Abstract
Recent experience suggests that reduced-intensity conditioning (RIC) regimens can improve the outcomes of patients with hemophagocytic lymphohistiocytosis (HLH) undergoing allogeneic hematopoietic cell transplantation (HCT). However, studies directly comparing RIC to myeloablative conditioning (MAC) regimens are lacking. Forty patients with HLH underwent allogeneic HCT between 2003-2009 at Cincinnati Children's Hospital. Fourteen patients received MAC consisting of busulfan, cyclophosphamide, and antithymocyte globulin plus or minus etoposide. Twenty-six patients received RIC consisting of fludarabine, melphalan, and alemtuzumab. All patients engrafted. Acute graft-versus-host disease grades II to III occurred in 14% of MAC patients and 8% of RIC patients (P = .3171). Posttransplantation mixed donor/recipient chimerism developed in 18% of MAC patients and 65% of RIC patients (P = .0110). The majority of patients with mixed chimerism received intervention with reduction of immune suppression plus or minus donor lymphocyte infusion or stem cell boost, which stabilized or increased donor contribution to hematopoiesis and prevented relapse of HLH in all but 1 patient. Grade II to III graft-versus-host disease occurred in 5 of 14 RIC patients after donor lymphocyte infusion. The overall estimated 3-year survival after HCT was 43% (confidence interval = ± 26%) for MAC patients and 92% (confidence interval = ± 11%) for RIC patients (P = .0001). We conclude that RIC significantly improves the outcome of patients with HLH undergoing allogeneic HCT.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHLH) is a collection of primary immune deficiencies that are the result of genetic defects that affect granule-mediated cytotoxicity. Known autosomal recessive genetic causes of FHLH include mutations in PRF1, UNC13D, STX11, and STXBP2,1-4 though many patients with FHLH lack a known genetic defect. Recently, it has also been suggested that mutations in X-linked inhibitor of apoptosis (XIAP)/BIRC4 may be best classified as a cause of FHLH.5
HLH is a severe and often overwhelming systemic hyperinflammatory syndrome classically described to include the development of fever, splenomegaly, and cytopenias.6 Other associated symptoms can include jaundice, hepatitis, liver failure, rash, and effusions.6 Seizures, mental status changes, and developmental regression can occur in association with central nervous system involvement.6 Common laboratory findings include hypertriglyceridemia, hypofibrinogenemia, hyperferritinemia, elevated levels of soluble interleukin (IL)-2 receptor, and decreased or absent natural killer (NK) cell function. Hemophagocytosis is observed on pathologic inspection of bone marrow or other tissues in many cases.6 HLH is typically life-threatening in the absence of appropriate treatment.
Currently, the only cure for FHLH is allogeneic hematopoietic cell transplantation (HCT). Over the past 10 years, several published series have documented the outcomes of patients with FHLH treated with allogeneic HCT after myeloablative conditioning (MAC) regimens, with overall long-term survival ranging from 45% to 65%.7-12 Survival among patients with genetically related disorders that are also associated with HLH (such as X-linked lymphoproliferative [XLP] disease, Chediak-Higashi syndrome, and Griscelli syndrome) are similar.13-15
In 2006, Cooper et al reported improved survival of patients with HLH using reduced-intensity conditioning (RIC), noting a survival rate of 75% among a group of 12 patients with FHLH, XLP disease, and Chediak-Higashi syndrome.16 A follow-up report with additional patients demonstrated survival of 84%.17 The results of these reports were very encouraging. However, very little information has been available regarding direct comparisons of RIC to MAC. In addition, contrary to the general conclusion that RIC is preferred to MAC, Ohga et al11 noted no difference in survival between HLH patients treated at multiple institutions with RIC (n = 11) versus MAC (n = 31). We therefore analyzed our single-center experience using RIC compared with MAC in 40 consecutive patients with FHLH undergoing allogeneic HCT to determine whether there is a significant survival advantage with RIC.
Methods
Patients
Approval for this retrospective study was granted by the Cincinnati Children's Hospital Institutional Review Board. Between April 2003 and April 2009, 40 patients underwent their first allogeneic HCT for definitive treatment of HLH with either a myeloablative preparative regimen consisting of busulfan, cyclophosphamide, and antithymocyte globulin (ATG) either with or without a single dose of etoposide, or a reduced-intensity preparative regimen consisting of alemtuzumab, fludarabine, and melphalan. Patients included in this study possessed mutations in PRF1, UNC13D, STX11, STXBP2, or BIRC4, or had genetically undefined HLH (Table 1). Informed consent was obtained from patients or parents for treatment with allogeneic HCT in all cases. Patient pretransplantation characteristics are listed in Table 1.
Preparative regimens, transplantation, and routine transplant care
Of 40 patients, 14 received MAC consisting of busulfan (16 mg/kg, adjusted if needed based on pharmacokinetics), cyclophosphamide (120 or 200 mg/kg), and horse (90 mg/kg) or rabbit (9 mg/kg) ATG. In addition, 12 patients received a single dose of etoposide (30 mg/kg) as part of the preparative regimen. Twenty-six patients received RIC consisting of fludarabine (150 mg/m2 if weight > 10 kg, or 5 mg/kg if weight < 10 kg), melphalan (140 mg/m2 if weight > 10 kg, or 4.7 mg/kg if weight < 10 kg), and alemtuzumab. The RIC regimens were based on existing European16 and American18 regimens. Alemtuzumab was administered to 10 patients proximal to HCT as 48 mg or 33 mg total dose (depending on weight ≥ 10 kg, respectively) divided over days −12 to −9, −11 to −8, or −9 to −5; and to 8 patients as 1 mg/kg total dose over days −9 to −5 or days −8 to −4. Alemtuzumab was administered to 6 patients distal to HCT as 48 mg or 33 mg total dose (depending on weight greater than or less than 10 kg, respectively) divided over days −22 to −19. Two patients received an increased total of 90 mg or 100 mg of alemtuzumab starting on day −22 because of recalcitrant HLH, and 1 of these patients also received etoposide during the pretransplant conditioning period to control disease.
All patients received graft-versus-host disease (GVHD) prophylaxis with either cyclosporine or tacrolimus. MAC patients additionally received either methotrexate (7 patients, administered on days 1, 3, and 6) or methylprednisolone (7 patients), and all RIC patients additionally received methylprednisolone. One RIC patient also received methotrexate. Patients received acyclovir for cytomegalovirus (CMV; if recipient or donor were seropositive) and herpes simplex virus (HSV; if recipient seropositive) prophylaxis if appropriate unless the patient was already being treated with another antiviral agent. Voriconazole, weekly amphotericin, or caspofungin were used for antifungal prophylaxis, depending on physician preference and preexisting or therapy-associated organ toxicities. All patients received pentamidine prophylaxis of Pneumocystis jiroveci, which was changed to cotrimoxazole after marrow recovery based on physician preference, and amoxicillin was administered for pneumococcal prophylaxis after transplantation. All patients received intravenous immunoglobulin. Patients with CMV infection or reactivation received CytoGam. Intravenous fluid and parenteral nutrition were given as needed. Granulocyte colony stimulating factor was administered to all patients until neutrophil recovery. Blood samples were taken once or twice a week to screen for Epstein-Barr virus (EBV), CMV, and adenovirus viremia by polymerase chain reaction.
All patients received either fully matched or single allele mismatched related or unrelated grafts based on typing of human leukocyte antigen (HLA) A, B, C, and DR (Table 2). Marrow was the stem cell source in all patients except 4, who received either cord blood or peripheral blood stem cell grafts. Graft characteristics in the MAC and RIC groups are shown in Table 2. Neutrophil engraftment was considered to be the day the neutrophil count reached 0.5 × 106/L. Engraftment studies were done using either XY fluorescence in situ hybridization (FISH), in the case of sex mismatched donor, or variable number of tandem repeat (VNTR) analysis in the case of same sex donor. Mixed chimerism is defined in this study as having 5% or more host-derived cells in the whole blood on more than one occasion. Acute and chronic GVHD were assessed by standardized published criteria.19,20 If GVHD occurred after donor lymphocyte infusion, it was considered acute DLI-associated GVHD regardless of the timing in relationship to the original transplant. Patients who received donor lymphocyte infusion received doses based on number of CD3+ lymphocytes per kilogram of recipient weight (CD3+ percent determined by flow cytometry), and were not receiving immunosuppressive therapy when administered.
Statistical analysis
Multivariate logistic regression analysis was used to examine the association between the odds of mixed chimerism and GVHD with independent variables that include donor source (sibling versus unrelated donor), match (full versus single allele mismatch), total nucleated cell dose (in logarithmic scale), preparative regimen (MAC versus RIC) and age at HCT (in logarithmic scale). For the survival analysis of overall survival time and the impact of the above variables, Cox proportional hazard regression analysis was used. The Kaplan-Meier product-limit estimator was used to estimate the survival rates of the MAC and RIC groups. The Kaplan-Meier survival estimate for the RIC group became less reliable beyond 3 years after HCT because of the limited sample available for the RIC group, and the survival rates are reported up to 3 years after HCT. For the analysis of the effect of the alemtuzumab schedule used in the RIC group (distal versus proximal) with regard to incidence of mixed chimerism and GVHD, logistic regression analysis considering match, total nucleated cell dose (in logarithmic scale), and CD3+ cell dose (in logarithmic scale) was used. For the analysis of the effect of the alemtuzumab dose within the proximal alemtuzumab subgroup with regard to incidence of mixed chimerism, logistic regression analysis was applied considering alemtuzumab dose (in logarithmic scale), match, donor type (sibling versus unrelated donor), total nucleated cell dose (in logarithmic scale), and CD3+ cell dose (in logarithmic scale). For the analysis of the incidence of EBV, CMV, and adenovirus viremia and infection after HCT with regard to preparative regimen, logistic regression analysis was performed, and Fisher exact test was also used for the analysis of association of adenoviremia with preparative regimen. We did not adjust for the multiplicity of the statistical analysis, as this was an observational study. Statistical significance was considered for P < .05.
Results
Patients
The first allogeneic HCT for HLH using RIC was performed at our center in April 2006. We chose to review the outcomes of patients with FHLH transplanted during the 3 years before and after April 2006, to control for variations in routine patient transplant care (such as antimicrobial prophylaxis and screening for CMV, adenovirus, and EBV reactivation by polymerase chain reaction [PCR]) and HLA typing and donor selection. Forty patients were eligible for inclusion in the study. Pretransplantation patient characteristics are shown in Table 1. All MAC patients underwent HCT between April 2003 and April 2006 except for 2 patients who received MAC in 2009 because they received cord blood grafts as a result of physician concern for possible cord blood graft rejection after RIC. RIC patients underwent HCT between April 2006 and April 2009. Overall, the RIC and MAC groups were similar with regard to pretransplantation disease severity and treatment. Of patients in the RIC group, 61% were in remission from HLH (defined as possessing normal neutrophil and platelet counts and lacking fever or clinically significant hepatitis immediately before HCT), and 64% of MAC patients were in remission from HLH. Fewer patients were treated with cyclosporine before transplantation in the MAC group given the timing of patient presentation in relation to the HLH 2004 study that included cyclosporine as part of HLH therapy protocol. More patients in the RIC group received alemtuzumab for salvage therapy, both because of failure to completely respond to protocol therapy, and also because of an increasingly favorable experience at our center with the use of alemtuzumab for salvage treatment. The median ages of presentation and at transplantation were higher in the RIC group, but the age ranges of all patients in each group were similar. Notably, XIAP/BIRC4 mutation was predominant in the RIC group, but male patients in the MAC group were not tested for XIAP/BIRC4 mutation.
Engraftment
Bone marrow was the source of stem cells for all patients except for 2 patients in the MAC group, who received cord blood grafts, and 2 patients in the RIC group, who received peripheral blood stem cells (Table 2). All patients received fully matched or single-allele mismatched grafts. The average total nucleated cell (TNC) dose per kilogram was lower in the MAC group, 3 × 108 TNCs/kg, versus 6 × 108 TNCs/kg in the RIC group. All patients engrafted, at a median of 14.5 days in the MAC group and 10 days in the RIC group.
Infectious complications of HCT
Documented serious bacterial infections such as abscesses, pneumonias, or sepsis (excluding uncomplicated bacteremias) occurred in 14% of MAC patients and 15% of RIC patients. Aspergillus infection occurred in 1 MAC patient, and no RIC patients. Toxoplasmosis occurred in 1 RIC patient, and no MAC patients. Collectively, infection or viremia caused by RSV, HHV6, Varicella, or HSV occurred in 29% of MAC patients and 8% of RIC patients. Because of the limited occurrence of each of these viral infections or viremias, we were unable to determine whether there were any statistically significant differences. EBV infection or viremia occurred 29% of MAC patients and 15% of RIC patients (P = .3339). CMV infection or viremia occurred in 29% of MAC patients and 27% of RIC patients (P = .7795). Adenovirus infection or viremia occurred in 15% of MAC patients and 38% of RIC patients. This difference initially appeared statistically significant (P = .0353), but comparing only patients in the MAC and RIC groups who received steroid immunosuppression for GVHD prophylaxis, this difference was not statistically significant (P > .99).
Acute graft-related GVHD
Grades II to III acute GVHD related to the primary graft occurred in 2 of 14 (14%) MAC patients and in 2 of 26 (8%) RIC patients (there were no cases of grade IV GVHD). This difference was not statistically significant (P = .3171).
Within the RIC group, differences were observed between the distal and proximal alemtuzumab groups. Four cases of grades I to III GVHD were observed in the RIC group, accounting for 43% (3/7) of distal alemtuzumab patients and 5% (1/19) of proximal alemtuzumab patients. The single case of GVHD in the proximal alemtuzumab group occurred only after this patient was abruptly weaned from GVHD prophylaxis as treatment for mixed donor chimerism one month after HCT. While controlling for the TNC dose, a multivariate analysis including alemtuzumab timing, degree of match, and CD3+ dose revealed a trend toward increased incidence of GVHD in the distal alemtuzumab group (P = .0680).
Chimerism
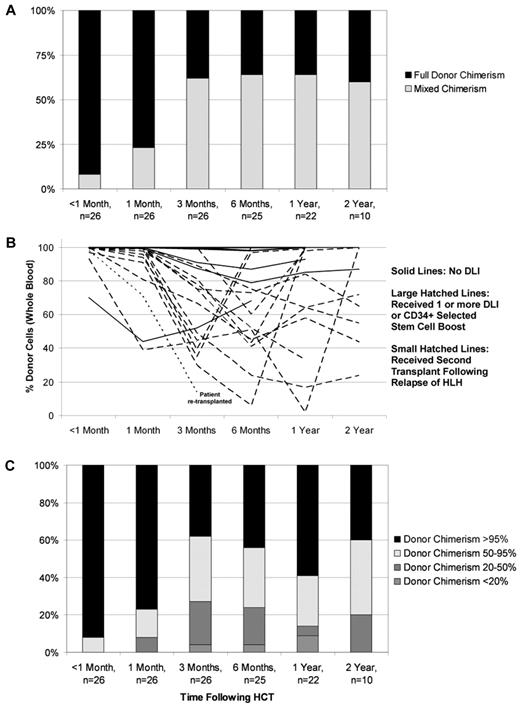
Routinely performed chimerism studies revealed the development of mixed chimerism (< 95% donor cells detected in peripheral blood on more than 1 occasion) in 2 of 11 evaluated MAC patients (18%; 3 patients died before assessment) and 17 of the 26 RIC patients (65%; all patients were evaluated) within the first 6 months after transplant (Figure 1A). All patients with mixed chimerism in whole blood samples who underwent T-cell sorting for chimerism analysis possessed mixed chimerism within the T-cell compartment (n = 10). Mixed chimerism was significantly less frequent in patients treated with MAC (P = .0110) and also in older patients (P = .0190; Table 3). The graft source, HLA match, and TNC dose did not significantly alter the odds of mixed chimerism.
Donor and recipient chimerism within the RIC group. (A) Cumulative incidence of mixed chimerism within the RIC group. Full donor chimerism = 95% or greater donor cells detected in whole blood samples. Mixed donor/recipient chimerism ≤ 95% donor cells detected in whole blood samples on more than one occasion. (B) Individual patient donor chimerism kinetics within RIC group patients. (C) Distribution of donor chimerism over time within the RIC group.
Donor and recipient chimerism within the RIC group. (A) Cumulative incidence of mixed chimerism within the RIC group. Full donor chimerism = 95% or greater donor cells detected in whole blood samples. Mixed donor/recipient chimerism ≤ 95% donor cells detected in whole blood samples on more than one occasion. (B) Individual patient donor chimerism kinetics within RIC group patients. (C) Distribution of donor chimerism over time within the RIC group.
Within the RIC group, we observed a decreased incidence of mixed chimerism among patients who received distal alemtuzumab, 29% (2/7), as opposed to 79% (15/19) of patients who received proximal alemtuzumab (P = .0225). This remained significant after multivariate analysis including the degree of match and CD3+ dose, and controlling for TNC dose (Table 4). Alemtuzumab dose was not included in the multivariate analysis of all RIC patients because alemtuzumab dose timing highly correlated with the dose: the median alemtuzumab dose per kilogram of patients who received distal alemtuzumab was twice that of the patients who received proximal alemtuzumab (and thus inclusion of alemtuzumab dose would have introduced an issue of collinearity in the analysis). However, an analysis including all RIC patients showed no significant relationship between alemtuzumab dose and the development of mixed chimerism, accounting for match. In addition, multivariate analysis within only the proximal alemtuzumab subgroup revealed no significant relationship between alemtuzumab dose per kilogram and the development of mixed chimerism (P = .5292) including match, donor type (sibling versus unrelated donor), TNC dose, and CD3+ dose in the analysis.
Of the 2 patients in the MAC group who developed mixed chimerism, 1 patient experienced recurrence of severe central nervous system (CNS) HLH unresponsive to treatment. Multiple DLIs administered to this patient were not successful in converting chimerism to full donor chimerism, and eventually, CNS HLH was the cause of death. The second patient maintained stable donor chimerism over time of greater than 30%, without recurrence of HLH.
Of the 17 patients who developed mixed chimerism within the RIC group, GVHD prophylaxis was weaned and/or withdrawn sooner than typically initiated at our center in an effort to allow allogeneic donor T-cell recovery and stabilization of donor chimerism. Eighty-two percent of patients with mixed chimerism received a CD34+ selected stem cell boost and/or one or more donor lymphocyte infusion(s). Three patients (18%) received a CD34+ selected stem cell boost with a median CD34+ cell dose of 6.35 × 106 CD34+ cells/kg. Seventy-two percent of patients with mixed chimerism received a first or only DLI with a median CD3+ cell dose of 2 × 106/kg. Patients who continued to experience decline of donor contribution to hematopoiesis over the 2 weeks after the DLI were considered for additional DLI, with or without a CD3+ T-cell dose escalation. Fifty-three percent of patients with mixed chimerism received a second DLI with a median CD3+ cell dose of 6 × 106/kg. Forty-seven percent of patients with mixed chimerism received a third DLI with a median CD3+ cell dose of 10 × 106/kg. Thirty-five percent, eighteen percent, and six percent of patients received a fourth, plus or minus fifth, plus or minus sixth DLI with median CD3+ cell doses of 31, 60, or 200 × 106/kg, respectively. Stabilization or an increase in percent donor chimerism was observed in the majority of patients (Figure 1B). One patient developed recurrence of HLH at day 71 despite DLI administered after the donor chimerism had reached 9% (the lowest recorded whole blood donor chimerism for this patient was 1%). This patient had experienced 3 relapses of HLH and CNS disease before the RIC-HCT. After treatment of HLH, this patient received a second transplant using MAC and is currently HLH-free with full donor engraftment. There were no other occurrences of HLH relapse after RIC-HCT, and the majority of patients have maintained whole blood donor chimerism greater than 20% over time (Figure 1C).
DLI-associated acute GVHD
Of the 2 patients in the MAC group who developed mixed chimerism, 1 patient received multiple DLIs without occurrence of GVHD. Of the 17 patients in the RIC group who developed mixed chimerism, 14 patients received 1 or more DLI(s) and/or CD34+ selected stem cell boost, and 5 patients developed grades II or III acute DLI-associated GVHD (there were no cases of grade IV GVHD after DLI).
Chronic GVHD
Chronic GVHD was not observed in the MAC group (incidence likely affected by the limited number of surviving patients). Within the RIC group, 3 patients (12%) developed limited chronic GVHD of the skin or mouth. Two of these patients received primary peripheral blood stem cell grafts and developed de novo chronic limited GVHD in the absence of acute GVHD. One patient (4%) developed extensive chronic GVHD after grade 1 skin GVHD.
Survival and quality of life
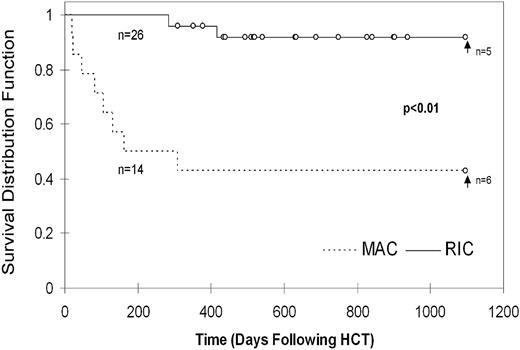
At the time of analysis, 43% of MAC patients are surviving at a median follow-up of 2282 days after HCT. Within the RIC group, 89% of patients are currently surviving at a median of 634 days after HCT. This difference between MAC and RIC groups is statistically significant (P = .0036) using Cox proportional hazards model considering match, cell dose, and age at HCT. Notably, 4/8 patients who died in the MAC group did so before 100 days after HCT, with 7/8 deaths occurring before 6 months as a result of complications including sepsis, multiorgan failure, pulmonary hemorrhage, gastrointestinal hemorrhage, GVHD, pneumonitis, and acute respiratory distress syndrome (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The remaining patient died later from complications of severe CNS HLH. In the RIC group, all patients survived beyond 6 months. The 3 deaths that occurred in the RIC group were caused by complications of infections between 9 months to over 3 years after HCT in the setting of significant immune suppression for treatment of GVHD (supplemental Table 1).
The survival rates up to 3 years after HCT were significantly different, with the 3 year probability of survival being 92% (confidence interval [CI]) = ± 11%)in the RIC group and 43% (CI = ± 26%) in the MAC group (P = .0001, Figure 2). Because the RIC patients were followed for significantly less time than the MAC patients (median of 634 days versus median of 2282 days at the time of analysis), estimation of survival probability beyond 3 years was unreliable (supplemental Figure 1).
The Lansky score at last follow-up was 100 for all 6 MAC survivors at a median of 2282 days after HCT at the time of analysis. Of the 23 survivors in the RIC group, 1 (4%) and 20 (87%) patients had a Lansky score of 90 or 100, respectively, at a median of 634 days after HCT. The remaining 2 patients had Lansky scores of 60 or less because of central nervous system compromise related to pre-HCT HLH complications.
Discussion
This is the first direct comparison demonstrating a significant survival advantage for patients undergoing RIC-HCT compared with MAC-HCT for definitive cure of HLH, which supports the initial observation of good outcomes of RIC-HCT for HLH reported by Cooper et al.16,17 We have observed that RIC eliminates the early mortality (< 180 days) that is often observed in patients with HLH undergoing MAC HCT,21 and RIC significantly improves survival, with a 3-year survival rate of 92%. As in a previous cohort of HLH patients treated with RIC-HCT,17 we observed no cases of hepatic veno-occlusive disease, which can occur in 5%-30% of patients with HLH undergoing MAC HCT,8-10 and there were no cases of fatal pneumonitis/acute respiratory distress syndrome or fatal hyperinflammatory syndromes. Notably, we observed no differences in infectious complications between the MAC and RIC groups. This is in agreement with a previous study, which also found no differences in infectious complications between alemtuzumab and ATG-containing regimens in pediatric patients.22
We observed a high incidence of mixed chimerism among the RIC patients reported here (65%) compared with the MAC group (18%). Mixed chimerism often occurs in patients with HLH undergoing allogeneic HCT, and has been reported in 10%-50% of HLH patients who are long-term survivors of MAC regimens,8-10 and 29% of HLH patients receiving a RIC regimen similar to the one reported here (Cooper et al).17
There are several possible explanations for the increased incidence of mixed chimerism observed in our cohort compared with the cohort of HLH patients previously reported who received RIC. All patients treated with RIC-HCT at our center received steroids as part of their GVHD prophylaxis whereas the HLH patients undergoing RIC-HCT reported by Cooper et al17 received only cyclosporine for GVHD prophylaxis. Although the addition of steroids to GVHD prophylaxis may contribute to the increased incidence of mixed chimerism observed in our cohort, this is likely less significant given that mixed chimerism has been reported in 63% of 120 adult patients with a variety of malignancies receiving alemtuzumab as part of preparative regimens containing fludarabine/melphalan, fludarabine/busulfan, or clofarabine/melphalan, who received only tacrolimus for GVHD prophylaxis.23 In addition, mixed donor chimerism including the T-cell compartment has been reported in half of adult patients with acute myeloid leukemia and myelodysplastic syndromes after allogeneic HCT after alemtuzumab, fludarabine, and busulfan using only cyclosporine for GVHD prophylaxis.24
Another possible explanation is that the patients reported by Cooper et al received 1 mg/kg total alemtuzumab, which is less than the average alemtuzumab dose given to our patients, 2.1 mg/kg. Although we did not observe a direct effect of alemtuzumab dose on the incidence of mixed chimerism, we did observe that mixed chimerism is particularly common among patients treated with the proximal alemtuzumab schedule (79%). This suggests that the timing of alemtuzumab administration affects the incidence of mixed chimerism, likely because of higher systemic levels of alemtuzumab at the time of graft infusion and resultant increased graft lymphocyte/T-cell depletion. Younger age was also associated with the development of mixed chimerism. It is possible that there could be differences in the pharmacokinetics of alemtuzumab in the youngest patients who may result in higher levels of alemtuzumab at the time of graft administration.
No primary graft-associated acute GVHD was observed in patients receiving proximal alemtuzumab, with the exception of 1 patient who developed GVHD only after abrupt cessation of GVHD prophylaxis because of rapidly waning donor chimerism. This again suggests more effective lymphocyte/T-cell depletion of the graft with proximal alemtuzumab administration. A previous study noted no occurrence of GVHD among patients receiving alemtuzumab compared with ATG-containing regimens,22 and mixed chimerism has also been shown to be associated with decreased risk of GVHD in other studies.23,24
Despite the development and persistence of mixed chimerism in the RIC group, RIC-HCT was successful in 96% of patients, who remain free of HLH. Similar to a previous report,9 we observed a relapse of HLH in a RIC patient as whole blood donor chimerism decreased to below 10%, and this patient subsequently received a second successful HCT after MAC. The majority of all other patients with mixed donor chimerism received intervention with early wean from GVHD prophylaxis and/or were given DLI to stabilize or increase donor chimerism in an active effort to maintain whole blood donor chimerism above 20%.
The greatest benefit of RIC-HCT is the significantly improved patient survival. The 3-year probability of survival more than doubles with RIC in our series, increasing from 43% for patients undergoing MAC-HCT to 92% for patients undergoing RIC-HCT. The survival that we observed with MAC is similar to that reported by Baker et al in 1997,12 but lower than the survival observed in more recent reports. This may be because our center is a tertiary referral center, and it has been our experience that patients with straightforward clinical courses often undergo HCT at other US institutions, whereas patients with difficult or recalcitrant disease are often referred. However, even with comparison to the highest reported survival rate among HLH patients undergoing MAC-HCT, 64%,8 the survival that we have observed here is still significantly improved in comparison.
The improved survival may be a result of the “reduced intensity” of fludarabine and melphalan compared with traditional MAC regimens, or it is also possible that survival is improved simply because of the inclusion of alemtuzumab in the regimen. Alemtuzumab is efficient at depleting T cells and also CD52-expressing antigen-presenting cells, which may effectively treat any residual/smoldering HLH at the time of transplantation. Parenthetically, we have observed a beneficial effect of alemtuzumab for treatment of refractory HLH (unpublished data). The fatal periengraftment hyperinflammatory syndromes that have been observed in patients with HLH may also be prevented by alemtuzumab via efficient depletion of both host and donor antigen-presenting and effector cells.
Given the significantly improved survival of patients with HLH undergoing RIC-HCT, we recommend that all patients with HLH receive RIC regimens if a suitable bone marrow or peripheral blood stem cell source is identified. Choice of preparative regimen when a cord blood graft is the only option should be carefully considered, as the lack of any ready source of DLI is a concern given the high percentage of mixed chimerism observed with RIC. It appears that whole blood donor chimerism above 10%-20% does indeed protect against HLH, as the only patient to relapse HLH did so only after donor chimerism fell to less than 10%. Frequent monitoring of chimerism studies is essential, and centers should be prepared to stabilize donor chimerism with early withdrawal of GVHD prophylaxis or administration of DLI if needed. Future studies to more closely measure donor chimerism within the myeloid and lymphoid compartments, and specifically within the lymphocyte subsets, are needed to determine which cell populations are most critical for protection against HLH.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and their families for their support of our work, Denise Bellman and Rebekah Kennedy for assistance with data collection, all of the physicians, nurses, and staff who provided care for patients at Cincinnati Children's Hospital, and the Cincinnati Children's Hospital Diagnostic Immunology and Genetics Laboratories.
Authorship
Contribution: R.A.M. collected and analyzed data and wrote the manuscript; G.V. collected data; M.K. and D.L. performed the statistical analysis; S. Jodele, S. Joshi, and P.A.M. edited the manuscript; S.M.D., M.B.J., and J.J.B. edited the manuscript and contributed invaluable expertise; and A.H.F. designed the study and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rebecca A. Marsh, Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: rebecca.marsh@cchmc.org.