Abstract
Germline and somatic gain-of-function mutations in tyrosine phosphatase PTPN11 (SHP-2) are associated with juvenile myelomonocytic leukemia (JMML), a myeloproliferative disease (MPD) of early childhood. The mechanism by which PTPN11 mutations induce this disease is not fully understood. Signaling partners that mediate the pathogenic effects of PTPN11 mutations have not been explored. Here we report that germ line mutation Ptpn11D61G in mice aberrantly accelerates hematopoietic stem cell (HSC) cycling, increases the stem cell pool, and elevates short-term and long-term repopulating capabilities, leading to the development of MPD. MPD is reproduced in primary and secondary recipient mice transplanted with Ptpn11D61G/+ whole bone marrow cells or purified Lineage−Sca-1+c-Kit+ cells, but not lineage committed progenitors. The deleterious effects of Ptpn11D61G mutation on HSCs are attributable to enhancing cytokine/growth factor signaling. The aberrant HSC activities caused by Ptpn11D61G mutation are largely corrected by deletion of Gab2, a prominent interacting protein and target of Shp-2 in cell signaling. As a result, MPD phenotypes are markedly ameliorated in Ptpn11D61G/+/Gab2−/− double mutant mice. Collectively, our data suggest that oncogenic Ptpn11 induces MPD by aberrant activation of HSCs. This study also identifies Gab2 as an important mediator for the pathogenic effects of Ptpn11 mutations.
Introduction
Juvenile myelomonocytic leukemia (JMML), a myeloproliferative disease (MPD) of young children characterized by cytokine hypersensitivity of myeloid progenitors, is associated with mutations in the rat sarcoma viral oncogene (RAS) pathway.1,2 Thirty-five percent of patients with JMML have activating mutations in tyrosine phosphatase PTPN11 (SHP-2), a known positive regulator of the Ras pathway (see next paragraph), while activating mutations in RAS (N-RAS or K-RAS) and homozygous inactivation of NF1, a GTPase activating protein that negatively regulates RAS output, account for 20% and 15% of JMML cases, respectively.1,2 More recently, loss-of-function mutations in a E3 ubiquitin ligase c-CBL have been identified in 10%-15% of patients with JMML.3,4 PTPN11, RAS, NF1, and c-CBL mutations are usually mutually exclusive in patients. Remarkably, mutations in these genes play a causal role in the pathogenesis of JMML. Single disease mutations, such as Ptpn11D61G/Y, K-RasG12D, Nf1 deficiency, and Cbl deficiency, are necessary and sufficient to induce cytokine hypersensitivity in myeloid progenitors and JMML-like MPD in mice.5-12
Ptpn11 (Shp-2), a ubiquitously expressed protein tyrosine phosphatase, is involved in multiple cell signaling processes, such as the RAS-MAP kinase, JAK/STAT, PI3K/AKT, NF-κB, and NFAT pathways.13-15 Intriguingly, despite its direct function in protein dephosphorylation, Shp-2 generally plays a positive role in transducing signals initiated from receptor and cytosolic kinases. This is particularly the case for the RAS pathway. The underlying mechanism, however, is unknown. Shp-2 interacts with a number of cell signaling intermediates. Of these partners, some are the targets of Shp-2 enzymatic activity. However, none of the putative substrates identified to date can fully account for the overall positive signaling effects of Shp-2 on the many biological processes with which it has been implicated. The scaffolding proteins Gab1 and Gab2 are prominent targets of Shp-2 phosphatase activity.16,17 Yet, Gab proteins form stable complexes with Shp-2 and play critical roles in growth factor/cytokine signal transduction, especially in RAS and PI3K/AKT pathways.16,17 Shp-2 is highly expressed in hematopoietic cells. Our previous studies have shown that Shp-2 plays an overall positive role in hematopoietic cell development18-20 and that it promotes cytokine (IL-3) signaling in both catalytically dependent and independent manners.21,22
PTPN11 is the most common target of genetic mutations in JMML.23,24 These mutations, such as congenital mutation D61G and somatic mutation E76K, disrupt inhibitory intramolecular interaction between the N-terminal SH2 (N-SH2) and catalytic domains, leading to hyperactivation of SHP-2.23,25 Furthermore, interactions of mutant Shp-2 with tyrosine phosphorylated signaling partners, such as Gab1 and Gab2, are enhanced by the mutations in the N-SH2 domain.26,27 However, as the biochemical basis for the positive role that Shp-2 phosphatase plays in the Ras pathway is entirely unclear, the cellular and molecular mechanisms by which gain-of-function (GOF) mutations in PTPN11 induce JMML are poorly defined. It is not completely understood how germ line and somatic mutations in PTPN11 impact hematopoietic stem cells (HSC) function. Furthermore, signaling partners that mediate the pathogenic effects of PTPN11 mutations have not been characterized.
To address these important questions and to further investigate the pathogenesis of JMML, we used Ptpn11D61G knock-in mouse model9 to analyze effects of germ line Ptpn11 GOF mutations on HSC function. We found that Ptpn11D61G mutation aberrantly enhanced HSC activity, leading to the development of MPD. MPD was reproduced in primary and secondary recipient mice transplanted with Ptpn11D61G/+ whole bone marrow (BM) cells or purified Lineage− Sca-1+c-Kit+ (LSK) cells that are enriched for HSCs, but not lineage committed progenitors. The deleterious effects of Ptpn11D61G mutation on HSCs were attributable to enhancing cytokine/growth factor signaling. The aberrant HSC activities caused by Ptpn11D61G mutation were largely corrected by deletion of Gab2, and MPD phenotypes were markedly ameliorated in Ptpn11D61G/+/Gab2−/− double mutant mice, suggesting Gab2 as an important mediator for the pathogenic effects of Ptpn11D61G mutation.
Methods
Mice
Ptpn11D61G /+ mice9 were originally imported from Beth Israel Deaconess Medical Center. Gab2+/− mice28 were obtained from Dr Toshio Hirano (Osaka University, Japan). Gab2+/− mice had been backcrossed with C57BL/6 mice at least 8 generations.29 Ptpn11D61G/+ mice were backcrossed with C57BL/6 mice for 3 generations and then used to cross Gab2+/− mice to generate Ptpn11D61G/+/Gab2+/− mice. Ptpn11D61G/+/Gab2+/− mice were intercrossed to produce various types of mice (4th generation backcross to the C57BL6 background) for this study. Backcrossing of Ptpn11D61G/+ or Ptpn11D61G/+/Gab2+/− mice to C57BL/6 mice could not be continued because of the complete penetrance of embryonic lethality in F5 Ptpn11D61G/+ mice.30 C57BL/6 (CD45.2+) mice and congenic strain B6.SJL-PtprcaPep3b/BoyJ (BoyJ, CD45.1+) mice were originally obtained from The Jackson Laboratory and subsequently bred in house. All mice were kept under specific pathogen–free conditions in the Animal Resources Center at Case Western Reserve University. All animal procedures complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Flow cytometric analysis and cell sorting
Multiparameter fluorescent-activated cell sorting (FACS) analysis was performed to determine percentages of stem cell-enriched LSK cells, long-term HSCs (LT-HSC), short-term HSCs (ST-HSC), and lineage progenitors, such as common myeloid progenitors (CMP), common lymphoid progenitors (CLP), granulocyte-macrophage progenitors (GMP), and megakaryocyte-erythroid progenitors (MEP) in the bone marrow (BM) cell population. BM cells were freshly harvested by flushing femurs and tibias following standard procedures. Cell harvesting efficiencies of the procedures in wild-type (WT), Ptpn11D61G/+, Gab2−/−, and Ptpn11D61G/+/Gab2+/− mice were nearly identical (data now shown). BM cells were first stained with anti–Flk2-biotin (eBioscience) and subsequently stained with antibodies labeled with various fluorochromes: streptavidin-APC-Cy7, c-Kit-APC, Sca-1-PE, CD34-Pacific Blue, CD16/32-PE-Cy7, CD127 (IL-7Rα)–PE-Cy5 (eBioscience), and FITC-labeled antibodies for lineage markers including Mac-1, Gr-1, Ter119, CD4, CD8a, CD3, and B220 (BD Biosciences). Specific cell populations were gated based on immunophenotypes for quantification or cell sorting as previously reported.31,32 Fluorescence minus 1 (FMO) was used for setting the gating on control samples.
To determine engraftment and contribution of donor/test cells in peripheral blood or BM of the transplant recipients, populations of various lineages derived from donor/test cells were quantified by double or triple immunostaining using anti-CD45.2–FITC in combination with Mac-1-APC, Gr-1-PE, B220-PE, Terr119-PE, or CD4-PE. Antibodies used in this study were purchased from BD Biosciences. Flow cytometric analyses were performed on BD LSR II or BD LSR I (BD Biosciences). Cell sorting was conducted using BD FACSAria. Data were analyzed using FlowJo software Version 8.5 (TreeStar).
Apoptosis and cell-cycle analysis
Fresh BM cells were stained with biotin-labeled antibodies to lineage markers (Gr-1, Mac-1, B220, Ter119, CD4, CD8, and CD3), followed by staining with streptavidin-conjugated APC-Cy7, anti–c-Kit–APC, and anti–Sca-1–FITC. The cells were subsequently stained with anti–annexin V–PE and 7-amino-actinomycin D (7-AAD) using the BD annexin V–PE Apoptosis Detection Kit I (BD Biosciences). Apoptosis was analyzed by quantification of the annexin V/7-AAD positive cell population by FACS. For HSC cell-cycle analysis, Pyronin Y and Hoechst 33342 staining and population gating were performed as previously reported.33,34
Generation of BM-derived mast cells and macrophages
BM cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) and mouse recombinant IL-3 (10 ng/mL) for 3-4 weeks. Mast cell phenotype was confirmed by FACS analysis with antibodies specific for c-Kit and FcγRII/RIII. At the time of use, greater than 98% of the cultured cells were mast cells. To generate BM-derived macrophages, BM cells were cultured in Dulbecco modified Eagle medium supplemented with 10% FBS and 20% L cell conditioned medium (as a source of mouse colony-stimulating factor 1). After 24 and 48 hours, nonadherent cells were collected and seeded into new tissue culture plates. After 5 to 7 days of culture, cells were confirmed as macrophages as more than 90% of semiadherent cells were positive for Mac-1 and F4/80.
CFU-S and transplantation assays
The colony-forming unit-spleen (CFU-S) assay was carried out as described.35 In brief, BM cells (1 × 105) were injected through the lateral tail vein into sub-lethally irradiated (9.5 Gy) C57BL/6 mice. Twelve days after the transplantation, recipients were killed and spleens were dissected and fixed with Telleyesniczky solution. Colonies on the spleen were counted under a dissecting microscope. For noncompetitive repopulation assays, 1 × 106 BM cells from 12 week old WT or Ptpn11D61G/+ littermates (CD45.2+) were directly injected through the lateral tail vein into lethally irradiated (11.0 Gy) BoyJ (CD45.1+) recipients. Donor cell reconstitution was monitored by FACS analyses of peripheral blood cells at 4, 8, and 16 weeks after transplantation. Sixteen weeks after transplantation recipients were killed and used as donors for the subsequent transplantation cycle. For competitive repopulation assays, 1 × 106 BM cells (test cells) from 12 week old WT or Ptpn11D61G/+ littermates (CD45.2+) were transplanted with the same number of BoyJ (CD45.1+) BM cells (competitor cells) into lethally irradiated BoyJ (CD45.1+) recipients. Test cell reconstitution was determined at 4, 8, 12, and 16 weeks by FACS analyses of peripheral blood. For secondary transplantations, BM cells (2 × 106) harvested from 3-5 primary recipient mice were pooled and transplanted into lethally irradiated secondary recipients. Contributions of test cells to the hematopoietic system were determined at 16 to 24 weeks after the secondary transplantation.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was assessed using the unpaired, 2-tailed Student t test. P values of < .05 were considered to be significant. Statistical significance among 4 groups was determined by 2-way analysis of variance (ANOVA) followed by Bonferroni or 1-way ANOVA followed by the Tukey posttest.
Results
Germline mutation Ptpn11D61G increases HSC and lineage progenitor populations
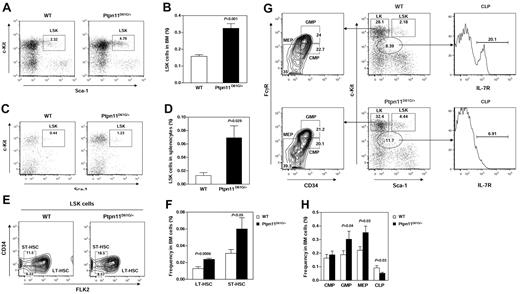
To investigate the mechanism by which GOF mutations in Ptpn11 induce MPD, we determined effects of Ptpn11 mutation on HSCs in Ptpn11D61G/+ knock-in mice, which develop JMML-like MPD characterized by excessive myeloid expansion in the BM and spleen (Ptpn11D61G/D61G mice die at embryonic day 13.5-15.5 due to heart developmental defects).9 Lineage−Sca-1+c-Kit+ (LSK) cells that are enriched for HSCs were quantified by flow cytometry. The frequency (Figure 1A-B) and the absolute number (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) of LSK cells were doubled in the bone marrow (BM) of Ptpn11D61G/+ mice. The difference in the percentages (Figure 1C-D) of LSK cells in the spleen between mutant and WT mice was even more dramatic. To further define the effects of Ptpn11D61G/+ mutation on hematopoietic cell development, phenotypic long-term HSCs (LT-HSC), short-term HSCs (ST-HSC), and later stage progenitor populations, including common myeloid progenitors (CMPs), common lymphoid progenitors (CLPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs), were quantified using multiparameter FACS analyses based on well-defined cell surface immunophenotypes31,32,36 (Figure 1E,G). We found that both LT-HSCs and ST-HSCs were significantly increased in Ptpn11D61G/+ mice (Figure 1F, supplemental Table 1). Percentages of GMPs and MEPs were also increased. The percentage of CLPs was relatively decreased in the mutant mice (Figure 1H, supplemental Table 1). The increase in primitive hematopoietic cell population and later stage myeloid progenitors suggests that Ptpn11D61G/+ mutation promotes expansion of both HSCs and the myeloid lineage.
Aberrant hematopoietic cell development in Ptpn11D61G/+ mice. (A-B) BM cells freshly harvested from femurs and tibias (both hind limbs) from 5- to 7-month-old Ptpn11D61G/+ and WT littermates (n = 10 per group) were assayed by multiparameter flow cytometric analysis to determine frequencies of hematopoietic cell populations of various stages and lineages. The cells were stained with antibodies against lineage markers, c-Kit, Sca-1, CD34, Flk2, CD16/32, and IL-7R (CD127). The proportion of BM cells corresponding to the HSC-containing LSK (Lineage−Sca-1+c-Kit+) cell population was quantified. (C-D) Frequencies of LSK cells in spleens of Ptpn11D61G/+ (n = 5) and WT (n = 4) littermates were determined as above. (E) BM LSK cells were sub-fractioned according to CD34 and Flk2 expression to yield phenotypic assessments of LT-HSC (LSK, Flk2−CD34−) and ST-HSC (LSK, Flk2−CD34+) fractions. (G) More differentiated BM LK cell (Lineage−c-Kit+Sca-1−) population was sub-fractioned based on CD16/32 and CD34 expression to identify CMP, GMP, and MEP progenitors (left panel). CLP progenitors were identified by gating L−KlowSlow (Lineage−c-Kit low Sca-1 low) cells followed by assessing CD127 expression (right panel). (F,H) Frequencies of LT-HSC, ST-HSC, CMP, GMP, MEP, and CLP populations in BM cells were quantified (n = 10 per group).
Aberrant hematopoietic cell development in Ptpn11D61G/+ mice. (A-B) BM cells freshly harvested from femurs and tibias (both hind limbs) from 5- to 7-month-old Ptpn11D61G/+ and WT littermates (n = 10 per group) were assayed by multiparameter flow cytometric analysis to determine frequencies of hematopoietic cell populations of various stages and lineages. The cells were stained with antibodies against lineage markers, c-Kit, Sca-1, CD34, Flk2, CD16/32, and IL-7R (CD127). The proportion of BM cells corresponding to the HSC-containing LSK (Lineage−Sca-1+c-Kit+) cell population was quantified. (C-D) Frequencies of LSK cells in spleens of Ptpn11D61G/+ (n = 5) and WT (n = 4) littermates were determined as above. (E) BM LSK cells were sub-fractioned according to CD34 and Flk2 expression to yield phenotypic assessments of LT-HSC (LSK, Flk2−CD34−) and ST-HSC (LSK, Flk2−CD34+) fractions. (G) More differentiated BM LK cell (Lineage−c-Kit+Sca-1−) population was sub-fractioned based on CD16/32 and CD34 expression to identify CMP, GMP, and MEP progenitors (left panel). CLP progenitors were identified by gating L−KlowSlow (Lineage−c-Kit low Sca-1 low) cells followed by assessing CD127 expression (right panel). (F,H) Frequencies of LT-HSC, ST-HSC, CMP, GMP, MEP, and CLP populations in BM cells were quantified (n = 10 per group).
Ptpn11D61G mutation promotes cell cycling and decreases apoptosis in HSCs
To delineate the mechanism by which Ptpn11D61G/+ mutation causes HSC expansion, cell-cycle status of LSK cells in Ptpn11D61G/+ mice was analyzed and compared with that in WT littermates. This analysis revealed an approximately 2-to-3-fold reduction in the percentage of the G0 phase LSK cells and an increase in the percentages of the G1 and S/G2/M phase LSK cells in Ptpn11D61G/+ mice (Figure 2A-B). These results suggest an enhanced entry of quiescent stem cells (G0 phase) into the cell cycle in the mutant mice. Furthermore, we evaluated the impact of Ptpn11D61G/+ mutation on stem cell survival. As shown in Figure 2C and D, apoptotic LSK cells in Ptpn11D61G/+ mice were markedly decreased. Thus, the expansion of the stem cell population in mutant mice appears to be attributed to both enhanced cell proliferation and decreased programmed cell death.
Enhanced cycling and decreased apoptosis in Ptpn11D61G/+ LSK cells. (A) BM cells freshly harvested from 5- to 7-month-old Ptpn11D61G/+ (n = 6) and WT (n = 5) littermates were assayed by multiparameter flow cytometric analysis to determine cell-cycle status of HSC-enriched LSK cells as described in “Methods.” (B) Percentages of LSK cells in G0, G1, and S/G2/M phases identified based on Pyronin Y and Hoechst staining profiles were determined. (C-D) BM cells from Ptpn11D61G/+ and WT littermates (n = 5 per group) were analyzed by multiparameter flow cytometric analysis to determine apoptosis (annexin V positive cells) in the LSK population as described in “Apoptosis and cell-cycle analysis.”
Enhanced cycling and decreased apoptosis in Ptpn11D61G/+ LSK cells. (A) BM cells freshly harvested from 5- to 7-month-old Ptpn11D61G/+ (n = 6) and WT (n = 5) littermates were assayed by multiparameter flow cytometric analysis to determine cell-cycle status of HSC-enriched LSK cells as described in “Methods.” (B) Percentages of LSK cells in G0, G1, and S/G2/M phases identified based on Pyronin Y and Hoechst staining profiles were determined. (C-D) BM cells from Ptpn11D61G/+ and WT littermates (n = 5 per group) were analyzed by multiparameter flow cytometric analysis to determine apoptosis (annexin V positive cells) in the LSK population as described in “Apoptosis and cell-cycle analysis.”
Ptpn11D61G mutation enhances short-term and long-term repopulating activities of BM cells
To functionally test the effects of Ptpn11D61G/+ mutation on HSCs, both short-term and long-term in vivo repopulating activities of BM hematopoietic cells were determined. Spleen colony-forming unit (CFU-S) assays, a well-established approach to evaluate short-term repopulating activity of hematopoietic cells,35 showed that the number of day 12 CFU-S was increased by 3-fold in Ptpn11D61G/+ cell–transplanted mice (Figure 3A-B). Moreover, the size of CFU-S colonies in these mice was markedly increased. These data clearly indicate an enhanced short-term repopulating activity of Ptpn11D61G/+ BM cells.
Greater short-term and long-term repopulating activities of Ptpn11D61G/+ BM cells. (A-B) BM cells (1 × 105) freshly harvested from 3-month-old Ptpn11D61G/+ and WT littermates were injected into sub-lethally irradiated C57BL/6 mice (n = 9 per group). Twelve days after the transplantation, colonies on the spleen (CFU-S) were counted. Spleens were photographed using a Canon PowerShot SD600 digital camera. (C) BM cells (test cells; 1 × 106) harvested from WT or Ptpn11D61G/+ littermates (CD45.2+) were transplanted with the same number of BoyJ (CD45.1+) BM cells (competitor cells) into lethally irradiated BoyJ (CD45.1+) mice (n = 5 per group). Test cell reconstitution (CD45.2+) was determined at 4, 8, 12, and 16 weeks by FACS analyses of peripheral blood cells of the recipient mice. (D) Sixteen weeks after transplantation, percentages of test cell–derived CD45.2+ cells in the whole BM population were determined (n = 5 per group). (E) Contributions of test cells (CD45.2+) to each lineage (Mac-1, Gr-1, B220, Ter119, and CD4 positive cells) in the BM were also quantified (n = 5 per group). (F) BM cells (2 × 106) harvested from the primary recipients were transplanted into lethally irradiated BoyJ (CD45.1+) mice. Sixteen weeks after the transplantation, BM cells of the secondary recipients were analyzed for the contribution of test cells in the BM (n = 5 per group). (G) Reconstitution from test cells in each lineage of the BM was determined as described in panel E (n = 5 per group). (H-I) BM cells harvested from WT or Ptpn11D61G/+ littermates were transplanted with the same number of BoyJ BM cells into lethally irradiated BoyJ mice (n = 5 per group) as in panel C. Sixteen weeks after the transplantation, recipient mice were killed and frequencies of LSK cells renewed from test cells (CD45.2+) in the BM (H) and spleen (I) were quantified by multiparameter FACS analyses as described in Figure 1. BM cells (2 × 106) harvested from primary recipient mice were transplanted into lethally irradiated BoyJ mice. Sixteen weeks after transplantation, frequencies of LSK cells renewed from test cells (CD45.2+) were quantified in the BM (J) and spleen (K) of the secondary recipient mice.
Greater short-term and long-term repopulating activities of Ptpn11D61G/+ BM cells. (A-B) BM cells (1 × 105) freshly harvested from 3-month-old Ptpn11D61G/+ and WT littermates were injected into sub-lethally irradiated C57BL/6 mice (n = 9 per group). Twelve days after the transplantation, colonies on the spleen (CFU-S) were counted. Spleens were photographed using a Canon PowerShot SD600 digital camera. (C) BM cells (test cells; 1 × 106) harvested from WT or Ptpn11D61G/+ littermates (CD45.2+) were transplanted with the same number of BoyJ (CD45.1+) BM cells (competitor cells) into lethally irradiated BoyJ (CD45.1+) mice (n = 5 per group). Test cell reconstitution (CD45.2+) was determined at 4, 8, 12, and 16 weeks by FACS analyses of peripheral blood cells of the recipient mice. (D) Sixteen weeks after transplantation, percentages of test cell–derived CD45.2+ cells in the whole BM population were determined (n = 5 per group). (E) Contributions of test cells (CD45.2+) to each lineage (Mac-1, Gr-1, B220, Ter119, and CD4 positive cells) in the BM were also quantified (n = 5 per group). (F) BM cells (2 × 106) harvested from the primary recipients were transplanted into lethally irradiated BoyJ (CD45.1+) mice. Sixteen weeks after the transplantation, BM cells of the secondary recipients were analyzed for the contribution of test cells in the BM (n = 5 per group). (G) Reconstitution from test cells in each lineage of the BM was determined as described in panel E (n = 5 per group). (H-I) BM cells harvested from WT or Ptpn11D61G/+ littermates were transplanted with the same number of BoyJ BM cells into lethally irradiated BoyJ mice (n = 5 per group) as in panel C. Sixteen weeks after the transplantation, recipient mice were killed and frequencies of LSK cells renewed from test cells (CD45.2+) in the BM (H) and spleen (I) were quantified by multiparameter FACS analyses as described in Figure 1. BM cells (2 × 106) harvested from primary recipient mice were transplanted into lethally irradiated BoyJ mice. Sixteen weeks after transplantation, frequencies of LSK cells renewed from test cells (CD45.2+) were quantified in the BM (J) and spleen (K) of the secondary recipient mice.
To assess long-term repopulating activity of Ptpn11D61G/+ mutant BM cells, competitive repopulating assays were then performed. BM cells (test cells) from Ptpn11D61G/+ or WT littermates (CD45.2+) were mixed with competitor BM cells from congenic BoyJ mice (CD45.1+) at the ratio of 1:1. Mixed cells were transplanted into lethally irradiated BoyJ recipients. After 4, 8, 12, and 16 weeks, recipients were bled and contributions of the test cells to peripheral leukocytes were determined by FACS analyses based on cell surface immunophenotypes. In this setting, the contribution of WT test cells was higher than expected (∼ 60%) possibly because of the slight difference in the genetic backgrounds of test and competitor cells (F4 WT and Ptpn11D61G/+ mice from the backcrosses to the C57BL6 background were used in this study because F5 Ptpn11D61G/+ mice were completely lethal at the embryonic stage30 ). Importantly, percentages of test cell-derived nucleated cells (CD45.2+) in the peripheral blood were greatly increased at all time points in the recipients of Ptpn11D61G/+ test cells compared with those in the mice transplanted with WT test cells (Figure 3C). Lineage contribution analyses suggest that this was primarily attributed to excess expansion of myeloid cells (Mac-1/Gr-1 double positive cells) from transplanted Ptpn11D61G/+ cells (data not shown). BM cells were analyzed 16 weeks after transplantation. Similar increases in the percentages of CD45.2+ cells in the whole BM cells (Figure 3D) and spleen (data not shown) and enhanced myelopoiesis in the CD45.2+ population (Figure 3E) were observed in Ptpn11D61G/+:BoyJ transplants. The contribution of Ptpn11D61G/+ cells to lymphoid lineages was decreased in the BM (Figure 3E) and spleen (data not shown), implicating an impact of Ptpn11D61G/+ mutation on lineage determination. To further evaluate effects of Ptpn11D61G/+ mutation on long-term stem cell repopulating activity, BM cells harvested from primary recipients were transplanted into lethally irradiated secondary recipient mice (BoyJ). Sixteen weeks after secondary transplantation, BM cells and splenocytes of secondary recipients were analyzed. Again, Ptpn11D61G/+ recipients manifested a substantial increase in test cell reconstitution in the BM (Figure 3F) and spleen (data not shown) and in test cell-derived myelopoiesis in the BM (Figure 3G) and spleen (data not shown). These results together suggest that Ptpn11D61G/+ BM stem cells have a competitive advantage and a greater long-term repopulating ability compared with WT counterparts.
To gain a better understanding of the underlying mechanisms, we analyzed test cell-derived LSK cells (CD45.2+) in both primary and secondary recipient mice of competitive repopulation assays. As shown in Figure 3H, the percentage of Ptpn11D61G/+ cell-derived LSK cells (CD45.2+) in the BM of primary recipients was significantly increased compared with that of the LSK cells derived from WT test cells. This difference was even more dramatic in the spleen (Figure 3I). In the secondary recipients, Ptpn11D61G/+ cell-derived LSK cells in the bone marrow (Figure 3J) and spleen (Figure 3K) were still significantly higher than those generated from WT cells. These LSK cell quantification data from serial transplantation experiments suggest that the increased repopulation activity and the enhanced myelopoiesis of Ptpn11D61G/+ mutant BM cells occur largely at the HSC level.
MPD phenotypes are reproduced in primary and secondary recipient mice transplanted with Ptpn11D61G cells
To determine whether the pathogenic effects of Ptpn11 GOF mutations initiate at stem cells, we first directly transplanted Ptpn11D61G/+ or WT BM cells (CD45.2+) into lethally irradiated BoyJ (CD45.1+) recipients. Peripheral blood of recipients was analyzed 8, 12, and 16 weeks after transplantation. In this setting, both WT and Ptpn11D61G/+ cells reconstituted the entire hemato-poietic system in the recipient mice as evidenced by nearly 100% contribution of donor-derived cells in peripheral blood 16 weeks after transplantation (data not shown). Ptpn11D61G/+ but not WT cell transplants developed MPD. White blood cell (WBC) counts were markedly increased in Ptpn11D61G/+ recipients, which was mainly due to excess expansion of neutrophils (Figure 4A-B, supplemental Table 2). Spleens of Ptpn11D61G/+ cell-reconstituted mice were enlarged (Figure 4C). Furthermore, percentages of Mac-1/Gr-1 double positive myeloid cells in the BM (Figure 4D), and spleen (Figure 4E) were substantially elevated. BM cells harvested from primary recipients were further transplanted into secondary recipient mice to test for long-term effects of Ptpn11D61G/+ mutation. Similar results were obtained in the secondary transplantation (supplemental Figure 1A-D). We then monitored MPD development in the mice transplanted with a mixture of BM cells from Ptpn11D61G/+ or WT mice and BM cells from BoyJ mice at the ratio of 1:1. MPD phenotypes were also reproduced by Ptpn11D61G/+ cells even though these cells were competing against the same amount of BoyJ cells in recipients (Figure 4F-H). The Ptpn11D61G/+:BoyJ recipient mice displayed elevated WBC (mainly neutrophils) counts (Figure 4F-G, supplemental Table 3) and splenomegaly (Figure 4H). Further, BM cells harvested from primary recipients were transplanted into secondary recipient mice. The secondary recipients of Ptpn11D61G/+:BoyJ cells also developed similar MPD phenotypes (supplemental Figure 1E-F). Collectively, the transplantability of MPD, especially in the recipients transplanted with mixed Ptpn11D61G/+ and BoyJ cells, supports the idea that the pathogenic effects of Ptpn11D61G/+ mutant cells are cell autonomous. Furthermore, as these data were collected from long-term assays in both primary and secondary recipients, the results suggest that the deleterious effects of Ptpn11D61G/+ mutation start at the stem cell level.
MPD is reproduced in Ptpn11D61G/+ BM cell-reconstituted primary and secondary recipients. BM cells (1 × 106) freshly harvested from 3-month-old Ptpn11D61G/+ and WT littermates (CD45.2+) were directly transplanted into lethally irradiated BoyJ (CD45.1+) mice (noncompetitive repopulation assay; n = 3 and 7 for WT and Ptpn11D61G/+ groups, respectively). White blood cell (WBC) counts in peripheral blood (A), percentages of neutrophils and lymphocytes (B), spleen weights (C), and percentages of donor cell-derived myeloid (Mac-1+/Gr-1+) cells in BM (D) and spleen (E) of the recipients were determined at 16 weeks or the indicated time points after the transplantation. BM cells (1 × 106) harvested from 3-month-old WT or Ptpn11D61G/+ littermates were transplanted with the same number of BoyJ BM cells into lethally irradiated BoyJ mice (n = 5 per group). Sixteen weeks after transplantation, WBC counts (F), percentages of neutrophils and lymphocytes (G), and spleen weights (H) of the recipient mice were determined. Spleens were photographed using a Canon PowerShot SD600 digital camera.
MPD is reproduced in Ptpn11D61G/+ BM cell-reconstituted primary and secondary recipients. BM cells (1 × 106) freshly harvested from 3-month-old Ptpn11D61G/+ and WT littermates (CD45.2+) were directly transplanted into lethally irradiated BoyJ (CD45.1+) mice (noncompetitive repopulation assay; n = 3 and 7 for WT and Ptpn11D61G/+ groups, respectively). White blood cell (WBC) counts in peripheral blood (A), percentages of neutrophils and lymphocytes (B), spleen weights (C), and percentages of donor cell-derived myeloid (Mac-1+/Gr-1+) cells in BM (D) and spleen (E) of the recipients were determined at 16 weeks or the indicated time points after the transplantation. BM cells (1 × 106) harvested from 3-month-old WT or Ptpn11D61G/+ littermates were transplanted with the same number of BoyJ BM cells into lethally irradiated BoyJ mice (n = 5 per group). Sixteen weeks after transplantation, WBC counts (F), percentages of neutrophils and lymphocytes (G), and spleen weights (H) of the recipient mice were determined. Spleens were photographed using a Canon PowerShot SD600 digital camera.
To further test the pathogenic capabilities of Ptpn11D61G/+ mutant HSCs, BM cells were transplanted into BoyJ mice through limiting dilution with competitor BM cells from BoyJ mice (Table 1). MPD development in the recipient mice was determined 16-to-24 weeks after transplantation as described in the previous paragraph. In these experiments, Ptpn11D61G/+ mutant BM cells could still reproduce MPD in 2/5 and 1/5 recipient mice when competing against BoyJ BM cells at the ratios of 4 × 104/2 × 105 (1:5) and 8000/2 × 105 (1:25), respectively (Table 1). Ptpn11D61G mutation is insufficient to cause MPD phenotypes when present in a small subset (1600 or 300) of cells in competition against 2 × 105 WT cells. This is likely because too few mutant long-term repopulating stem cells were actually transplanted into the recipients. Another possibility is that the congenital D61G mutation is a mild activating mutation. Its pathogenic effect is relatively mild because effects of Ptpn11 GOF mutations on MPD development are likely dependent on Shp-2 catalytic activity. We next determined whether the MPD phenotypes were transplanted via HSCs or lineage progenitors. Purified LSK cells, CMPs, and GMPs were transplanted into recipient mice with or without competitor cells of the same cell types. Whole BM cells or lineage-positive cells (carrier cells) from BoyJ mice were included to radioprotect the recipient mice (Table 1). The results clearly showed that Ptpn11D61G/+ mutant LSK cells outcompeted normal LSK cells even at the ratio of 1:2.5 and led to MPD development in the recipients (Table 1). Furthermore, only LSK cells, not CMP or GMP progenitors, could confer disease phenotypes in recipient mice. These data together reaffirm aberrantly enhanced activities and pathogenic effects of Ptpn11D61G/+ mutant stem cells.
Increased response of Ptpn11D61G stem cells/multipotent progenitors to IL-3
Cytokine hypersensitivity of myeloid progenitors is the hallmark of JMML.1,2 To determine whether increased cytokine sensitivity also occurs at the level of stem cells/multipotent progenitors, we purified LSK cells from WT and Ptpn11D61G/+ mice and cultured the cells (5 × 103/well) in the presence of IL-3. After 7 days of culture, Ptpn11D61G/+ mutant cells were increased 300- to 400-fold as opposed to 40-fold increase in WT cells (supplemental Figure 2A). The accompanying myeloid differentiation induced by this cytokine was enhanced by Ptpn11D61G/+ mutation. Mac-1 single positive and Mac-1/Gr-1 double positive myeloid cells were substantially increased in the culture of Ptpn11D61G/+ cells (supplemental Figure 2B-C), recapitulating MPD phenotypes, that is, exces-ive myeloid expansion. These data further verify cell autonomous effects of Ptpn11D61G/+ mutation on sensitizing HSCs/multipotent progenitors to cytokine-induced differentiation.
Ptpn11-associated MPD appears to be attributable to aberrantly enhanced cytokine/growth factor signaling in HSCs and the myeloid compartment. However, the molecular mechanisms by which GOF mutations in Ptpn11 enhance cell signaling are poorly understood as the biochemical basis for the positive role of Shp-2 phosphatase in cell signaling remains unclear. Signaling partners that mediate the pathogenic effects of Ptpn11 mutations have not been characterized. To address these questions, we generated BM-derived mast cells from 12-week-old WT and Ptpn11D61G/+ mice, which allowed for large scale biochemical analyses. IL-3 signaling activities in these cells were assessed. As seen in Figure 5A, IL-3-induced tyrosine phosphorylation of cellular proteins was increased in Ptpn11D61G/+ cells although D61G mutation increases phosphatase activity of Shp-2. Activation of Erk and Akt kinases was substantially elevated in Ptpn11D61G/+ cells (Figure 5B). We also generated BM-derived macrophages from WT and Ptpn11D61G/+ mice and assessed GM-CSF signaling in these cells. Similar effects of Ptpn11D61G mutation on GM-CSF-induced activation of Erk kinases were observed (supplemental Figure 3). Furthermore, we found that the interaction between Shp-2 and Gab2, a prominent interacting protein of Shp-2 and an important scaffolding protein for cytokine/growth factor signaling,16,17 was dramatically enhanced by Ptpn11D61G/+ mutation (Figure 5C). These observations point to the possibility that Gab2 may mediate the pathogenic effects of Ptpn11D61G/+ mutation.
IL-3-induced interaction between Shp-2 and Gab2 is substantially enhanced by Ptpn11D61G/+ mutation. BM-derived mast cells were generated as described in “Generation of BM-derived mast cells and macrophages.” The cells were starved in serum and cytokine free medium for 5 hours and then stimulated with IL-3 (2 ng/mL) for the indicated periods of time. (A-B) Whole cell lysates were prepared and examined for tyrosine phosphorylation, Akt and Erk activities by immunoblotting with antiphospho-tyrosine (pY), antiphospho-Erk, and antiphospho-Akt Abs. Blots were stripped and reprobed with anti-pan–Erk to check protein loading. Panels A and B were derived from the same blot. (C) The cell lysates were also immunoprecipitated with anti–Shp-2 Ab followed by anti-pY immunoblotting. Blots were stripped and reprobed with anti-Gab2 and then anti–Shp-2 Abs.
IL-3-induced interaction between Shp-2 and Gab2 is substantially enhanced by Ptpn11D61G/+ mutation. BM-derived mast cells were generated as described in “Generation of BM-derived mast cells and macrophages.” The cells were starved in serum and cytokine free medium for 5 hours and then stimulated with IL-3 (2 ng/mL) for the indicated periods of time. (A-B) Whole cell lysates were prepared and examined for tyrosine phosphorylation, Akt and Erk activities by immunoblotting with antiphospho-tyrosine (pY), antiphospho-Erk, and antiphospho-Akt Abs. Blots were stripped and reprobed with anti-pan–Erk to check protein loading. Panels A and B were derived from the same blot. (C) The cell lysates were also immunoprecipitated with anti–Shp-2 Ab followed by anti-pY immunoblotting. Blots were stripped and reprobed with anti-Gab2 and then anti–Shp-2 Abs.
Important role for Gab2 in mediating the pathogenic effects of Ptpn11D61G mutation
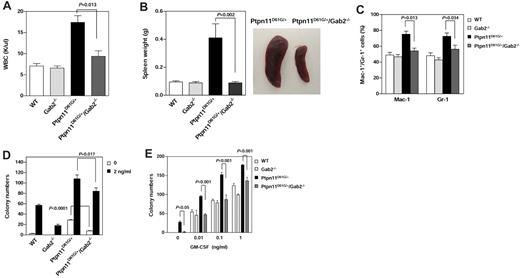
We next generated Ptpn11D61G/+/Gab2−/− double mutant mice from Ptpn11D61G/+9 and Gab2+/−28 mice, and compared the phenotypes of the double mutants with those of Ptpn11D61G/+ single mutant mice at 5-7 months after birth when MPD was fully developed in Ptpn11D61G/+ mice.9 Gab2 deficiency did not rescue the embryonic lethality induced by homozygous Ptpn11D61G/D61G mutation9 or the developmental defects (Noonan syndrome) induced by Ptpn11D61G/+ mutation9 (data not shown). However, gross health signs of Ptpn11D61G/+/Gab2−/− double mutants were significantly improved. MPD phenotypes, such as elevated WBC counts (Figure 6A, supplemental Table 4) and overproduction of myeloid cells in the BM (Figure 6C), were substantially alleviated. Splenomegaly was diminished in the double mutants (Figure 6B). Furthermore, IL-3 or GM-CSF-stimulated colony formation of myeloid progenitors from Ptpn11D61G/+/Gab2−/− mice was significantly attenuated compared with that of Ptpn11D61G/+ cells (Figure 6D-E). Notably, cytokine-independent baseline colony formation of Ptpn11D61G/+ mutant progenitors was also greatly decreased by additional Gab2 deficiency, indicating that decreased basal activities also contributed to the diminished response of Ptpn11D61G/+/Gab2−/− myeloid progenitors to cytokines.
MPD phenotypes induced by Ptpn11D61G/+ mutation are substantially attenuated by deletion of Gab2. Ptpn11D61G/+ mice were used to cross Gab2+/− mice to generate Ptpn11D61G/+/Gab2+/− mice. The double heterozygous mice were then intercrossed to produce WT, Gab2−/−, Ptpn11D61G/+, and Ptpn11D61G/+/Gab2−/− mice. These mice were analyzed at 5-7 months after birth. WBC counts (A; n = 10-13), spleen weights (B; n = 5-8), and percentages of Mac-1+ and Gr-1+ cells in BM (C; n = 4-10) were determined. Spleens were photographed using a Canon PowerShot SD600 digital camera. (D) BM cells (2 × 104 cells/mL) freshly harvested from 4 genotypes of mice were assayed for colony forming units (CFUs) in 0.9% methylcellulose Iscove Modified Dulbecco medium containing 30% FBS, glutamine (10-4 M), β-mercaptoethanol (3.3 × 10-5 M), and IL-3 (D) or GM-CSF (E) at the indicated concentrations. After 7 days of culture at 37°C in a humidified 5% CO2 incubator, hematopoietic cell colonies (primarily CFU-GM) were counted under an inverted microscope. Experiments described in panels D and E were performed 3 times and similar results were obtained in each. Results shown are the mean ± SEM of triplicates from 1 experiment. P values for comparisons between Ptpn11D61G/+/Gab2−/− and Ptpn11D61G/+ mice were determined by Student t tests. Statistical significance among 4 groups in all panels was verified by 2-way ANOVA followed by Bonferroni or 1-way ANOVA followed by the Tukey posttest.
MPD phenotypes induced by Ptpn11D61G/+ mutation are substantially attenuated by deletion of Gab2. Ptpn11D61G/+ mice were used to cross Gab2+/− mice to generate Ptpn11D61G/+/Gab2+/− mice. The double heterozygous mice were then intercrossed to produce WT, Gab2−/−, Ptpn11D61G/+, and Ptpn11D61G/+/Gab2−/− mice. These mice were analyzed at 5-7 months after birth. WBC counts (A; n = 10-13), spleen weights (B; n = 5-8), and percentages of Mac-1+ and Gr-1+ cells in BM (C; n = 4-10) were determined. Spleens were photographed using a Canon PowerShot SD600 digital camera. (D) BM cells (2 × 104 cells/mL) freshly harvested from 4 genotypes of mice were assayed for colony forming units (CFUs) in 0.9% methylcellulose Iscove Modified Dulbecco medium containing 30% FBS, glutamine (10-4 M), β-mercaptoethanol (3.3 × 10-5 M), and IL-3 (D) or GM-CSF (E) at the indicated concentrations. After 7 days of culture at 37°C in a humidified 5% CO2 incubator, hematopoietic cell colonies (primarily CFU-GM) were counted under an inverted microscope. Experiments described in panels D and E were performed 3 times and similar results were obtained in each. Results shown are the mean ± SEM of triplicates from 1 experiment. P values for comparisons between Ptpn11D61G/+/Gab2−/− and Ptpn11D61G/+ mice were determined by Student t tests. Statistical significance among 4 groups in all panels was verified by 2-way ANOVA followed by Bonferroni or 1-way ANOVA followed by the Tukey posttest.
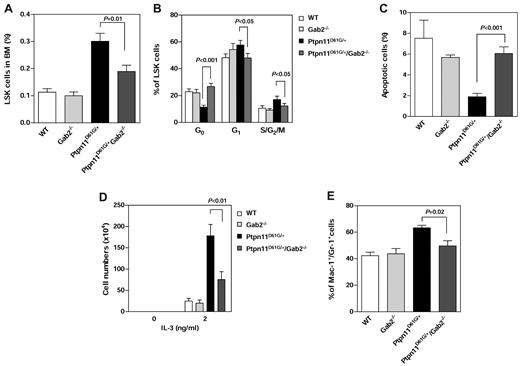
To further determine whether the rescue effects of Gab2 deletion occurred at the stem cell level, we analyzed HSC homeostasis in the double mutants and compared with that in Ptpn11D61G/+ single mutants. The frequency (Figure 7A) and absolute number (supplemental Figure 4) of LSK cells in the BM and spleen (data not shown) of Ptpn11D61G/+/Gab2−/− mice were significantly decreased compared with those in Ptpn11D61G/+ mice. Cell-cycle analyses showed that the disrupted LSK cell-cycle distribution, especially cell quiescence, was largely rescued (Figure 7B). Programmed cell death in LSK cells of Ptpn11D61G/+/Gab2−/− mice was fully restored (Figure 7C). Furthermore, we sorted LSK cells from the 4 types of mice and assessed their responses to cytokine-induced differentiation. Compared with those of Ptpn11D61G/+ cells, cell expansion (Figure 7D) and myeloid differentiation (Figure 7E) of Ptpn11D61G/+/Gab2−/− cells in response to IL-3 were markedly attenuated. These data are fully consistent with the observation that MPD in Ptpn11D61G/+/Gab2−/− mice was ameliorated (Figure 6A-C) and support the notion that Gab2 is an important mediator for the MPD induced by Ptpn11D61G mutation.
An important role of Gab2 in mediating the pathogenic effects of Ptpn11D61G/+ mutation in stem cells. WT, Gab2−/−, Ptpn11D61G/+, and Ptpn11D61G/+/Gab2−/− mice were generated as described in Figure 6. The mice were analyzed for the percentage of LSK cells in the BM (A; n = 6 per group), cell-cycle status (B; n = 5-8), and apoptosis (C; n = 5 per group) as described in Figure 1. (D) LSK cells were sorted from these mice and cultured in IL-3 (0 or 2 ng/mL) and 10% FBS-containing Iscove Modified Dulbecco media (5 × 103 cells/well). After 7 days of in vitro culture, total cell numbers were determined (n = 3 per group). (E) Percentages of myeloid (Mac-1+/Gr-1+) cells differentiated from the sorted LSK cells in the presence of IL-3 (2 ng/mL) were assessed by FACS analysis (n = 6 per group). P values for comparisons between Ptpn11D61G/+/Gab2−/− and Ptpn11D61G/+ mice were determined by Student t tests. Statistical significance among 4 groups in all panels was verified by 2-way ANOVA followed by Bonferroni or 1-way ANOVA followed by the Tukey posttest.
An important role of Gab2 in mediating the pathogenic effects of Ptpn11D61G/+ mutation in stem cells. WT, Gab2−/−, Ptpn11D61G/+, and Ptpn11D61G/+/Gab2−/− mice were generated as described in Figure 6. The mice were analyzed for the percentage of LSK cells in the BM (A; n = 6 per group), cell-cycle status (B; n = 5-8), and apoptosis (C; n = 5 per group) as described in Figure 1. (D) LSK cells were sorted from these mice and cultured in IL-3 (0 or 2 ng/mL) and 10% FBS-containing Iscove Modified Dulbecco media (5 × 103 cells/well). After 7 days of in vitro culture, total cell numbers were determined (n = 3 per group). (E) Percentages of myeloid (Mac-1+/Gr-1+) cells differentiated from the sorted LSK cells in the presence of IL-3 (2 ng/mL) were assessed by FACS analysis (n = 6 per group). P values for comparisons between Ptpn11D61G/+/Gab2−/− and Ptpn11D61G/+ mice were determined by Student t tests. Statistical significance among 4 groups in all panels was verified by 2-way ANOVA followed by Bonferroni or 1-way ANOVA followed by the Tukey posttest.
Gab2 belongs to the Gab scaffolding protein family that also contains Gab1 and Gab3.16,17,37 To test whether Gab1 and Gab3 may compensate for Gab2 function in Gab2−/− or Ptpn11D61G/+/Gab2−/− double mutant mice, we determined expression levels of Gab1,Gab2, and Gab3 in LSK cells purified from WT, Gab2−/−, Ptpn11D61G/+, and Ptpn11D61G/+/Gab2−/− mice. As shown in supplemental Figure 5, Gab1 and Gab3 were expressed at comparable levels in LSK cells across the 4 genotypes, indicating that there is no significant up-regulation of other Gab proteins in Ptpn11D61G/+/Gab2−/− double mutant mice. Furthermore, we knocked down Gab2 from Ptpn11D61G/+ BM cells and evaluated the effect of acute knockdown of Gab2 on attenuating myeloid over-production. As seen in supplemental Figure 6B, both cytokine-independent and cytokine (IL-3 and GM-CSF)–stimulated colony formations were significantly decreased in Gab2 knockdown cells. In addition, the size of the colonies derived from Gab2 knockdown cells was much smaller (supplemental Figure 6C). These results reaffirm the important role of Gab2 in mediating Ptpn11D61G mutation-induced pathogenesis.
Discussion
While significant advances have been made in understanding the genetic lesions responsible for the development of JMML, the underlying cellular and molecular mechanisms of leukemogenesis associated with these mutations are not completely understood. This is particularly the case for PTPN11 (SHP-2) mutations because the molecular mechanism for the positive role of SHP-2 in cell signaling remains unclear. The effects of PTPN11 mutations on hematopoietic cell development, especially those on HSC activities, are not well characterized. Signaling partners that mediate the pathogenic effects of PTPN11 mutations have not been investigated. Our studies in this report now show that oncogenic Ptpn11 aberrantly activates HSCs, leading to the development of MPD. MPD phenotypes were reproduced by Ptpn11D61G/+ mutant whole BM cells, LSK cells, but not myeloid progenitors, in primary and secondary recipients in both noncompetitive and competitive transplantation analyses (Figure 4, Table 1, supplemental Figure 1). Furthermore, we have identified Gab2, a prominent interacting protein of Shp-2 that plays an essential role in cell signaling, as an important mediator for Ptpn11D61G mutation-induced pathogenesis. The aberrant HSC activities caused by Ptpn11D61G mutation were largely corrected by deletion of Gab2 and MPD phenotypes were markedly ameliorated in Ptpn11D61G/+/Gab2−/− double mutant mice.
HSCs are believed to be relatively quiescent as a reservoir for blood cell development. Recent studies suggest that maintenance of HSC quiescence is coupled to the prevention of leukemia development. Aberrant overactive cycling of HSCs can lead to leukemogenesis. Numerous tumor suppressors and proto-oncogenes, such as Pten,38,39 Bmi-1,40 c-Myc,41 and JunB,42 have been implicated in the regulation of HSC quiescence and leukemia development. Ptpn11D61G/+ mutation appears to induce MPD also by activating HSCs. The percentage of cycling LSK cells in G1 or S/G2/M phases was increased in Ptpn11D61G/+ mutant mice (Figure 2A-B). As a result of the accelerated cycling, the percentage of quiescent stem cells at the G0 phase was decreased (Figure 2A-B). Alternatively, increased mobilization of Ptpn11D61G/+ mutant cells43 could also contribute to the lower abundance of quiescent stem cells. Activation of stem cells usually leads to a decrease in the stem cell pool due to exhaustion of the reserve capacity. However, the absolute number of quiescent HSCs was not significantly changed in Ptpn11D61G/+ mice because the total number of LSK cells was doubled in the mutant mice (supplemental Table 1). This might explain why enhanced cycling increased the overall HSC pool in the BM and the spleen in Ptpn11D61G/+ mutant mice (Figure 1B,D). Both phenotypic LT-HSCs and ST-HSCs were increased (Figure 1F), consistent with the greater short-term and long-term repopulating activities demonstrated by the mutant BM cells in noncompetitive and competitive repopulation assays (Figure 3A-G). Remarkably, LSK cells derived from Ptpn11D61G/+ donor cells in the primary and secondary recipient mice of competitive repopulation assays were also significantly increased (Figure 3H-K), suggesting that the pathogenic effects of Ptpn11D61G/+ mutation start at the HSC level. In addition, later stage myeloid progenitors, especially GMPs and MEPs in Ptpn11D61G/+ mutant mice were increased (Figure 1H), indicating a general impact of Ptpn11D61G/+ mutation on cell survival or proliferation. In support of this idea, apoptosis of LSK cells in Ptpn11D61G/+ mutant mice was markedly decreased (Figure 2D). The aberrant hematopoietic cell development induced by Ptpn11D61G/+ mutation is similar to that of Cbl,12 JunB,42 or p18INK4C (cyclin-dependent kinase inhibitor)44 knockout mice, in which HSCs are expanded with enhanced cycling.
In contrast to the global Ptpn11D61G knock-in model, inducible expression of Ptpn11D61Y in conditional knock-in mice decreases HSCs in the BM although both lines of mice similarly show increased cycling and decreased apoptosis in HSCs, and increased HSCs in the spleen.11 The mice used in the current study were F4 mice backcrossed to the C57BL/6 background, whereas Ptpn11D61Y knock-in mice were on pure C57BL/6.11 It is possible that the genetic background modifies HSC homeostasis such that activation of HSCs by Ptpn11 GOF mutations in C57BL/6 mice may cause faster HSC exhaustion. Another possibility is that although the 2 mutations occur at the same amino acid residue, the D61Y mutation is more potent than the D61G mutation in enhancing Shp-2 catalytic activity.25 The pathogenic effects of Ptpn11 mutations on HSC homeostasis may be dependent on the level of Shp-2 catalytic activity and excessive activation of HSCs by the Ptpn11D61Y mutation eventually leads to HSC exhaustion. Finally, the difference in the stem cell microenvironments of the 2 mouse models may also contribute to the different HSC phenotypes. Recent studies have shown that tumor suppressor Rb must be inactivated in both stromal cells and the hematopoietic cell compartment for MPD development,45 and that the stem cell microenvironment but not HSCs is responsible for the development of MPD in retinoic acid receptor γ deficient mice.46 The stem cell microenvironment in Ptpn11D61G/+ mice also contains the same mutation, unlike inducible Ptpn11D61Y knock-in mice in which the mutation is primarily present in hematopoietic cells. Ptpn11D61G mutation in the components of the microenvironment may exert certain detrimental effects on HSC homeostasis by altering cytokine/growth factor secretion. Thus, the HSC phenotypes caused by Ptpn11D61G germ line mutation may represent a combined effect of Ptpn11 mutation in HSCs and in the microenvironment. Further studies are required to clarify these possibilities, and more importantly, to compare stem cell phenotypes in JMML patient samples with somatic and germline PTPN11 mutations.
HSC homeostasis and function are tightly controlled by environmental cues, such as cytokines and growth factors. Shp-2 phosphatase appears to play a key role in the regulation of cytokine/growth factor response of HSCs. A recent gene expression microarray and systems biology study showed that SHP-2 (PTPN11), like GATA2, PTEN, and STAT5, acted as a hub to maintain the stability and connectivity of the whole genetic network in human HSCs.47 Shp-2 is known to be involved in the signal transduction of multiple cytokines and growth factors, and it plays an overall positive role in the signaling processes.13-15 GOF mutations in Ptpn11, including D61G mutation, may disturb HSC function by altering cytokine or growth factor signaling. This notion is supported by the observations that signaling activities were greatly increased in Ptpn11D61G/+ hematopoietic cells in response to IL-3 (Figure 5B) or GM-CSF (supplemental Figure 3) and that IL-3–induced differentiation of LSK cells was drastically enhanced by Ptpn11D61G/+ mutation (supplemental Figure 2).
It appears that Gab2 plays an important role in mediating the effects of Ptpn11D61G/+ mutation on baseline or cytokine-induced hematopoietic cell activities. Gab2 is the most prominent substrate of Shp-2 in cell signaling.16,17 It also forms a stable complex with Shp-2.16,17 Previous studies have shown that this scaffolding protein plays an essential role in coupling downstream pathways to the proximity of cytokine receptors48 and is required for Bcr-Abl oncogenic tyrosine kinase to induce chronic myeloid leukemia.49,50 Deficiency in Gab2 decreases mast cell development28 and multilineage repopulating activity of HSCs.29 In the present study we found that D61G mutation in the N-SH2 domain of Shp-2 greatly enhanced the interaction between Shp-2 and tyrosine phosphorylated Gab2 in IL-3 signaling (Figure 5C). Elevated baseline and cytokine (IL-3 and GM-CSF)–stimulated differentiation of Ptpn11D61G/+ myeloid progenitors (Figure 6D-E, supplemental Figure 6B) and LSK cells (Figure 7D) was corrected by deletion of Gab2 in vivo and in vitro. HSC homeostasis was also largely restored in Ptpn11D61G/+/Gab2−/− double mutants (Figures 6–7). Furthermore, MPD phenotypes were attenuated in Ptpn11D61G/+/Gab2−/− mice. These findings demonstrate an essential role of Gab2 in the pathogenesis of Ptpn11 GOF mutation-induced MPD, implicating a potential therapeutic benefit of targeting GAB2 or GAB2-dependent pathways for the treatment of PTPN11-associated JMML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr Toshio Hirano for Gab2+/− mice and Dr James Jacobberger for technical assistance with FACS analysis.
This work was supported by National Institutes of Health grants HL068212, HL095657, and HL082670 (to C.K.Q.), DK059380 (to K.D.B.), and CA114945 (to B.G.N.).
National Institutes of Health
Authorship
Contribution: D.X., S.W., W.-M.Y., G.C., and T.A. conducted the research and summarized the data; K.D.B. and B.G.N. provided critical reagents, discussed the work, and edited the manuscript; C.-K.Q. designed the experiments and provided technical training to the first 3 authors; and D.X. and C.-K.Q. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng-Kui Qu, Case Western Reserve University, 10900 Euclid Ave, Wolstein Bldg, Rm 2-134, Cleveland, OH 44106; e-mail: cxq6@case.edu.