppressive drugs that cause predictable, dose-dependent thrombocytopenia were excluded unless the drugs had been identified as the cause of acute immune-mediated thrombocytopenia. Heparin and heparin analogs were excluded from this analysis because heparin-induced thrombocytopenia has a distinct pathogenesis and clinical course manifested predominantly by thrombosis, and also because a standardized diagnostic evaluation for heparin-induced thrombocytopenia has been established.9,17,18 Some agents were listed in the AERS database individually and in combination with other drugs. Drug combinations were included as separate items when they were documented as distinct approved drugs by one of the resource databases.
Classification based on case reports of patients with suspected drug-induced thrombocytopenia
We systematically reviewed all published case reports of drug-induced thrombocytopenia initially in 1998,9 and we repeated our systematic reviews every 2 years, most recently on October 6, 2008.10 In each review, we used a defined set of clinical criteria to assess the level of evidence for a causal association with thrombocytopenia.9,19 Criteria for article identification and selection, data extraction, and assessment of the clinical evidence have been described previously.9 Four levels of evidence were defined according to the following criteria. (1) “Definite” evidence for a causal association of a drug with thrombocytopenia in a reported patient required 4 criteria, within the limitations of secondary documentation: (A) the suspected drug was taken before thrombocytopenia occurred, and recovery from thrombocytopenia was complete and sustained after the drug was discontinued. (B) The suspected drug was the only drug taken before the onset of thrombocytopenia, or other drugs were continued or reintroduced with a sustained normal platelet count. (C) To the extent possible with secondary documentation, all other potential causes of thrombocytopenia were reasonably excluded. (D) Re-exposure to the suspected drug resulted in recurrent thrombocytopenia. (2) A “probable” association required 3 criteria (A, B, and C above). (3) If criterion A was met but both criteria B and C were not met, the drug was interpreted as having a “possible” association. (4) If criterion A was not met, or if re-exposure to the suspected drug did not result in recurrent thrombocytopenia, the drug was interpreted as having an “unlikely” association. Reasons for failure to meet criterion A included reports of patients with pre-existing thrombocytopenia or patients in whom recovery to a normal platelet count was not described. For the present study, drugs were considered to have a causal association with thrombocytopenia if they were described in 1 or more case reports as having “definite” evidence; however, reports that describe a rechallenge with the suspected drug are uncommon. Therefore, in the absence of any case reports with “definite” evidence, drugs were also considered to have a causal association with thrombocytopenia if they were described in 2 or more case reports as having “probable” evidence. The rationale for this arbitrary rule was that “probable” was the next highest level of evidence, and replicated, independent findings were considered to provide confirmation of a causal association. Analysis of tests for drug-dependent antibodies was not included in these assessments.
Because the goal of the present study was to include all drugs that had been suspected by a clinician of causing immune-mediated DITP, all drugs that were identified in published case reports, regardless of their level of evidence, were included in the analysis. Therefore, drugs that had been excluded from our previous analyses9 because of insufficient data, platelet counts not less than 100 000/μL, and patient age less than 16 years were included in the present analysis. Case reports that described the use of an established drug in a nontherapeutic manner (such as an overdose) and that described drug-induced disease that included thrombocytopenia but predominantly involved other organ system abnormalities were excluded from the present analysis.
Classification based on detection of drug-dependent, platelet-reactive antibodies
Testing for drug-dependent, platelet-reactive antibodies was performed in the Platelet and Neutrophil Immunology Laboratory of the BloodCenter of Wisconsin on samples submitted by clinicians for testing because of a suspicion of DITP from 1995 through December 31, 2008. These data from the BloodCenter of Wisconsin are similar to previously published data from 1998-2008.2 Data from this single laboratory were used to provide consistency of the testing procedure and therefore consistent data over a 14-year period from a laboratory that does referral testing for drug-dependent, platelet-reactive antibodies on a regular basis and on a large scale (approximately 600 samples per year [excluding heparin], predominantly from the United States). On the basis of extensive inquires, we believe that the BloodCenter of Wisconsin is the largest resource for drug-dependent, platelet-reactive antibody testing in the United States. Drug-dependent binding of antibodies to intact normal platelets was demonstrated by flow cytometry as described previously, with methodology that has been standardized across a wide range of drugs.11,12 The cut point that defined a positive result was defined by the mean result of a healthy donor population plus 3 standard deviations, providing a confidence level of 99%. All positive reactions were confirmed by an independent repeat assay. For some drugs, such as acetaminophen, ibuprofen, and naproxen, testing required use of drug metabolites, because antibodies were specific for metabolites of the drug and did not react with the unmodified drug.2 All drugs that met the previously described inclusion criteria for which drug-dependent antibody testing was requested were included in the present analysis, whether or not a drug-dependent antibody had been detected.
Classification based on data mining of the FDA AERS database
The AERS database is coded with the Medical Dictionary for Regulatory Activities (MedDRA), a standardized and widely used adverse event dictionary.20 Reporting of adverse drug reactions to the AERS is typically required of pharmaceutical companies; reports are also voluntarily submitted by healthcare professionals, patients, and others using the MedWatch Web site (https://www.accessdata.fda.gov/scripts/medwatch/). The principal purpose of the AERS database is to detect signals of possible adverse events that are novel in terms of their clinical nature, frequency, and/or severity. For the present analysis, we used the quarterly extracts of the AERS data, available from 1968 through June 2009, provided by the FDA in response to a Freedom of Information Act request. These data extracts contain quantitative information covering all drugs and all events from the core data fields in the form of aggregate reporting frequencies. The clinical narratives of the individual MedWatch forms are not applicable for the data mining techniques used in the present analysis and are not included in these quarterly data releases.
Reports of adverse drug reactions to the AERS are numerous and uncensored. Therefore, analytic processes (data mining) may be used to quantitatively explore these data in a search for systematic relationships between variables to help safety reviewers identify possible adverse events. Data mining is a screening procedure that identifies an association of drugs with adverse events and provides a possible starting point for further evaluation if the magnitude of the association is sufficiently large and the clinical context is appropriate. We used previously reported data mining methods13,21,22 based on 2 × 2 contingency tables that classify reports according to the presence or absence of the drug and the presence or absence of the adverse event of interest, which in the present study was thrombocytopenia. We then calculated the number of reports that might be expected if the drug and thrombocytopenia were independently recorded in the database; this expected independent recording of a drug and thrombocytopenia was considered to be a baseline or null level of association. There are various measures of association that may be calculated from 2 × 2 contingency tables that represent a ratio of observed reports to the expected number of reports. One commonly used measure in drug safety surveillance is described as the proportional reporting ratio (PRR). The greater the ratio of the observed number of reports to the expected number of reports exceeds predefined thresholds, described as disproportionality or a signal of disproportionate reporting (SDR),21 the more numerically distinctive is the association between the drug and thrombocytopenia. Disproportionality analysis is a commonly used data mining approach for pharmacovigilance studies.13
To search comprehensively for adverse events that could indicate DITP, we used multiple terms from the Medical Dictionary for Regulatory Activities that could be related to DITP to search the AERS database: (1) thrombocytopenia, (2) platelet count decreased, (3) platelet destruction increased, (4) thrombocytopenic purpura, (5) idiopathic thrombocytopenic purpura, (6) antiplatelet antibody, and (7) antiplatelet antibody positive. To search comprehensively for statistical reporting associations, we used data mining algorithms with both frequentist13 and Bayesian13 methods of analysis. Both methods of analysis were performed on subsets of data grouped by individual years and by sequential cumulative years (ie, 1968, 1968-1969, 1968-1970, 1968-1971, …, 1968-2009). With frequentist analysis of disproportionality, the signal of disproportionate reporting was significant for a reporting association between a drug and thrombocytopenia if (1) the drug-thrombocytopenia combination was reported 3 or more times in the analyzed stratum, (2) the proportional reporting ratio (ratio of observed reports to the baseline or expected reports) was greater than or equal to 2, and (3) χ2 was greater than 4. With Bayesian analysis of disproportionality, the signal of disproportionate reporting was significant for a reporting association between a drug and thrombocytopenia if (1) the drug-thrombocytopenia combination was reported 1 or more times in the analyzed stratum and (2) the observed-to-expected number of reports was significantly large, as defined by the fifth percentile of the posterior empirical Bayes gamma mixture (EB05) of 2 or more, a Bayesian formulation of one of the available association measures for 2 × 2 tables (the reporting ratio).13 These analyses were performed with Empirica version 7.0 software (Phase Forward).
Results
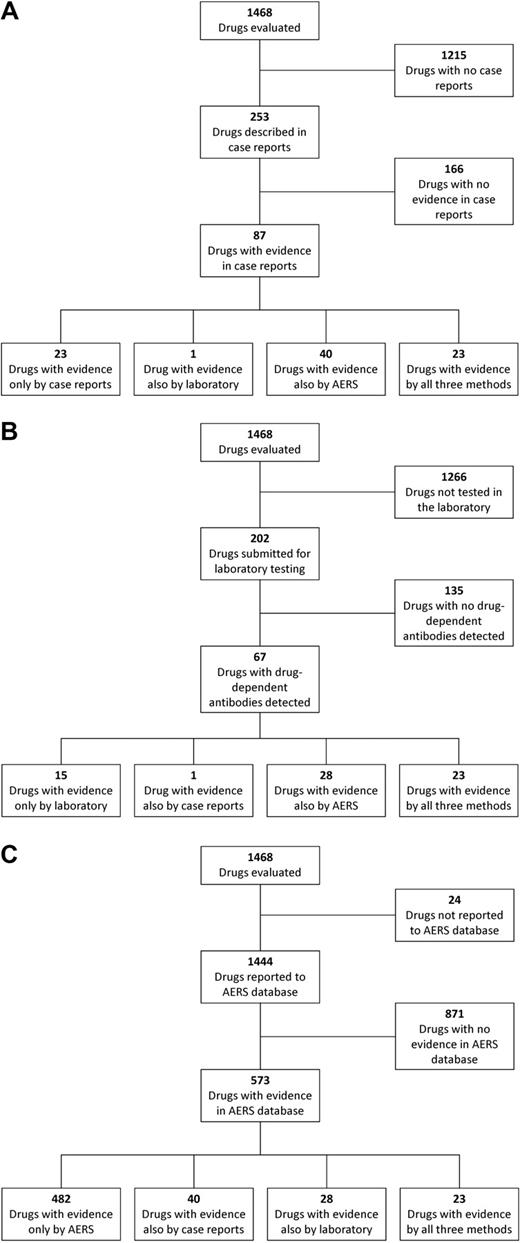
Case reports have described 253 drugs suspected of causing thrombocytopenia. There was evidence for a causal association between the drug and thrombocytopenia for 87 (34%) of these drugs. In patients whose blood samples were submitted to the BloodCenter of Wisconsin for testing for drug-dependent, platelet-reactive antibodies, 202 different drugs were suspected as possible causes of thrombocytopenia. Drug-dependent antibodies were identified for 67 (33%) of these drugs. A total of 351 drugs were described in case reports and/or tested for drug-dependent antibodies; 104 drugs were analyzed by both methods. One hundred thirty (37%) of these 351 drugs had evidence for a causal association with thrombocytopenia either by clinical evidence from case reports, by detection of drug-dependent antiplatelet antibodies, or by both methods. These analyses are summarized in Table 1. Table 2 lists the 24 drugs that had evidence for a causal association with thrombocytopenia by both methods. These 24 drugs included 6 of the 7 drugs that have been described in 10 or more case reports with “definite” or “probable” evidence for a causal association between the drug and thrombocytopenia. Gold has also been identified in 11 case reports as having a “probable” association with thrombocytopenia, but no case reports described a “definite” association. Gold has been statistically associated with thrombocytopenia by data mining but has not been tested for drug-dependent, platelet-reactive antibodies at the BloodCenter of Wisconsin since 1995. Previous testing for gold-dependent antibodies at the BloodCenter of Wisconsin was negative (data not shown). These 24 drugs also included 11 of the 12 drugs for which drug-dependent antibodies have been identified in 10 or more patients. Ceftriaxone also had evidence for a causal association with thrombocytopenia by detection of drug-dependent, platelet-reactive antibodies in 10 or more patients. Ceftriaxone has been statistically associated with thrombocytopenia by data mining but has not been reported in any case reports with “definite” or “probable” clinical evidence.
The AERS database contained reports of thrombocytopenia for 2062 agents; 1444 (70%) met our inclusion criteria of currently approved drugs. These 1444 drugs included 327 (93%) of the 351 drugs that were described in case reports and/or tested for drug-dependent antibodies; 24 (7%) of the drugs described in case reports and/or tested for drug-dependent antibodies were not identified in the AERS database. Five hundred seventy-three (40%) of the 1444 drugs were identified as having a statistically significant reporting association with thrombocytopenia; only 91 (16%) of these 573 drugs had been identified with evidence for a causal association with thrombocytopenia by published case reports in the medical literature or identification of drug-dependent antibodies. These analyses are summarized in Table 3.
Of all 1468 drugs analyzed in the present study, only 102 (7%) were evaluated by all 3 methods (Table 4). Because these drugs were suspected of causing thrombocytopenia and were evaluated by all 3 methods, they may be the most commonly suspected causes of DITP. However, of these 102 drugs, only 23 had evidence for a causal association with thrombocytopenia by all 3 methods; 14 drugs had no evidence for an association by any method; and some drugs were identified by only one method or by a combination of 2 of the methods. Only 1 drug, amiodarone (Table 2), was identified by both case reports and laboratory testing as having evidence for a causal association with thrombocytopenia but did not have a statistical reporting association with thrombocytopenia detected by data mining, even though it had been reported to the AERS. The results with each of the 3 methods are summarized in Figure 1. Supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) lists all 1468 drugs analyzed in the present study with the results of analysis by each of the 3 methods.
Results of analysis by the 3 distinct methods. (A) Results of analysis by published case reports and the relation of the results to analysis by the other 2 methods. Not all of the 87 drugs with evidence in case reports were also analyzed by the other 2 methods. (B) Results of analysis by laboratory detection of drug-dependent, platelet-reactive antibodies and the relation of the results to analysis by the other 2 methods. Not all of the 67 drugs with evidence by documentation of drug-dependent antibodies were also analyzed by the other 2 methods. (C) Results of analysis by data mining of the AERS database and the relation of the results to analysis by the other 2 methods. Not all of the 573 drugs with evidence in the AERS database were also analyzed by the other 2 methods.
Results of analysis by the 3 distinct methods. (A) Results of analysis by published case reports and the relation of the results to analysis by the other 2 methods. Not all of the 87 drugs with evidence in case reports were also analyzed by the other 2 methods. (B) Results of analysis by laboratory detection of drug-dependent, platelet-reactive antibodies and the relation of the results to analysis by the other 2 methods. Not all of the 67 drugs with evidence by documentation of drug-dependent antibodies were also analyzed by the other 2 methods. (C) Results of analysis by data mining of the AERS database and the relation of the results to analysis by the other 2 methods. Not all of the 573 drugs with evidence in the AERS database were also analyzed by the other 2 methods.
Discussion
Acute immune-mediated thrombocytopenia is a potentially severe adverse reaction to many drugs; therefore, the diagnosis of DITP is often considered in patients with unexpected thrombocytopenia. However, there are few data describing the relative risk of individual drugs for causing thrombocytopenia, and there are no routine diagnostic procedures for documenting that a drug is the cause of thrombocytopenia. Pharmacoepidemiologic studies have described methods to identify patients with potential DITP23 and have reported quinine, trimethoprim-sulfamethoxazole, and sulfisoxazole to be common causes of DITP.6,24 However, there is no evidence-based comprehensive database comprising all drugs that have been associated with thrombocytopenia and estimating their relative risk of causing thrombocytopenia. Our goal was to assist the initial evaluation of patients with suspected DITP by identifying drugs that may have a high likelihood of being causative based on assessment by 3 distinct methods of analysis.
We began our effort to document drugs that can cause thrombocytopenia with a systematic analysis of all published case reports of DITP.9,10,19 Analysis of case reports has limitations because (1) it is logical to assume that very few occurrences of DITP are published as case reports; (2) in many published case reports, it is difficult to apply and interpret the assessment criteria, and the available data presented in the concise reports are often insufficient to determine whether a causal association with thrombocytopenia existed; (3) patients with complex illnesses who develop possible DITP while being treated with multiple drugs may be relatively underreported, because clinicians may not be able to implicate a single, potentially causative drug; and (4) rechallenge with a suspected drug may not definitely prove causation. Although in the present study, we used a reported positive rechallenge as a criterion of a “definite” causal association of a drug with thrombocytopenia, a rechallenge is not necessarily accepted as proof of causation by drug safety surveillance specialists, because the persuasiveness of a reported rechallenge may be highly situation-dependent.25
A second distinct and informative way to identify drugs that can cause thrombocytopenia is to identify antibodies that react with platelets only in the presence of the clinically suspected drug. A positive finding provides very strong evidence that drug sensitivity is the cause of thrombocytopenia and can be helpful for understanding the mechanisms of DITP.26 Because antibody testing is ordered only when a particular drug is suspected of being a possible cause of thrombocytopenia, identification of drug-dependent, platelet-reactive antibodies reflects both clinical judgment and the laboratory test result. However, tests to identify drug-dependent, platelet-reactive antibodies are performed in few specialized laboratories, and physicians may not be aware of the availability of these analyses. Even when laboratory analysis for drug-dependent, platelet-reactive antibodies is performed, tests can be negative in patients with DITP because (1) assay methods may be insufficiently sensitive to detect some antibodies; (2) some drugs are relatively insoluble in water and are difficult to incorporate into in vitro assays; and (3) a metabolite of the drug formed in vivo, rather than the primary drug, may be responsible for the thrombocytopenia.2 A limitation of the present data may be that we report results from only a single laboratory that primarily tests samples from the United States. There are other case reports of drug-induced thrombocytopenia that describe drug-dependent antibodies. For example, Kroll et al27 described 5 patients with drug-induced thrombocytopenia and carbimazole-dependent antibodies. Gold has been reported to induce drug-independent autoantibodies to platelets, resulting in a syndrome similar to autoimmune thrombocytopenic purpura.28 However, for the present analysis, data from the BloodCenter of Wisconsin, perhaps the largest resource for drug-dependent, platelet-reactive antibody testing in the United States, provided results from many samples submitted from across the United States using consistent methods over a 14-year period.
When the results of these 2 distinct methods of drug identification were combined, 351 drugs were identified that were suspected of causing thrombocytopenia. Although 130 (37%) of the 351 drugs had evidence for a causal association with thrombocytopenia by at least 1 of the 2 methods, only 104 drugs (30%) had been evaluated by both methods, and only 24 drugs were identified as being causal by both methods.
The third approach used was data mining of the FDA's AERS database. Because reporting has been simplified by the MedWatch Web site, the AERS database may provide a more comprehensive list of drugs that can cause thrombocytopenia. The FDA's quarterly reports of AERS data include all drugs and all adverse events but do not incorporate case-level information from the clinical narratives of the individual MedWatch reports. AERS data cannot be used to assess actual risk or incidence, because (1) reports are uncontrolled, are not peer reviewed, and are of uneven quality; (2) they are subject to numerous biases and reporting artifacts that may lead to both underreporting and overreporting of drug-event associations; and (3) they do not contain information on the number of persons exposed or at risk. Therefore, data mining cannot determine whether a drug is causally associated with thrombocytopenia. Data mining algorithms to search the AERS database are a screening tool of existing data that can identify a statistical reporting association of drugs with thrombocytopenia. Therefore, data mining may detect an initial signal that a drug is associated with thrombocytopenia that might be confirmed by subsequent clinical evidence and laboratory detection of drug-dependent antibodies. In addition, if a drug had been associated with thrombocytopenia by clinical or laboratory data, additional detection of its association with thrombocytopenia by data mining may increase the level of suspicion for the drug's risk for causing thrombocytopenia. The sensitivity and/or lack of specificity of data mining was apparent by the identification of a statistically significant reporting association with thrombocytopenia for 482 drugs that did not have evidence for a causal relation with thrombocytopenia by either the clinical or laboratory methods.
The 3 methods for identifying drugs that can cause thrombocytopenia used in the present study each have unique perspectives. No method was inclusive of all drugs that could cause thrombocytopenia, and there was relatively little overlap among the drugs identified by each method. A potential limitation of the present data was that data from each method were current with regard to a different time in 2008 or 2009. Furthermore, the suspicion of drugs as a cause of thrombocytopenia and the resulting publication of case reports, submission of samples for laboratory testing, and reporting to the FDA MedWatch were not systematic but were only the result of the initiative of individual clinicians. There is no basis for assuming that these data are representative of the US population.
Even among the 102 drugs tested by all 3 methods, there was little overlap among the drugs identified by each method. Some drugs had evidence for an association with thrombocytopenia by 1 method but not others, by only 2 of the methods, by all 3 methods, or by none of the 3 methods; however, the combined use of all 3 methods and their datasets to identify drugs with evidence for an association with thrombocytopenia provides the most complete useful current information for evaluation and management of patients with suspected DITP. The 612 drugs identified as being associated with thrombocytopenia by any of the 3 methods should be considered as possible causes of thrombocytopenia, provided that the associated clinical features, such as timing of the onset of thrombocytopenia in relation to drug administration, are also consistent with a diagnosis of DITP for the drug in question (supplemental Table 1). Perhaps important for patient evaluations, the 23 drugs found to be associated with thrombocytopenia by all 3 methods should be considered to be a likely cause of otherwise unexplained thrombocytopenia in any patient receiving them (Table 2). This interpretation is supported by the observation that 6 of the 7 drugs that have been described in 10 or more case reports with “definite” or “probable” evidence for a causal association between the drug and thrombocytopenia, and 11 of the 12 drugs for which drug-dependent, platelet-reactive antibodies have been identified in 10 or more patients are included among these 23 drugs.
However, data obtained by the 3 methods are insufficient to accurately rank drugs against each other according to their relative risk for causing thrombocytopenia. Future research may incorporate more quantitative information and epidemiologic analyses to develop algorithms that can inform physicians about the relative risks for thrombocytopenia with individual drugs. Improvement in causality-assessment algorithms may be supported by future research to determine the risk of thrombocytopenia associated with each drug related to the number of patients exposed to the drug. Because drug-dependent, platelet-reactive antibodies typically develop within 1 to 2 weeks after a new drug is begun (or over a longer period when a drug is taken intermittently),1 the number of patients who have been exposed to each drug is the important observation, not the duration of exposure to the drug. Currently, only limited data have been reported from population surveillance5,29 and case-control studies.6 With wider use of electronic medical records, it may be possible for future studies to estimate these data.
The limited overlap among these 3 methods for identifying drugs that can cause thrombocytopenia and the difficulty of establishing an unequivocal diagnosis of DITP emphasize the responsibility of clinicians to carefully consider possible drug causes in their evaluation of patients with thrombocytopenia. Careful clinical evaluation should include explicit questions about drug use, including the occasional use of over-the-counter drugs, quinine-containing beverages, and other nonprescription remedies.30 The evaluation should also document the clinical criteria that are required to establish evidence for a causal association of the suspected drug with thrombocytopenia.9 If clinical evidence is not “definite,” or if a positive test for drug-dependent, platelet-reactive antibodies has not been documented previously, consideration should be given to testing the patient for the presence of drug-dependent antibodies.11,12
If there is evidence that a drug caused thrombocytopenia, the physician should report this experience to the FDA MedWatch Web site (https://www.accessdata.fda.gov/scripts/medwatch/) for inclusion in the AERS database and also to the drug manufacturer. Finally, if there are few case reports describing “definite” or “probable” clinical evidence for the drug's association with thrombocytopenia, it is important to publish this experience. The data derived from an increased effort to document drugs as the cause of thrombocytopenia will help in the development of causality-assessment algorithms; these algorithms can then provide clinicians with more accurate information about which drugs are most likely to cause thrombocytopenia. Accurate information about drugs that can cause DITP will strengthen the ability of clinicians to assess future patients with suspected DITP and will support the efforts of health professionals to provide drug safety surveillance.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shari C. Clifton, MLIS, AHIP, Professor/Head, Reference & Instructional Services, Robert M. Bird Health Sciences Library, University of Oklahoma Health Sciences Center, for her assistance with identifying resources for identification of drugs that are currently approved worldwide.
This work was supported in part by the National Institutes of Health (HL-13629 and HL-44612; R.H.A. and D.W.B.).
National Institutes of Health
Authorship
Contribution: All authors designed the research, analyzed the data, and wrote the paper. R.H.A., D.W.B., and B.R.C. performed tests for drug-dependent antibodies; M.H. performed the data mining; and J.A.R., X.L., J.N.G., and S.K.V. synthesized the data.
Conflict-of-interest disclosure: R.H.A. and B.R.C. are supported in part by the BloodCenter of Wisconsin and its Laboratory of Platelet Immunology. M.H. is Medical Director of Risk Management Strategy for Pfizer, Inc, owns stock and stock options in Pfizer, Inc, and owns stock in other pharmaceutical companies that may market drugs mentioned in this article or competing drugs from the same pharmacologic/therapeutic classes as drugs mentioned in this article. The remaining authors declare no competing financial interests.
Correspondence: Sara K. Vesely, PhD, Department of Biostatistics and Epidemiology, Room CHB 358, The University of Oklahoma Health Sciences Center, PO Box 26901, Oklahoma City, OK 73190; e-mail: sara-vesely@ouhsc.edu.