Abstract
Abstract 3144
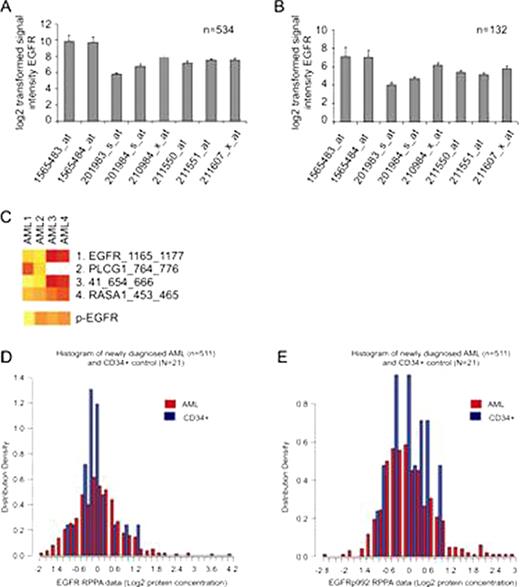
It was previously described that Epidermal Growth Factor Receptor (EGFR) inhibitors can induce a clinical response in AML patients (Boehrer et al., 2008; Chan and Pilichowska, 2007; Pitini et al., 2008). In contrast, it was reported that EGFR is not expressed in AML cells both at protein and mRNA level and that the phenotypic effects of the EGFR inhibitors are off-target effects on the spleen tyrosine kinase (SYK) (Lindhagen et al., 2008; Stegmaier et al., 2005; Hahn et al., 2009). We have, however, detected EGFR mRNA expression in gene expression array data of 534 publicly available adult AML patient samples and 132 publicly available pediatric AML patient samples (obtained from the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo, accession number GSE6891 for adult AML patients and GSE22056 for pediatric AML patients) (Fig. A, and B). EGFR kinase activity was measured in four AML samples using the PamChip® tyrosine kinase microarray system and EGFR phosphorylation measured using Phospho-receptor tyrosine kinase antibody microarray (R&D). EGFR kinase activity could be detected in 2 of 4 of the samples (Fig. C) In addition, protein expression of EGFR and phosphorylated (Tyr-992) EGFR was measured using Reverse Phase Protein Array in 511 cases of newly diagnosed AML. Total EGFR levels were equivalent to normal bone marrow CD34+ cells in 72% and above and below normal in 13.5 and 13.7% of AML patient samples respectively (Fig. D). The phosphorylation of the EGF receptor was equivalent to that observed in normal bone marrow CD34+ cells in 80% and above and below normal in 8 and 11.7% of cases respectively (Fig. E). EGFR protein expression was correlated with the level of NF2, CIAP, Survivin, Stat5p694, CyclinD1, Her3, NRP1, PLAC1, CateninA, IGF1, TAZ, SMAD6, YAP, NF2 and PI3Kp110. The level of phospho-EGFR was correlated with the level of expression of P53, RB, pRB, Cyclin B1 and E, BCLXL, BIM, Cleaved caspase 7 and 9, GATA3, Stat1p701 and ODC. Levels of EGFR protein expression and phosphorylation did not correlate with FAB classification, cytogenetics, or the presence of FLT3-ITD or NPM1 or RAS mutation or with age, gender, or the presence of an antecedent hematological disorder. Neither total or phospho-EGFR levels were prognostic for remission attainment, remission duration, overall or event free survival. These results indicate that EGFR is expressed at both the mRNA and the protein level in AML. In addition, EGFR is commonly phosphorylated and active in primary AML patient samples. Activity of EGFR receptor was correlated with higher expression of proliferation regulating genes (Cyclins, P53, RB) and anti-apoptotic proteins suggesting that signaling through the EGFR axis might be affecting proliferation and apoptosis sensitivity in AML blasts. This suggests that modulation of the EGFR axis in different settings could be used to stimulate proliferation and sensitivity to cell cycle specific agents or inhibited to inhibit proliferation or to reduce anti-apoptotic forces. Future experiments to correlate the responsiveness of AML blasts to therapy with EGFR activators/inhibitors with the endogenous activity of the EGFR receptor and to determine the optimal way to target cells through this pathway are underway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.