Abstract
The natural cell type(s) that synthesize and release factor VIII (FVIII) into the circulation are still not known with certainty. In vitro studies indicate that artificial expression of FVIII in endothelial cells produces an intracellular pool of FVIII that can be mobilized together with its carrier protein, von Willebrand factor (VWF), by agonists. Here, we show that expression of human B-domain deleted FVIII (hFVIII) in the vascular endothelium of otherwise FVIII-deficient mice results in costorage of FVIII and VWF in endothelial Weibel-Palade bodies and restores normal levels and activity of FVIII in plasma. Stored FVIII was mobilized into the circulation by subcutaneous administration of epinephrine. Human FVIII activity in plasma was strictly dependent on the presence of VWF. Endothelial-specific expression of hFVIII rescued the bleeding diathesis of hemophilic mice lacking endogenous FVIII. This hemostatic function of endothelial cell–derived hFVIII was suppressed in the presence of anti-FVIII inhibitory antibodies. These results suggest that targeting FVIII expression to endothelial cells may establish a releasable pool of FVIII and normalize plasma FVIII level and activity in hemophilia A, but does not prevent the inhibitory effect of anti-FVIII antibodies on the hemostatic function of transgene-derived hFVIII as is seen with platelet-derived FVIII expression.
Introduction
The hereditary deficiency of factor VIII (FVIII) leads to hemophilia A, a severe X-linked bleeding disorder.1 The precise site of FVIII biosynthesis and the cellular origin of the regulated releasable pool of FVIII remain unclear.2-5 It has been proposed that synthesis of FVIII occurs in a subpopulation of endothelial cells.6-11 Targeting FVIII expression to lung endothelial cells12 or liver sinusoidal endothelial cells13 has been shown to result in phenotypic correction in hemophilia A mice. In vitro studies performed in our laboratory have demonstrated that FVIII traffics to storage granules in a von Willebrand factor (VWF)-dependent manner and is coreleased with VWF by agonist stimulation.14,15 We explored the hypothesis that directing FVIII synthesis to a cell type producing and storing VWF would facilitate the secretion and protection of FVIII, as well as assist in the formation of a secretory pool that could be released at the sites of injury and thereby achieve improved hemostatic effectiveness.
VWF is synthesized in 2 cell types within the body, endothelial cells and megakaryocytes, and is stored in Weibel-Palade bodies (WPBs) of endothelial cells and α-granules of megakaryocytes and platelets.16,17 Our previous studies have demonstrated that targeting FVIII expression to platelets results in storage of FVIII together with VWF in α-granules and that platelet-derived FVIII can correct the murine hemophilia A phenotype even in the presence of high-titer anti-FVIII inhibitory antibodies.18,19 Because endothelial cells are the only cells, other than megakaryocytes, that also synthesize and store VWF, we explored in the current study the properties and effectiveness of endothelial cell–derived FVIII in a hemophilia A mouse model. To this end, we generated a transgenic mouse strain (T2F8) expressing human B-domain–deleted FVIII under the transcriptional control of the endothelial cell–specific promoter/enhancer of the Tek (Tie2) receptor tyrosine kinase gene (Tie2 promoter/enhancer).20,21 This T2F8 mouse strain was used to determine the efficacy of the endothelial cell–derived FVIII in hemophilia A mice with or without inhibitory antibodies, the effect of FVIII expression in the context of the endothelial cell regulated secretory pathway, and the effect of VWF on T2F8 expression.
Methods
Construction of vector
Tie2 promoter and enhancer were from the vector pHPPSDKXK,22 a kind gift from Takashi Minami (Harvard Medical School, Boston, MA). The 2.1-kb murine Tie2 promoter was cloned into pCIneo (Promega) in place of the cytomegalovirus (CMV) promoter, giving the vector, Tie2-pCIneo. The human FVIII cDNA used in this study has the entire FVIII B-domain deleted (hBDDFVIII), removing amino acids 741-1648, and was a kind gift of Randal J. Kaufman (University of Michigan, Ann Arbor, MI). hBDDFVIII was excised from the pMT2 vector23 and used to create pCMV-BDDFVIIIneo vector as described in our previous study.15 The 4.6-kb XhoI/SalI BDDFVIII fragment was excised from pCMV-BDDFVIIIneo and inserted into Tie2-pCIneo yielding the vector named pTie2-FVIIIneo. The Tie2 enhancer, a 1.8-kb NotI/DraIII fragment, was inserted into pTie2-FVIIIneo, generating the vector, pTie2-FVIII-En-neo. The fragment containing Tie2-FVIII-En was released with PvuI and inserted into HindIII-digested vector p1338 using blunted ligation. The Tie2-FVIII-En-neo (T2F8) expression cassette was released from the resulting vector, p1338-T2F8, by NsiI digestion and used for generation of transgenic mice.
Generation of transgenic mice
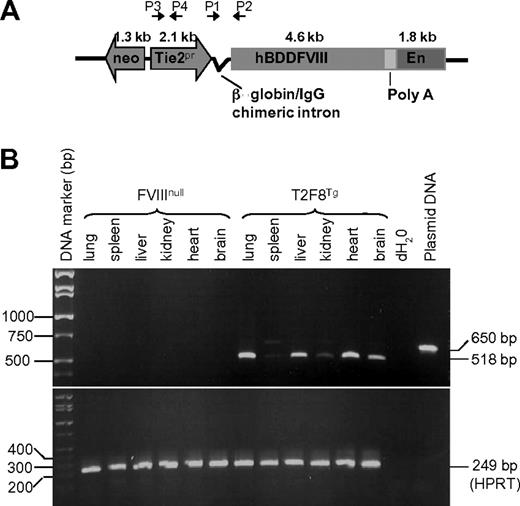
Studies were approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Transgenic mice were generated in the Transgenic Core Facility of the Blood Research Institute and Medical College of Wisconsin. A 10.7-kb fragment, containing a neomycin resistance cassette, the Tie2 promoter, β-globin/immunoglobulin G (IgG) chimeric intron, hBDDFVIII, SV40 poly A, and Tie2 enhancer, was electroporated into the D3 line of 129Sv embryonic stem (ES) cells.24 Colonies were selected for neomycin resistance using G418 and screened for the presence of transgene using the polymerase chain reaction (PCR) strategy shown in Figure 1A using 2 sets of primers (P1, forward 5′-AGGCCCTCAGAAGCTTTCCAGTAGGA-3′; P2, reverse 5′-AGGTAGCCTTGCAGAAGTTGGTCG-3′; P3, forward 5′-AGATGGATAGGGCTCGCTCTG-3′; P4, reverse 5′- TTGATACTTACCTGCCCAGTG-3′). Transgene-positive ES cells were propagated for microinjection into blastocysts from C57BL/6 mice (Charles River Laboratories International) to generate chimeric mice carrying the transgene. Upon germline transmission, the T2F8 transgene was then bred into the exon 17 FVIII knockout (FVIIInull) background to generate T2F8 transgenic mice expressing endothelial cell–derived FVIII without expression of murine FVIII [T2F8Tg, includes heterozygous (T2F8tg+/−) and homozygous (T2F8tg+/+) mice]. Genotype determination was performed by PCR analysis of blood-derived genomic DNA samples using the primers described above. All mice used in this study were in a C57BL/6 × 129S background except VWF deficient (VWF−/−) mice, which were in the C57BL/6 background.
Generation of T2F8tg+/+ and T2F8TgVWF−/− mice
T2F8tg+/+ mice were generated from T2F8tg+/− mating. Genotype was determined by PCR and quantitative real-time PCR as previously described using primers specific for human FVIII cDNA sequence.19,25 For quantitative real-time PCR, peripheral white blood cell (WBC)–derived genomic DNA was analyzed for quantitation of the T2F8 transgene copy number, with normalization to the Apo B gene using Platinum Quantitative PCR SuperMix-UDG (Invitrogen). Mating T2F8Tg and VWF−/− mice followed by sibling mating of offspring generated T2F8TgVWF−/− mice. VWF-deficient mice were identified by a solid phase capture enzyme-linked immunosorbent assay (ELISA) for mouse plasma VWF antigen (VWF:Ag), using rabbit anti–human VWF antibody that cross-reacts with murine VWF (Dako) for capture and horseradish peroxidase (HRP)–labeled VWF antibody for detection.
RT-PCR analysis of transgenic hFVIII mRNA
Total RNA was extracted from tissues including lung, spleen, liver, kidney, heart, and brain using the RNeasy Mini Kit (QIAGEN) after perfusion with phosphate-buffered saline (PBS). First strand cDNA was synthesized by reverse transcription (RT) using the Reverse Transcription System (Promega) with random primers. The same primers (P1 and P2) as described above for PCR were used for RT-PCR. PCR of HPRT (the hypoxanthine-guanine phosphoribosyl transferase gene) was used as an internal control to assess mRNA integrity. The primers used to detect mouse HPRT mRNA were 5′-GCTGGTGAAAAGGACCTCT-3′ (forward) and 5′-CACAGGACTAGAACACCTGC-3′ (reverse). RNA purified from FVIIInull mouse tissues were used as controls.
Immunofluorescent staining for hFVIII
Visual detection of human B-domain deleted FVIII (hFVIII) in transgenic endothelial cells was accomplished with immunofluorescent confocal laser scanning microscopy. Endothelial cells were isolated from lung and heart of 6- to 8-day-old mice as previously reported.26,27 Briefly, lungs and hearts were harvested from 2 to 3 pups, finely minced, and digested with 0.5 mg/mL collagenase (Sigma-Aldrich) in Dulbecco modified Eagle medium (DMEM; Invitrogen) at 37°C for 50 minutes to disperse cells. Endothelial cells were isolated by immunoselection using Dynabeads M-450 sheep anti–rat IgG beads (Dynal) coated with purified rat anti–mouse CD31 (PECAM-1) antibody (BD Biosciences). The bead-bound cells were collected using a magnetic separator (Invitrogen) and resuspended in endothelial cell growth medium [DMEM containing 20% fetal bovine serum (FBS), 100 μg/mL porcine heparin, 100 μg/mL endothelial cell growth supplement, 25mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1mM sodium pyruvate, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin], plated in human fibronectin-coated chamber slides (BD Biosciences) and cultured for 4 days.
Immunofluorecent staining was performed as described in our previous report.18 In brief, cells were fixed using 3.7% formalin, permeabilized with 5% Triton 100, and blocked in 2.5% normal goat serum for 1 hour at room temperature. Cells were incubated at 4°C overnight with 5 μg/mL rabbit anti–human FVIII polyclonal antibody (Conan; produced by our laboratory) directly conjugated to Alexa Fluor 488 and 5 μg/mL rabbit anti–human VWF polyclonal antibody (Dako), which cross-reacts with mouse VWF, directly conjugated to Alexa Fluor 568. Nonspecific isotype control antibody served as negative control. After extensive washes, cells were mounted under glass coverslips with Vectashield. Fluorescence detection was performed by confocal microscopy using a FV1000-MPE Multiphoton Microscope (Olympus).
FVIII assays
The expression levels of plasma FVIII in T2F8 mice were determined by FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) assays. Blood samples were collected from mice by tail bleeding into tubes containing 0.1 volume of 0.1M sodium citrate, and plasma was isolated for assays. FVIII:C levels in mouse plasma were quantitated by a modified FVIII chromogenic assay that we have developed using the Coatest SP4 FVIII kit (DiaPharma) as previously described.18 Briefly, plasma was diluted at least 1:40 in 1× Coatest buffer to overcome the inhibition of the assay by plasma components, and 25 μL diluted plasma was added to 96-well microtiter plates in duplicate. Assay components, including FIXa, FX, CaCl2, and phospholipid were added to each well, and the plate was incubated at 37°C for 10 minutes. The chromogenic FXa substrate S-2765 was added, and the plate was transferred immediately to a ThermoMax microplate reader (Molecular Devices) preset at 37°C. A standard curve was constructed by plotting known amounts of rhFVIII (Refacto; Wyeth Pharmaceuticals) in 1× Coatest buffer against Vmax (mOD/min) at 405-490 nm. Human FVIII antigen (FVIII:Ag) levels in mouse plasma were determined by ELISA as described in our previous report.25 Wild-type (WT) and FVIIInull mice served as controls.
Effect of epinephrine on the release of FVIII:C and VWF in T2F8 transgenic mice
To explore whether targeting FVIII expression to endothelial cells can establish a releasable pool of FVIII together with VWF, epinephrine (1 mg/mL; American Regent) was administrated by subcutaneous injection at a dose of 2 mg/kg body weight in 200 μL of 0.9% NaCl saline. Blood samples were collected from infused mice before infusion and 30 minutes after infusion, and plasmas were prepared for FVIII:C and VWF:Ag assays. NaCl saline (0.9%) was used as a control for infusion studies.
Assessment of phenotypic correction
To evaluate whether endothelial cell–derived FVIII improves hemostasis in hemophilia A mice, 3 in vivo injury models were used including tail clip survival tests, a FeCl3-induced arterial injury model, and an electrolytic venous injury model. The tail clip survival test was performed as described in our previous studies.18,19,25
FeCl3-induced arterial injury was induced according to published procedures.28 Briefly, the formation of thrombi in the carotid artery of animals was induced by application of a 20% FeCl3 solution to the exposed vessel for 1 minute. The field was flushed with saline, and blood flow was monitored for 30 minutes. The presence or absence of complete arterial occlusion at 30 minutes was recorded.
For the electrolytic venous injury model, the femoral vein was exposed through an incision, and the procedures were performed as previously reported.29 Briefly, a 75-μm–diameter steel microsurgical needle was inserted into the blood vessel, and a 1-mm length was held against the inner surface of the vessel. A 1-minute 1.5-volt anodal electrical stimulation was delivered from an AA battery. The needle was then withdrawn. After 10 minutes, the entire femoral vein was isolated and fixed in PBS with 4% formaldehyde. The vessel was then sectioned at 5 μm, stained with hematoxylin and eosin, and the clot region was digitally captured with a DMC-1 Polaroid digital camera. The clot areas per section were measured with ImageTool 3.0 software (NIH, public domain software; current version, ImageJ), and volume reconstructions were made to compute the volume of each clot. WT and FVIIInull mice were used as controls.
Evaluation of the efficacy of endothelial cell–derived FVIII in the presence of anti-FVIII inhibitory antibodies
Mice were immunized with 200 μL of rhFVIII (Refacto) at 600 U/kg in the presence of adjuvant (Sigma-Aldrich) by intraperitoneal injection to induce anti–human FVIII antibodies. To determine the titer of inhibitory antibodies, also known as inhibitors, a modified Bethesda assay was performed as described in our previous studies.18,19,25
T2F8 transgenic bone marrow transplantation (BMT)
It is known that the Tie2 gene is not only expressed in endothelial cells, but also in hematopoietic stem cells, some monocytes, and leukemia cells.30-32 We explored whether hematopoietic cells from T2F8Tg mice contributed to FVIII expression in the plasma. BMT from T2F8Tg mice into lethally irradiated FVIIInull mice was performed as previously described.18 Briefly, BM was harvested by flushing the femurs and tibias, and mononuclear cells were isolated with Fico/Lite-LM (mouse; ATLANTA Biological). FVIIInull mice (recipients) were conditioned with 1100 cGy total body irradiation. Twenty-four hours after irradiation, a cell dose of 1 × 107 cells from T2F8Tg mice in a volume of 300 μL/mouse was transplanted by retro-orbital injection. After allowing 3 weeks for BM reconstitution, blood samples were collected from recipients for analysis.
Statistical analysis
FVIII:C and FVIII:Ag results are presented as mean ± standard deviation (SD), and the significance of differences between groups of mice was evaluated by 2-tailed Student t test. The volumes of clots in venous thrombi are presented as mean ± SE, and the significance of differences between groups of mice were evaluated by Student-Newman-Keuls post hoc test. A value of P < .05 was considered statistically significant.
Results
FVIII expression in T2F8 transgenic mice
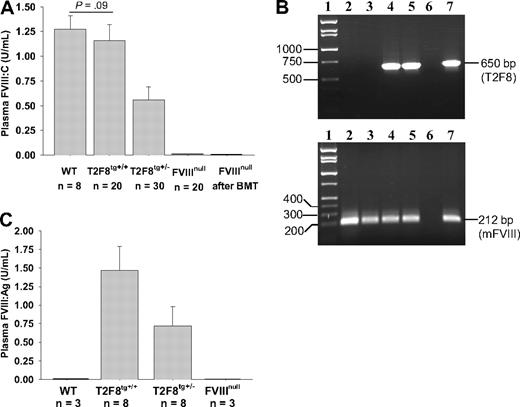
We generated transgenic mice expressing endothelium-specific hFVIII by using a 10.7-kb insert of mouse Tie2 promoter-driven hBDDFVIII with the Tie2 enhancer construct (T2F8; Figure 1A). Germ line transmission was established, and transgene positive offspring were mated with FVIIInull mice to generate mice that express the T2F8 transgene without expression of normal murine FVIII. The copy number of T2F8 transgene was determined by quantitative real-time PCR. There was one copy of T2F8 gene per cell in T2F8tg+/− and 2 copies per cell in T2F8tg+/+ mice.
T2F8 transgene construct and genetic analysis. (A) Schematic diagram of transgene construct. T2F8 cassette (from 5′ to 3′): neomycin resistance, the murine Tie2 gene promoter (Tie2 promoter), chimeric intron, human B-domain deleted factor FVIII, poly A, and the Tie2 enhancer. (B) RT-PCR analysis shows that spliced T2F8 transgene mRNA (518 bp) is detected in the tissues from T2F8 transgenic mice. The housekeeping gene, HPRT, was used as an internal control (bottom panel).
T2F8 transgene construct and genetic analysis. (A) Schematic diagram of transgene construct. T2F8 cassette (from 5′ to 3′): neomycin resistance, the murine Tie2 gene promoter (Tie2 promoter), chimeric intron, human B-domain deleted factor FVIII, poly A, and the Tie2 enhancer. (B) RT-PCR analysis shows that spliced T2F8 transgene mRNA (518 bp) is detected in the tissues from T2F8 transgenic mice. The housekeeping gene, HPRT, was used as an internal control (bottom panel).
The human FVIII transgene mRNA was amplified from total RNA by RT-PCR using primers designed to amplify a 518-bp fragment across the 132-bp β-globin/IgG intron, allowing us to distinguish spliced mRNA from the 650-bp genomic DNA product. With tissues from T2F8Tg mice, the 518-bp fragment was most abundant in lung and heart tissues, followed by liver, brain and kidney, and was weakest in spleen (Figure 1B). In contrast, no signal was amplified from similar FVIIInull tissue samples. The HPRT internal control was amplified from all samples, demonstrating the RNA quality for each sample.
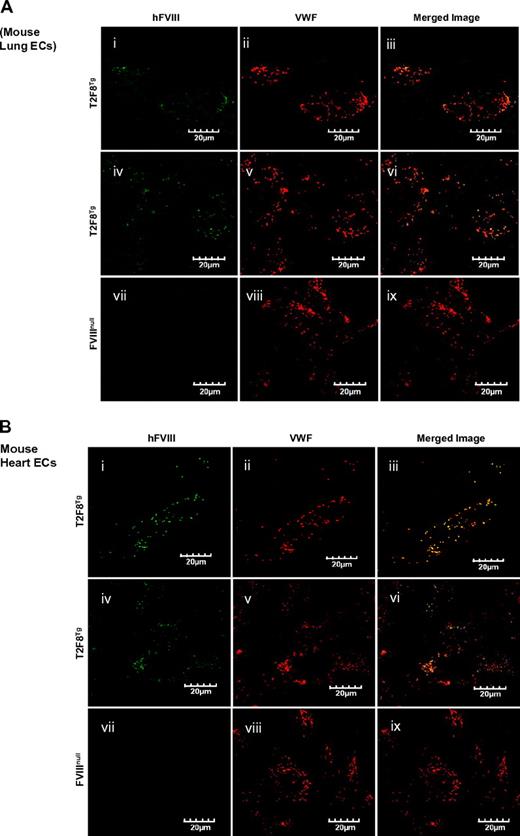
It has been shown that the Tie2 promoter results in transgene expression in endothelial cells.22,33-35 Immunostaining was performed to examine the expression of FVIII in tissue sections. Unfortunately, immunohistochemical and immunofluorescent staining of FVIII in tissue sections gave inconsistent results and could not be interpreted. We therefore isolated endothelial cells from neonatal lung and heart to examine whether FVIII was costored with VWF in the endothelial cells of T2F8 transgenic mice. Immunofluorescent confocal microscopy was performed and confirmed the expression of recombinant FVIII within endothelial cells isolated from T2F8Tg mouse lung (Figure 2Ai-vi) and heart (Figure 2Bi-v), and colocalization with mouse VWF in WPB as shown in yellow in the merged images (Figure 2Aiii,vi,B,iii,vi). FVIII was absent from endothelial cells of FVIIInull control mice, although a normal distribution of VWF was observed (Figure 2Avii-ix,Bvii-ix).
Immunostaining of hFVIII. Localization of transgene protein expression was determined by immunofluorescent confocal microscopy. Endothelial cells were isolated from lung (A) and heart (B) of neonatal mice by immunoselection using Dynabeads M-450 sheep anti-rat IgG beads coated with purified rat anti-mouse CD31 (PECAM-1) antibody. Cells were cultured in vitro for 4 days and then immunostained for either hFVIII using Conan-Alexa 488 or mouse VWF using Dako-Alexa 568. Transgene protein hFVIII was detected in endothelial cells from both lung (Ai-vi) and heart (Bi-vi) of T2F8Tg mice and was colocalized with mouse VWF in Weibel-Parade bodies as shown in yellow in merge images (iii, vi, xi, and xv). No hFVIII was detected in the endothelial cells from FVIIInull mice (Avii-ix,Bvii-x) These results demonstrate that targeting FVIII expression to endothelial cells results in FVIII storage together with VWF in endothelial cell WPB.
Immunostaining of hFVIII. Localization of transgene protein expression was determined by immunofluorescent confocal microscopy. Endothelial cells were isolated from lung (A) and heart (B) of neonatal mice by immunoselection using Dynabeads M-450 sheep anti-rat IgG beads coated with purified rat anti-mouse CD31 (PECAM-1) antibody. Cells were cultured in vitro for 4 days and then immunostained for either hFVIII using Conan-Alexa 488 or mouse VWF using Dako-Alexa 568. Transgene protein hFVIII was detected in endothelial cells from both lung (Ai-vi) and heart (Bi-vi) of T2F8Tg mice and was colocalized with mouse VWF in Weibel-Parade bodies as shown in yellow in merge images (iii, vi, xi, and xv). No hFVIII was detected in the endothelial cells from FVIIInull mice (Avii-ix,Bvii-x) These results demonstrate that targeting FVIII expression to endothelial cells results in FVIII storage together with VWF in endothelial cell WPB.
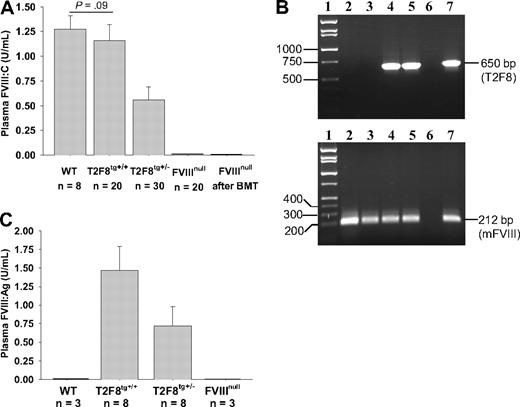
Functional FVIII activity (FVIII:C) in plasma of WT mice was 1.27 ± 0.14 U/mL when assayed against rhFVIII standards. Plasma FVIII:C was 0.56 ± 0.13 U/mL in T2F8tg+/− and 1.16 ± 0.16 U/mL in T2F8tg+/+ mice. The detection limit of this assay is 0.01 U/mL. The plasma FVIII:C levels in T2F8tg+/+ mice were not significantly different from WT control mice. FVIII:C was not detected in FVIIInull mice that received BMT from T2F8Tg mice (Figure 3A). Viable engraftment in recipients was confirmed by PCR (Figure 3B). Thus, plasma FVIII:C in T2F8Tg mice is derived from endothelial cells, with little, if any, contribution from bone marrow–derived hematopoietic cells.
FVIII expression in T2F8 transgenic mice. (A) Quantitative evaluation of FVIII activity levels in mouse plasma by chromogenic assay. FVIII:C was detected in the plasma of T2F8 transgenic mice. The level of FVIII:C in T2F8tg+/+ mice was similar to WT mice. FVIII:C was not detected in the plasma of FVIIInull mice that received BMT from T2F8Tg mice. (B) PCR analysis shows that the T2F8 transgene cassette (650 bp) is detected in FVIIInull mice after receiving BMT from T2F8Tg mice. Lane 1, DNA marker; lanes 2 and 3, FVIIInull pre-BMT; lanes 4 and 5, FVIIInull post-BMT; lane 6, H2O; lane 7, T2F8Tg control. DNA was purified from peripheral blood, and T2F8 transgene was amplified with primers P1 and P2 (upper panel). Mouse FVIII exon 4, which is not disrupted in FVIIInull mice, was amplified as an internal control (lower panel). (C) Quantitative evaluation of human FVIII antigen levels in mouse plasma by ELISA. hFVIII:Ag was detected in the plasma of T2F8Tg mice, but not in WT and FVIIInull mice. These results demonstrate that endothelial cell–derived FVIII can normalize plasma FVIII.
FVIII expression in T2F8 transgenic mice. (A) Quantitative evaluation of FVIII activity levels in mouse plasma by chromogenic assay. FVIII:C was detected in the plasma of T2F8 transgenic mice. The level of FVIII:C in T2F8tg+/+ mice was similar to WT mice. FVIII:C was not detected in the plasma of FVIIInull mice that received BMT from T2F8Tg mice. (B) PCR analysis shows that the T2F8 transgene cassette (650 bp) is detected in FVIIInull mice after receiving BMT from T2F8Tg mice. Lane 1, DNA marker; lanes 2 and 3, FVIIInull pre-BMT; lanes 4 and 5, FVIIInull post-BMT; lane 6, H2O; lane 7, T2F8Tg control. DNA was purified from peripheral blood, and T2F8 transgene was amplified with primers P1 and P2 (upper panel). Mouse FVIII exon 4, which is not disrupted in FVIIInull mice, was amplified as an internal control (lower panel). (C) Quantitative evaluation of human FVIII antigen levels in mouse plasma by ELISA. hFVIII:Ag was detected in the plasma of T2F8Tg mice, but not in WT and FVIIInull mice. These results demonstrate that endothelial cell–derived FVIII can normalize plasma FVIII.
The antigen level of human B-domain deleted FVIII in mouse plasma was determined by ELISA. Plasma FVIII:Ag was 0.72 ± 0.26 U/mL in T2F8tg+/− and 1.47 ± 0.33 U/mL in T2F8tg+/+ mice (Figure 3C). The detection limit of the antigen assay is 0.05 U/mL. As expected, no human FVIII:Ag was detected in WT and FVIIInull controls, demonstrating that the assay is specific for human FVIII:Ag.
The effect of VWF on T2F8 expression of FVIII
To explore whether coexpression with VWF is required for efficient biosynthesis of FVIII in endothelial cells, T2F8 trans-gene was bred into a VWF and FVIII double-knockout back-ground (T2F8TgVWF−/−). The levels of plasma FVIII:C in T2F8tg+/−VWF+/− animals were significantly decreased compared with T2F8tg+/− mice (0.36 ± 0.13 U/mL versus 0.56 ± 0.13 U/mL, P < .01). Of note, plasma FVIII:C was undetectable in both T2F8tg+/−VWF−/− and T2F8tg+/+VWF−/− mice. In contrast, the plasma levels of murine FVIII in VWF−/− mice were 0.12 ± 0.02 U/mL (Figure 4A).
The effect of VWF on plasma FVIII. (A) The level of FVIII:C is significantly decreased in T2F8tg+/−VWF+/− mice (P < .01) and was undetectable in T2F8TgVWF−/− mice. Approximately 10% of normal murine FVIII:C persisted in the plasma of VWF−/− mice. (B) Murine plasma-derived VWF was infused into T2F8TgVWF−/− mice to restore VWF levels to approximately 25% of normal. (C) Endothelial cell–derived plasma FVIII was rescued by the infused VWF. These results demonstrate that survival of endothelial cell–derived plasma hFVIII is VWF-dependent.
The effect of VWF on plasma FVIII. (A) The level of FVIII:C is significantly decreased in T2F8tg+/−VWF+/− mice (P < .01) and was undetectable in T2F8TgVWF−/− mice. Approximately 10% of normal murine FVIII:C persisted in the plasma of VWF−/− mice. (B) Murine plasma-derived VWF was infused into T2F8TgVWF−/− mice to restore VWF levels to approximately 25% of normal. (C) Endothelial cell–derived plasma FVIII was rescued by the infused VWF. These results demonstrate that survival of endothelial cell–derived plasma hFVIII is VWF-dependent.
To address whether VWF infusion can rescue endothelial cell–derived FVIII, plasma from FVIIInull mice was used as a source of murine VWF and infused into T2F8TgVWF−/− mice to restore VWF levels to approximately 25% of normal. Blood samples were collected before and after VWF infusion for FVIII:C and VWF:Ag assays. As expected, endothelial cell–derived plasma FVIII was stabilized by the infused VWF and was detected within 1 hour after infusion, with a peak at 4 hours in both T2F8tg+/−VWF−/− and T2F8tg+/+VWF−/− mice. Plasma FVIII was still detectable at 24 hours, when only a low level of VWF remained (Figure 4B-C). These results demonstrate that plasma VWF is essential in stabilizing endothelial cell–derived FVIII in plasma in this mouse model.
Epinephrine induces FVIII and VWF release from T2F8 transgenic mice
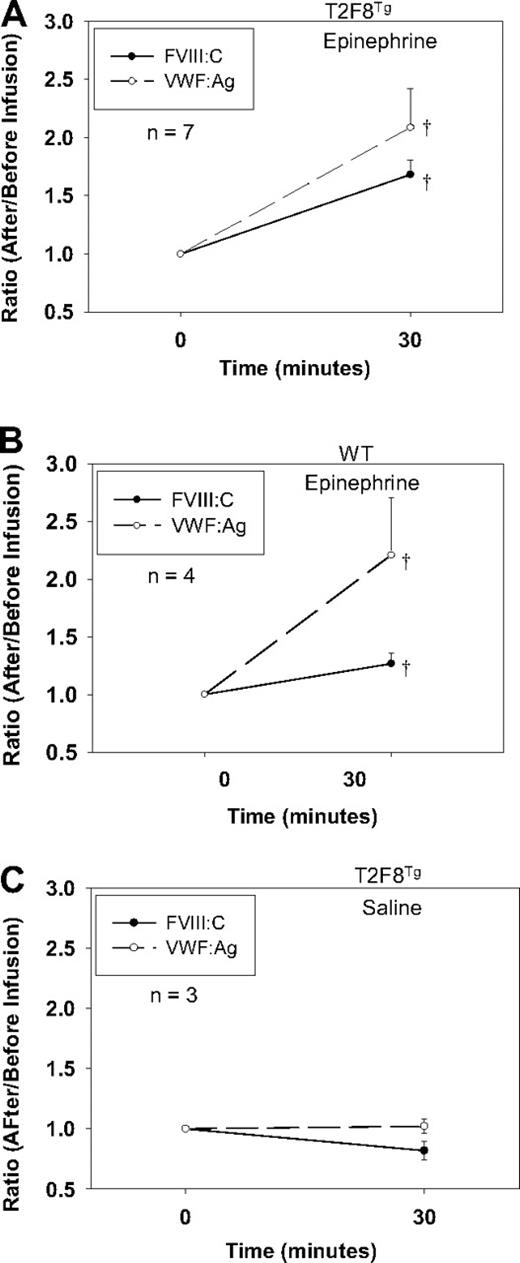
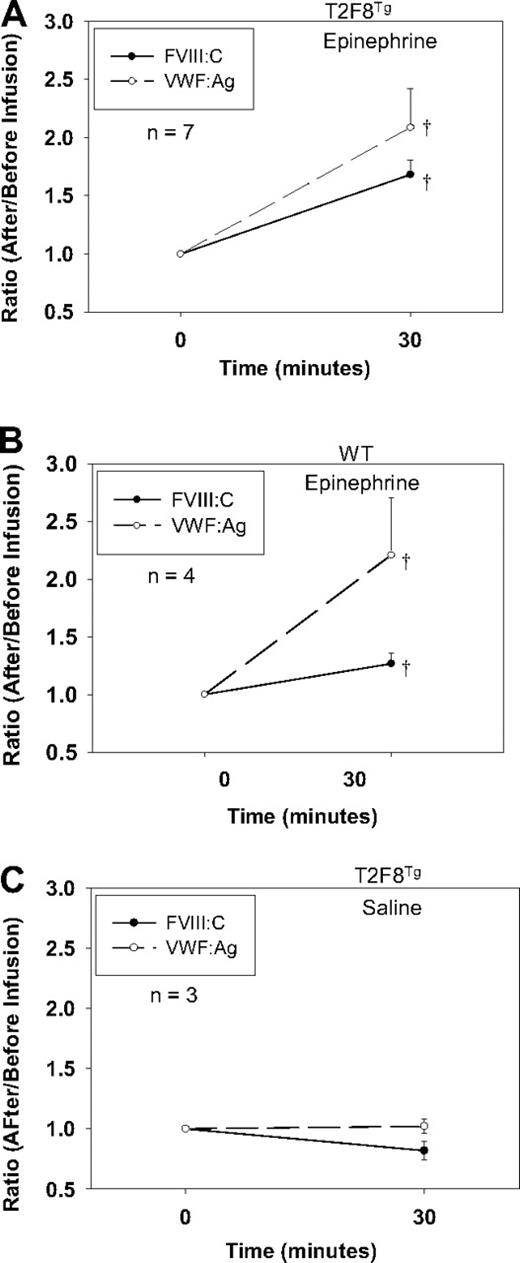
Although DDAVP (Desmopressin) releases both VWF and FVIII in humans, DDAVP does not release endothelial-stored proteins in mice. To explore if targeting FVIII to endothelial cells would establish a releasable pool of FVIII together with VWF in vivo, we treated T2F8Tg mice with epinephrine administration. Blood was collected before and after treatment, and plasma FVIII:C and VWF:Ag were quantitated. The levels of FVIII:C and VWF:Ag increased 1.68 ± 0.13- and 2.08 ± 0.34-fold, respectively, in the plasma from T2F8Tg mice in response to epinephrine (Figure 5A). Endogenous mouse FVIII:C increased 1.27 ± 0.09-fold in WT animals after epinephrine infusion. VWF:Ag increased 2.21 ± 0.5-fold in WT mice, which was not significantly different from T2F8Tg mice (Figure 5B). There was no significant difference in the levels of either FVIII:C or VWF:Ag in T2F8Tg mice before and after saline infusion (Figure 5C). No FVIII:C was detectable in T2F8TgVWF−/− mice either before or after epinephrine infusion (data not shown). These results demonstrate that targeting human FVIII expression to endothelial cells reestablishes a releasable pool of FVIII together with VWF.
Epinephrine releases FVIII. (A) Epinephrine releases FVIII and VWF from T2F8 transgenic mice. Epinephrine was administrated to animals and plasma samples were collected before and 30 minutes after infusion for FVIII:C and VWF:Ag assays. The levels of both FVIII and VWF significantly increased after epinephrine infusion in T2F8Tg mice; †P < .05. (B) Epinephrine releases endogenous mouse FVIII and VWF from WT mice. The levels of both FVIII and VWF significantly increased after epinephrine infusion in WT mice; †P < .05. (C) Saline administration control in T2F8Tg mice. There was no significant difference in the levels of either FVIII or VWF before versus after saline administration. These studies demonstrated that targeting FVIII to endothelial cells establishes a releasable pool of FVIII together with VWF.
Epinephrine releases FVIII. (A) Epinephrine releases FVIII and VWF from T2F8 transgenic mice. Epinephrine was administrated to animals and plasma samples were collected before and 30 minutes after infusion for FVIII:C and VWF:Ag assays. The levels of both FVIII and VWF significantly increased after epinephrine infusion in T2F8Tg mice; †P < .05. (B) Epinephrine releases endogenous mouse FVIII and VWF from WT mice. The levels of both FVIII and VWF significantly increased after epinephrine infusion in WT mice; †P < .05. (C) Saline administration control in T2F8Tg mice. There was no significant difference in the levels of either FVIII or VWF before versus after saline administration. These studies demonstrated that targeting FVIII to endothelial cells establishes a releasable pool of FVIII together with VWF.
Phenotypic correction of hemophilia by endothelial cell–derived FVIII
Mice expressing T2F8 transgene in endothelial cells were tested for correction of the hemophilia A phenotype. To assess correction of the FVIIInull coagulation defect, we determined the ability to clot and survive after inducing a minor wound by tail clipping. Clot formation and survival beyond 24 hours served as our criterion of correction of the murine hemophilia A phenotype. All T2F8 transgenic mice, including T2F8TgVWF−/− survived tail clipping, while only one of 6 FVIIInull mice survived under the same challenge (Figure 6A).
Assessment of phenotypic correction. (A) Tail clip survival test assessed phenotypic correction of hemophilia A mice. All T2F8 transgenic mice survived tail clipping. (B) FeCl3-induced thrombosis formation study. Four of 7 T2F8tg+/− mice developed complete occlusion in the FeCl3-induced carotid arterial injury model, which is not significantly different from WT controls (P = .41). (C) Electrical-induced femoral vein injury model. The volume of clot in T2F8tg+/− mice is 23-fold higher than in FVIIInull mice (P < .05). (D) Tail clip survival test of hFVIII immunized T2F8 transgenic mice. T2F8 transgenic mice were immunized once with rhFVIII (with adjuvant) by intraperitoneal injection. Two weeks after immunization, plasma was used for inhibitor quantitation, and tail clip survival test was performed. Only 20% of T2F8tg+/+ or T2F8tg+/− animals survived tail clip challenge. No T2F8TgVWF−/− mice survived tail clipping in the presence of inhibitors. These results demonstrate that endothelial cell–derived FVIII restores hemostasis to hemophilia A mice, but has limited clinical efficacy in the presence of anti-FVIII inhibitory antibodies.
Assessment of phenotypic correction. (A) Tail clip survival test assessed phenotypic correction of hemophilia A mice. All T2F8 transgenic mice survived tail clipping. (B) FeCl3-induced thrombosis formation study. Four of 7 T2F8tg+/− mice developed complete occlusion in the FeCl3-induced carotid arterial injury model, which is not significantly different from WT controls (P = .41). (C) Electrical-induced femoral vein injury model. The volume of clot in T2F8tg+/− mice is 23-fold higher than in FVIIInull mice (P < .05). (D) Tail clip survival test of hFVIII immunized T2F8 transgenic mice. T2F8 transgenic mice were immunized once with rhFVIII (with adjuvant) by intraperitoneal injection. Two weeks after immunization, plasma was used for inhibitor quantitation, and tail clip survival test was performed. Only 20% of T2F8tg+/+ or T2F8tg+/− animals survived tail clip challenge. No T2F8TgVWF−/− mice survived tail clipping in the presence of inhibitors. These results demonstrate that endothelial cell–derived FVIII restores hemostasis to hemophilia A mice, but has limited clinical efficacy in the presence of anti-FVIII inhibitory antibodies.
To further confirm the improvement of hemostasis in T2F8Tg mice, the FeCl3-induced arterial thrombosis and the electrical-induced venous thrombosis models were used. Four of 7 T2F8tg+/− mice developed complete occlusion in the FeCl3-induced carotid arterial injury model, which was not significantly different from WT controls (P = .41). In contrast, none of 6 FVIIInull mice developed stable occlusions (Figure 6B). In the electrical-induced femoral vein injury model, at 10 minutes T2F8tg+/− mice had clot volumes 23-fold higher than in FVIIInull mice as revealed by histomorphometric volume reconstruction of the clot (6.9 ± 2.5 μm3 versus 0.3 ± 0.2 μm3, respectively, P < .05). There was an apparent but not significant difference in clot volume between T2F8tg+/− mice versus WT mice (Figure 6C). Thus, both arterial and venous thrombus formation models demonstrated significant improvement of hemostasis at sites of injury in T2F8Tg mice.
The efficacy of endothelial cell–derived FVIII in the face of inhibitory antibodies
To assess whether endothelial cell–derived FVIII is still functional in the presence of anti-FVIII inhibitory antibodies, T2F8Tg mice were immunized with rhFVIII in the presence of adjuvant. All T2F8Tg and WT mice developed inhibitory antibodies after immunization and plasma FVIII:C dropped to undetectable levels (data not shown). When immunized mice were challenged with the tail clip survival test, only 20% of T2F8Tg animals survived tail clipping in both T2F8tg+/+ and T2F8tg+/− groups, which was not significantly different from immunized WT mice, in which one of 8 mice survived tail clipping (P = .44). No T2F8TgVWF−/− animals survived when anti-FVIII inhibitory antibodies were present (Figure 6D).
Discussion
Recently, studies by several groups have demonstrated that a subpopulation of endothelial cells naturally synthesize FVIII.2,7-11 Although it remains to be clarified whether endothelial cells from various vascular beds share this property, it is thought that nonhepatic endothelial cells might be a major source of FVIII secretion and contribute to the DDAVP-releasable pool in vivo. We wanted to know whether targeting FVIII expression to endothelial cells can reestablish a regulated releasable pool of FVIII and correct the hemophilia A phenotype. In the current studies, we generated transgenic animals with expression of FVIII under control of the endothelial cell–specific Tie2 promoter/enhancer, and we have demonstrated that endothelial production of FVIII normalizes plasma FVIII and reestablishes a releasable pool of FVIII. This endothelial cell–derived FVIII corrects hemostasis in hemophilia A mice.
There is release of functional recombinant FVIII from endothelium into plasma as well as storage of FVIII together with VWF in WPB in endothelial cells in T2F8 transgenic mice. The level of plasma FVIII is normalized in homozygous T2F8 mice, demonstrating that the Tie2 promoter/enhancer can efficiently direct FVIII expression in hemophilia A mice. The Tie2 promoter/enhancer has been used by several other groups and shown to direct vascular endothelial cell–specific gene expression in transgenic mouse models.22,33-35 It has been shown that Tie2 promoter activity is more pronounced in arteries, arterioles, and capillaries.34,35 At the mRNA level, T2F8 expression was most abundant in lung and heart tissues, which might be indicative that these organs contribute a significant proportion of the plasma FVIII. This could be due to the abundant vasculature in these tissues and/or to the intrinsic properties of the Tie2 promoter/enhancer. Immunofluorescent confocal microscopy confirmed that recombinant FVIII was expressed in endothelial cells and stored together with VWF in endothelial WPB when FVIII expression was driven by the Tie2 promoter/enhancer. It has been reported that the Tie2 gene is also expressed in hematopoietic stem cells, which play an important role in early hematopoiesis,30 and in some monocytes, which are involved in angiogenesis.31 De Palma and coworkers showed that the Tie2 promoter/enhancer directed transgene GFP expression in a significant fraction of human cord blood CD34+ cells, but this was effectively repressed in cell culture in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and virtually completely silenced in differentiating monocytic cells.31 Our BMT experiments supported our expectation that plasma FVIII in T2F8 mice is derived from endothelial cells, with little, if any, contribution from BM-derived hematopoietic cells.
FVIII stored together with VWF in WPB is releasable by epinephrine stimulation in T2F8 transgenic animals. The origin of the DDAVP-releasable pool of FVIII in humans is still not definitively known.6 Xu and coworkers have reported that liver-directed FVIII expression can normalize coagulation in hemophilia A dogs, but lack of a DDAVP response indicates that the DDAVP-releasable pool of FVIII in normal dogs may be from an extrahepatic source.4 Unlike humans and dogs, mice do not respond to DDAVP,36,37 and our studies support this conclusion. It has been shown that epinephrine, which affects vascular motility, increases plasma levels of FVIII:C and VWF:Ag in humans via a cAMP-dependent signaling pathway in responsive cells, which is the same pathway on which DDAVP acts.38,39 In T2F8Tg mice, FVIII and VWF release in response to epinephrine increased plasma levels by 68% and 108% respectively, demonstrating that a releasable pool of FVIII in parallel with VWF is reestablished in FVIIInull mice when FVIII expression is targeted to endothelial cells. In WT mice, plasma FVIII:C increased approximately 27% after stimulation with epinephrine, which is lower than in T2F8Tg mice, while VWF:Ag increased to similar levels (121%). These results demonstrate that both T2F8Tg and WT mice have a regulated secretory pool of FVIII but the extent of inducible release is different. This discrepancy could be due to differences between the cell-specificity of Tie2 and endogenous FVIII gene promoters, or because WT mice have full-length murine FVIII, while T2F8Tg mice express human B domain-deleted FVIII. No FVIII was detectable in T2F8TgVWF−/− mice in response to epinephrine infusion, indicating that the releasable pool of FVIII is VWF-dependent. In patients with mild VWD or hemophilia A, administration of the vasopressin analog DDAVP causes an almost immediate and parallel rise (2- to 5-fold) in both VWF and FVIII, but no FVIII or VWF is released in type 3 VWD.6,40 Studies done by our laboratory have demonstrated that FVIII is not internalized from culture media by endothelial cells in vitro41 and transfused FVIII does not reestablish the releasable pool in vivo.6 Taken together, our data support the notion that the DDAVP-releasable pool of FVIII in humans originates from endothelial cells.
Our studies have demonstrated that endothelial cell–derived FVIII can correct the murine hemophilia A phenotype, but its hemostatic efficacy is limited in the presence of inhibitory antibodies. Our previous studies have demonstrated that when FVIII expression is targeted to platelets, FVIII is sequestered within the platelets and that platelet-FVIII is protected from circulating inhibitory antibody inactivation and maintains its clinical efficacy even in the face of high-titer inhibitory antibodies.18,19 Theoretically, endothelial cells could provide local FVIII at sites of injury since both platelets and endothelial cells have stored pools of FVIII in the respective transgenic models. However, our findings show that endothelial delivery was not sufficient to correct the bleeding diathesis of hemophilic mice in the presence of anti-FVIII inhibitory antibodies. When FVIII expression is directed to endothelial cells, plasma FVIII is normalized, but drops to an undetectable level and loses its clinical effectiveness after only a single-dose immunization with rhFVIII in the presence of adjuvant, demonstrating that locally released FVIII from endothelial cells at sites of injury is not sufficient to rescue the murine hemophilia A phenotype when anti-FVIII inhibitory antibodies are present. We suspect that this difference can be attributed to a substantial difference in the amount of FVIII delivered by either activated platelets in the platelet-derived FVIII model18 or damaged local endothelial cells at the site of injury in the T2F8 model. Recruitment of additional platelets to the injury site provides a continuous supply of fresh FVIII, while endothelial cells are not sufficiently mobile to deliver additional FVIII. Why all T2F8 transgenic mice developed inhibitory antibodies after only a single-dose immunization with rhFVIII in the presence of adjuvant is still unknown and needs to be further investigated.
It is well known that plasma VWF is required to sustain normal plasma FVIII:C levels.42-44 The deficiency of FVIII in VWD is secondary to the VWF deficiency, which is explained by the fact that VWF forms a noncovalently linked complex with FVIII to stabilize and protect it from protease degradation. In the absence of VWF, free FVIII is rapidly degraded by proteases, reducing the plasma half-life of FVIII from 12 hours to 1-2 hours.6 In the clinic, a total absence of FVIII:C has only very rarely been reported in type 3 VWD. Plasma FVIII:C levels in severe type 3 VWD with no VWF in circulation generally range from 1% to 9%, where the FVIII synthesis is normal so this represents the steady state equilibrium of FVIII secretion and decay in the absence of VWF.45 In VWF knockout mice,44 in which normal endogenous murine FVIII is synthesized, there is approximately 10% FVIII activity persisting in plasma. When human FVIII is targeted to murine endothelial cells, the plasma FVIII level is normalized (T2F8tg+/+), but FVIII dropped to undetectable levels when T2F8 transgene was bred into the VWF and FVIII double-knockout background. This could be because of differential survival between murine FVIII and human FVIII or because the sources of endogenous mouse FVIII synthesis are not identical to sites of hFVIII production in T2F8Tg mice. Our studies did not establish which cells normally synthesize FVIII, but addressed the relative importance of synthesis in the context of VWF production and its regulated release.
Although plasma FVIII is undetectable in T2F8TgVWF−/− mice, all animals survived the tail clip challenge. This observation is reminiscent of a patient with type 3 VWD, which was recently reported by Mullah-Ali and coworkers, who had undetectable plasma FVIII:C, but the clinical bleeding tendency was remarkably mild.46 Both of these observations may be explained by the continuous synthesis and secretion of normal FVIII in T2F8TgVWF−/− mice and type 3 VWD. Although plasma FVIII is rapidly degraded in the absence of its carrier protein VWF, resulting in a profound steady-state deficiency in plasma, sufficient nascent FVIII must survive long enough to at least minimally meet localized needs. In contrast, in hemophilia A where FVIII synthesis is abnormal, resulting in qualitative and/or quantitative primary deficiencies of FVIII protein, the bleeding symptoms are often more severe. The secondary deficiency of plasma FVIII:C in T2F8TgVWF−/− mice can be rescued by VWF infusion, resulting in plasma FVIII:C stabilization and confirming the importance of VWF to maintenance of circulating FVIII:C.
In summary, our studies have demonstrated that targeting FVIII expression to endothelial cells in a transgenic mouse model cannot only normalize VWF-dependent plasma FVIII but also establishes a releasable pool of FVIII together with VWF. This endothelial cell–derived FVIII corrects the murine hemophilia A phenotype, but its efficacy in the presence of anti-FVIII inhibitory antibodies is significantly reduced compared with platelet-derived expression that maintains efficacy as we have previously reported.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the technical assistance of S. Kalloway, M. Kipp, J. Foeckler, and J. May (Transgenic Core Facility) in the generation of transgenic animals. We thank H. Kazazian at the University of Pennsylvania School of Medicine for the FVIIInull mice. The VWF−/− mice were developed by D. Wagner at Harvard Medical School and purchased from the Jackson Laboratory.
This work was supported by the American Heart Association National Center Scientist Development Award (0730183N; Q.S.), the National Hemophilia Foundation Career Development Award (Q.S.), the Hemophilia Association of New York grant (Q.S.), National Institutes of Health grants HL-102035 (Q.S.) and HL-44612 (R.R.M.), and the Goerlich Foundation (R.R.M.).
National Institutes of Health
Authorship
Contribution: Q.S. designed and performed the research, analyzed data, and wrote the manuscript; S.A.F. performed the research, analyzed data, and made comments on manuscript; H.W. assisted with the endothelial cell–specific Tie2 promoter construct, generated transgenic mice, and made comments on manuscript; E.L.K. and B.C.C. performed research; and R.R.M. helped in the design of the project, provided research support, critiqued results, and made comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: qizhen.shi@bcw.edu.