To the editor:
Patients with classic myeloproliferative neoplasms (MPNs) share a risk of transformation from a chronic phase (CP) to blast phase (BP; ie, acute myeloid leukemia, AML). Interestingly, JAK2V617F mutation detectable in most patients in CP can become undetectable upon transformation to BP, strongly suggesting that the event leading to transformation happens in JAK2V617F-negative cell clone. Recently, other mutations (eg, TET2, ASXL1, IDH1/2, RUNX1, and RAS) have been found in MPN, some possibly with higher incidence in BP than in CP, suggesting their role in the transformative process.1-5 Here we report a case that supports these recent observations of competing cell clones in MPN.
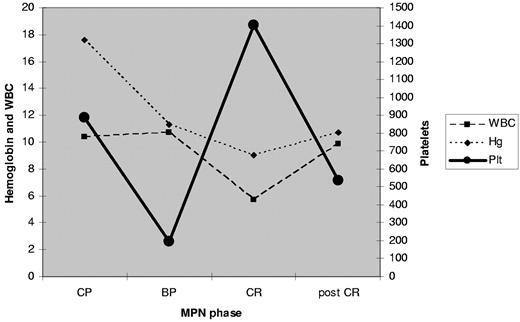
In October 2009, a 68-year-old Hispanic male with a 4-year history of hydroxyurea-treated JAK2V617F-positive polycythemia vera (PV) presented with AML. Bone marrow evaluation showed 30% myeloblasts, diploid cytogenetics, wild-type JAK2, and codon12 (G12D) NRAS mutation (wild-type NMP1 and FLT3 tested as part of AML workup). Complete remission (CR) was achieved after 2 cycles of standard 7 + 3 (cytarabine and idarubicin) induction chemotherapy; in CR, molecular testing revealed JAK2V617F positivity (48.8%) and wild-type NRAS. Re-evaluation after 4 cycles of consolidation chemotherapy confirmed CR, again with JAK2V617F positivity (47.05%) and wild-type NRAS (Table 1). There was a partial reappearance of MPN phenotype (increase of platelet count and bone marrow hyperplastic megakaryopoieisis; Figure 1).
CBC during MPN phases. MPN indicates myeloproliferative neoplasm; WBC, white blood cell count; Hg, hemoglobin; Plt, platelet; CP, chronic phase; BP, blast phase; and CR, complete remission.
CBC during MPN phases. MPN indicates myeloproliferative neoplasm; WBC, white blood cell count; Hg, hemoglobin; Plt, platelet; CP, chronic phase; BP, blast phase; and CR, complete remission.
BP develops in 5% to 8% of PV patients within 10 years after diagnosis.6 The mechanisms responsible for transformation in MPN are not well understood. Two major hypotheses have been proposed: (1) pre-JAK2 mutated clone still exists along with JAK2V617F-positive clone and acquires different mutation(s) leading to transformation and suppression of JAK2V617F-positive clone; both subclones are phylogenetically related; and (2) unrelated JAK2V617F-positive and -negative clones exist, with the JAK2V617F-negative clone more often acquiring mutation(s) resulting in transformation. Although many JAK2V617F-positive MPN patients become JAK2V617F-negative in BP, there is limited information on their molecular characteristics in CR, due in large part to their poor response and prognosis.7 In our patient, JAK2V617F-positive clone became dominant in CR as the mutant-NRAS became undetectable. This was confirmed at the end of consolidation chemotherapy strongly suggestive of coexistence of 2 cell clones, one more aggressive (mutated RAS) than the other (ie, permissive; JAK2V617F-positive). However, this finding does not distinguish between the 2 hypotheses. Recently, a few reports documented the coexistence of JAK2V617F and BCR-ABL clones.8
Much research is under way to understand the molecular chain of events that leads to the existence and progression of MPN. Abdel-Wahab et al recently found TET2 mutations in 43% of MPN in BP patients, and 0% in CP, which argues against TET2 being a pre-JAK2 mutation as previously suggested.9 Beer et al identified alterations of RUNX1, but not TP53 or CBL, to be associated with transformation to BP.10 It seems that multiple different paths exist that lead to the clinical entity we call MPN and its progression to BP. Our report of a case with evidence for existence of competing clones in MPN, and the strong suggestion that a mutation in RAS gene contributed to the transformation to BP, contributes further to the growing evidence of how complex MPN biology is.
Authorship
Contribution: A.A. and J.C. performed diagnosis and planned treatment, and wrote the manuscript; H.K and S.V. wrote the manuscript; and R.L. collected data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge Cortes, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX; e-mail: jcortes@mdanderson.org.