Abstract
Activation-dependent platelet granule release is mediated by integral membrane proteins called soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptors (SNAREs) and their regulators; however, the mechanisms for this process are ill-defined. To further characterize platelet secretion, we analyzed the function of platelets from Unc13dJinx mice. Platelets from these animals lack the putative vesicle priming factor, Munc13-4, and have a severe secretion defect. Release from dense granules was completely ablated and that from α-granules and lysosomes was severely compromised. Unc13dJinx platelets showed attenuated aggregation and, consequently, Unc13dJinx mice had prolonged tail-bleeding times. The secretion defect was not due to altered expression of SNAREs or SNARE regulators, defective granule biogenesis, or faulty platelet activation. The defective release could be rescued by adding recombinant Munc13-4 to permeabilized Unc13dJinx platelets. In wild-type mouse platelets, Munc13-4 levels were lower than those of SNAREs suggesting that Munc13-4 could be a limiting component of the platelets' secretory machinery. Consistently, Munc13-4 levels directly correlated with the extent of granule release from permeabilized platelets and from intact, heterozygous Unc13dJinx platelets. These data highlight the importance of Munc13-4 in platelets and indicate that it is a limiting factor required for platelet secretion and hemostasis.
Introduction
Platelets play a key role in hemostasis through their ability to respond to vascular injury. Damage to endothelial cells causes the exposure of agonists, for example, collagen and von Willebrand factor (VWF), which initiate platelet adhesion and activation. Platelet activation is marked by a rapid rise in intracellular [Ca2+]i that triggers secretion from 3 types of internal granule stores: dense granules, α-granules, and lysosomes.1,2 Each granule type carries specific molecules that promote hemostatic plug formation and the sequelae that are required to maintain a pressurized vasculature. Dense granules contain small molecules and ions such as adenosine triphosphate (ATP), adenosine 5′-diphosphate (ADP), serotonin, and Ca2+ which are important for thrombogenesis.3 Although few (3∼8/platelet), dense granule content is released much more rapidly than content from α-granules or lysosomes.4 α-Granules are the most abundant granules in platelets (40∼60/platelet) and their cargo is diverse, ranging from growth factors (eg, platelet-derived growth factor [PDGF]) and chemokines (eg, platelet factor IV [PF4]) to adhesive molecules (eg, VWF and fibrinogen).5 These factors are not only important for clot stabilization but also play a role in wound repair. Release of lysosomal cargo (eg, β-hexosaminidase) is thought to be involved in clot remodeling.1,2
Much like neurons and endocrine cells, platelet exocytosis is dependent on [Ca2+]i and mediated by soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptors (SNAREs) present on the granule/vesicle membranes (v-SNAREs) and on the plasma/target membrane (t-SNAREs). Cognate v- and t-SNAREs interact to form a trans-bilayer complex that juxtaposes vesicle and target membranes and provides the driving force for membrane fusion.6,7 Platelets express numerous v- and t-SNAREs. Data from our laboratory and from others suggest that the t-SNAREs, syntaxin and SNAP-23, and the v-SNARE, VAMP-8, play significant roles during platelet secretion.8-13 Based on in vivo studies of VAMP-8−/− animals3 and the analysis of human donors who are hyperresponsive to epinephrine,14 it is clear that modulation of platelet SNAREs can profoundly affect platelet function.
Although secretion is essential for thrombosis,3 little is known about the regulatory elements that control it. In addition to SNAREs, platelets contain numerous regulatory proteins that control where and when SNARE complexes assemble during activation-dependent secretion.15-18 Of particular interest are members of the Munc13 family, which are thought to affect SNARE complex formation and thus increase SNARE-mediated membrane fusion. The Munc13 family contains 4 distinct members, Munc13-1, -2 (2 splice variants), and -3, which are highly expressed in brain; Munc13-4 is ubiquitously expressed but enriched in cells of the hematopoietic lineage (eg, mast cells, neutrophils, eosinophils, and platelets).19-22 Like SNAREs, the Munc13 family is highly conserved across species indicating an evolutionarily important role.23-25 Munc13 proteins are generally thought to be vesicle-priming factors that control the fusion-competency of “docked” vesicles22,25,26 possibly through direct interactions with syntaxin t-SNAREs.27-29 Most studies of Munc13 proteins have focused on the role of Munc13-1 during Ca2+-dependent, excitatory secretion. Indeed, Munc13-1 is required for synaptic transmission, neuroendocrine secretion, and insulin secretion.25,26,30
Recent work suggests a role for Munc13-4 in hematopoietic cells. Overexpression of Munc13-4 in RBL-2H3 mast cells lead to increased degranulation31 while addition of Munc13-4 to permeabilized platelets mildly enhanced dense granule release.32 Moreover, neutrophils, natural killer (NK) cells, and cytotoxic T cells (CTLs) obtained from Unc13dJinx mice, which lack full-length Munc13-4, display severe secretion deficiencies.33,34 Munc13-4 colocalizes with secretory lysosomes in RBL-2H3 cells31 and translocates to the plasma membrane in neutrophils upon stimulation.35 Munc13-4 has been reported to be an effector of the small Guanosine triphosphate (GTP)–binding protein Rab27, which is essential for the biogenesis of certain granule types and for proper T-cell function.36 Dysfunction of Munc13-4 in humans is associated with familial hemophagocytic lymphohistiocytosis subset 3 (FHL3), a disease in which the lytic granules of NK cells and CTLs properly dock at immune synapses, but fail to fuse with the plasma membrane to release their cargo.22 Taken together, these data support a role for Munc13-4 as a positive regulator of granule secretion.
Although Munc13-4 has been suggested to be important for dense granule release,32 its mechanism of action and its potential role in the other platelet release events are unknown. Here we characterize the role of Munc13-4 in platelets. We show that platelets from Unc13dJinx mice lack Munc13-4. Functional analyses revealed that activation-dependent dense granule secretion was completely abolished in Unc13dJinx platelets while α-granule and lysosomal secretion were severely impaired. Consequently, Unc13dJinx mice display a severe bleeding diathesis. The secretion defects could be rescued by addition of full-length, recombinant Munc13-4 to permeabilized Unc13dJinx platelets but not by addition of a Munc13-4 truncation mutant that lacked the C-terminal, Ca2+-binding, C2 domain. Further analysis of platelets from Unc13dJinx heterozygous mice revealed that Munc13-4 is a limiting factor at a key control point in the secretory pathway that leads to platelet exocytosis.
Methods
Antibodies and reagents
Monoclonal antibodies against synaptobrevin/VAMP-2, TI-VAMP/VAMP-7, phosphotyrosine (clone 4G10), fibrinogen, β-actin, and polyclonal antibodies against cellubrevin/VAMP-3, endobrevin/VAMP-8, syntaxin 2, syntaxin 4, SNAP-23 were described previously.12 The monoclonal antibody against Munc13-1 (clone 266B1) was from Synaptic System. Polyclonal antibodies against Munc18a/b, Munc18c were described previously.16 The monoclonal anti-Rab27a antibody (clone 20.1/Rab27) was from BD Biosciences. The monoclonal anti–mouse PF4 antibody (clone 140910) was from R&D Systems. The monoclonal anti–α-SNAP antibody (clone 4E4) was described previously.15 The polyclonal anti-Doc2α antibody was generated using a Doc2α-specific peptide (CYLKDLEQAEQGQGL) conjugated to keyhole limpet hemocyanin as antigen. The polyclonal anti-Munc13-4 antibody was generated against an N-terminal fragment (human, 36-349 aa). Fluorescein isothiocyanate (FITC)–conjugated anti–mouse P-selectin antibody (clone RB40.34), anti–mouse CD61 antibody (clone 2C9.G2), and phycoerythrin (PE)–conjugated anti–mouse CD107a (LAMP-1) antibody (clone 1D4B), and appropriate isotype control immunoglobulin Gs (IgGs) were from BD PharMingen. PE-conjugated anti–mouse integrin αIIbβ3 (active form, clone Jon/A) antibody was from Emfret Analytics.
Plasmid construction and protein purification
DNAs encoding the human Munc13-4 open reading frame 1-1090 aa and a 1-910 aa fragment (lacking the C-terminal C2 domain; ΔC2B) were inserted into the pENTR/D-Topo vector (Invitrogen) and subcloned into the pDEST10 vector (Invitrogen). The pDEST10-Munc13-4 plasmid was then used to transform DH10Bac cells (Invitrogen) and the positive Munc13-4-Bacmid was isolated to infect Sf9 cells to produce baculovirus. Infected Sf9 cells were collected 72 hours later, and His6-tagged, recombinant proteins were first purified by Ni2+-NTA agarose chromatography using standard procedures followed by gel-filtration chromatography on Superose 6 (GE Healthcare; 25mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]/KOH pH 7.4, 150mM KCl, 2mM β-mercaptoethanol). The protein preparations were generally more than 90% pure (data not shown).
Mice and genotyping
The Unc13dJinx mice (MMRRC: 016137) on a C57Bl/6J background were purchased from Mutant Mouse Regional Resource Center at University of California Davis. Age- and sex-matched wild-type C57Bl/6J mice were purchased from The Jackson Laboratory. Unc13dJinx heterozygous mice were generated by a cross of C57Bl/6J and Unc13dJinx mice. All animal work was approved by the University of Kentucky Institutional Animal Care and Use Committee.
The genotype of each mouse was determined by polymerase chain reaction (PCR) using DNA from the tail tip, and the analysis was carried with primers: forward: 5′-ATCCCAACATGCCACTG-3′ and reverse: 5′-GGTGAAGTCAGAGCACA-3′ for the wild-type gene (394 bp), and primers: forward: 5′-ATCCCAACATGCCACTT-3′ and reverse: 5′-CAGAGAGGAGGGATCGG-3′ for the Unc13dJinx mutation (464 bp).
Platelet preparation
Blood collection and preparation of murine platelets were previously described.12 Human platelets were prepared from platelet-rich plasma (PRP) obtained from the Kentucky Blood Center (Lexington, KY).
Analysis of platelets
All the analyses of platelet function including measurements of calcium influx, aggregometry, and secretion of [3H]-5-HT (5-hydroxytryptamine/serotonin, dense granule marker), PF4 (α-granule marker), and β-hexosaminidase (lysosome marker) from intact and permeabilized platelets were performed as in Ren et al12 The biochemical and ultrastructural analyses have also been described previously.12 Quantitative Western blotting was done using enhanced chemifluorescence with the ECF substrate (GE Healthcare). Images were generated with a Typhoon 9400 Imager and quantified with ImageQuant 5.2 software (GE Healthcare). Recombinant proteins were used to generate the standard curves for quantification. Endogenous serotonin was measured using o-phthaldialdehyde (Sigma-Aldrich) as described by Holmsen and Dangelmaier.37 Tail-bleeding time measurements were performed as in Schraw et al.38
Flow cytometry
Flow cytometry was used to monitor secretion and to measure the activation of integrin, αIIbβ3.39 Five microliters of fluorescent-conjugated antibodies, isotype control IgG, or phosphate-buffered saline (PBS) were mixed with 45 μL of platelet suspension (1.2 × 106 platelets) and the cells were stimulated with the indicated concentrations of thrombin for 1 minute. The reactions were stopped with a 2-fold excess of hirudin, put on ice for 20 minutes in the dark, and then fixed with an equal volume of 2% formaldehyde/PBS for 45 minutes at room temperature (RT). The samples were diluted with 800 μL of HEPES/Tyrode buffer (10mM HEPES/NaOH, pH 7.4, 5.56mM glucose, 137mM NaCl, 12mM NaHCO3, 2.7mM KCl, 0.36mM KH2PO4, 1mM MgCl2) and flow cytometry was carried out with a FACSCalibur (BD Biosciences). The data were analyzed using WinMDI software (version 2.8).
Results
Characterization of platelets from Unc13dJinx mice
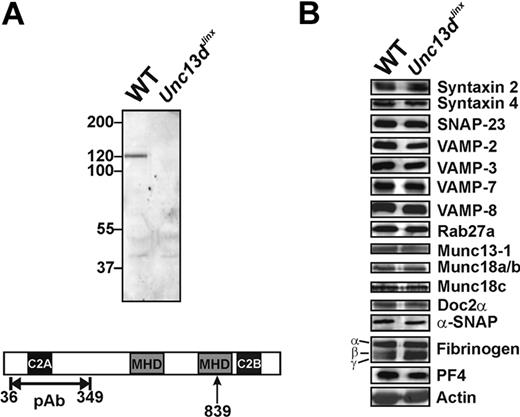
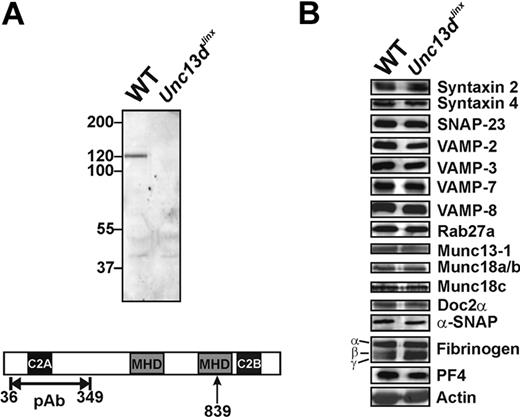
Munc13-4 is enriched in hematopoietic cells, including platelets. We first probed for Munc13-4 in wild-type platelets and for the predicted, truncated form in platelets from Unc13dJinx mice. Using a polyclonal antibody raised against an N-terminal fragment (36-349 aa; Figure 1A), a 120-kDa band, which represents the full-length Munc13-4, was detected in extracts from wild-type platelets; this was not visible in extracts from Unc13dJinx platelets. Based on the predicted splicing of the Munc13-4 RNA,33 an approximately 99-kDa fragment should be produced in Unc13dJinx mice (1-839 aa). No fragment of this size was detected in extracts from Unc13dJinx platelets, consistent with the data obtained from Unc13dJinx mast cells34 and suggesting that the Munc13-4 fragment (1-839 aa) is unstable in vivo. Thus, Unc13dJinx platelets are Munc13-4-deficient.
Western blotting analysis of platelets from wild-type and Unc13dJinx mice. (A) Equal amounts of platelet extract from wild-type (WT) and Unc13dJinx mice (5 × 107 platelet equivalents per lane) were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by Western blotting using a polyclonal, anti-Munc13-4 antibody. The epitope used for antibody generation is indicated in the schematic diagram of mouse Munc13-4 in panel A. The position of the frameshift mutation is indicated (839 aa, ↑). (B) The platelet extracts used in panel A were probed with antibodies against the indicated SNARE proteins, secretion regulators, and cargo.
Western blotting analysis of platelets from wild-type and Unc13dJinx mice. (A) Equal amounts of platelet extract from wild-type (WT) and Unc13dJinx mice (5 × 107 platelet equivalents per lane) were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by Western blotting using a polyclonal, anti-Munc13-4 antibody. The epitope used for antibody generation is indicated in the schematic diagram of mouse Munc13-4 in panel A. The position of the frameshift mutation is indicated (839 aa, ↑). (B) The platelet extracts used in panel A were probed with antibodies against the indicated SNARE proteins, secretion regulators, and cargo.
As Munc13-4 has been reported to interact with multiple SNARE regulators including Rab27a/b,32,40 Doc2α,41 and potentially syntaxins, we examined whether loss of Munc13-4 affected the levels of the core SNAREs or selected SNARE regulators. Of the 13 proteins examined, there were no obvious differences between wild-type and Unc13dJinx platelets (Figure 1B). Of specific note, Munc13-1 was present in mouse platelets, but its level was not changed in Unc13dJinx platelets.
Because Rab27b−/− mice have defects in dense granule biogenesis,40 we determined whether Munc13-4, a known Rab27a/b effector, was required for this process. Similar levels of serotonin were found in wild-type (2.67 ± 0.02 μg/109 platelets) and Unc13dJinx platelets (2.83 ± 0.27 μg/109 platelets) (p = .34, n = 3), suggesting the dense granule biogenesis is unaffected in Unc13dJinx mice. To confirm that Munc13-4 was not involved in α-granule biogenesis, we analyzed the levels of 2 abundant α-granule cargo proteins, PF4 (also calculated from secretion assay data) and fibrinogen by Western blot. There was no difference in the levels of these proteins between wild-type and Unc13dJinx platelets (Figure 1B). Platelets from Unc13dJinx mice also had wild-type levels β-hexosaminidase (calculated from secretion assay data). Consistently, there was no difference in granule number or morphology seen in the ultrastructural analyses (see Figure 3A and data not shown). Taken together, these data show that loss of Munc13-4 does not overtly affect the biogenesis of any of the 3 classes of platelet granules.
Thrombin-stimulated secretion is defective in platelets from Unc13dJinx mice
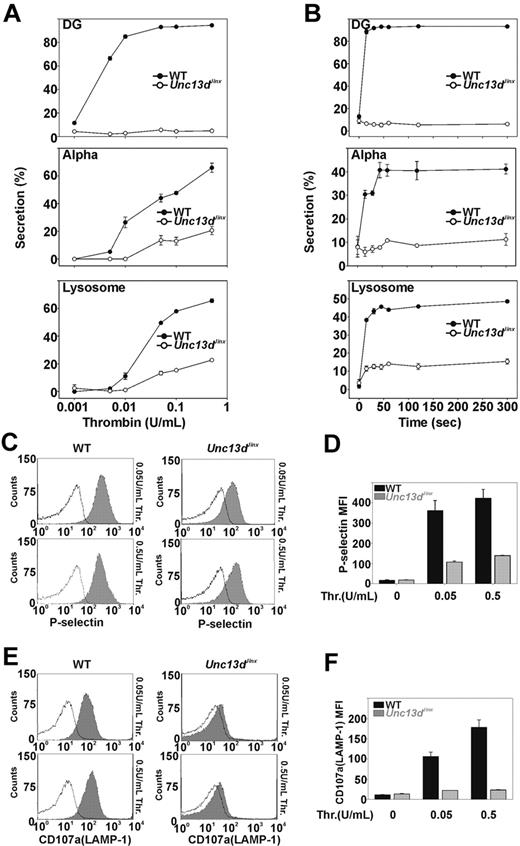
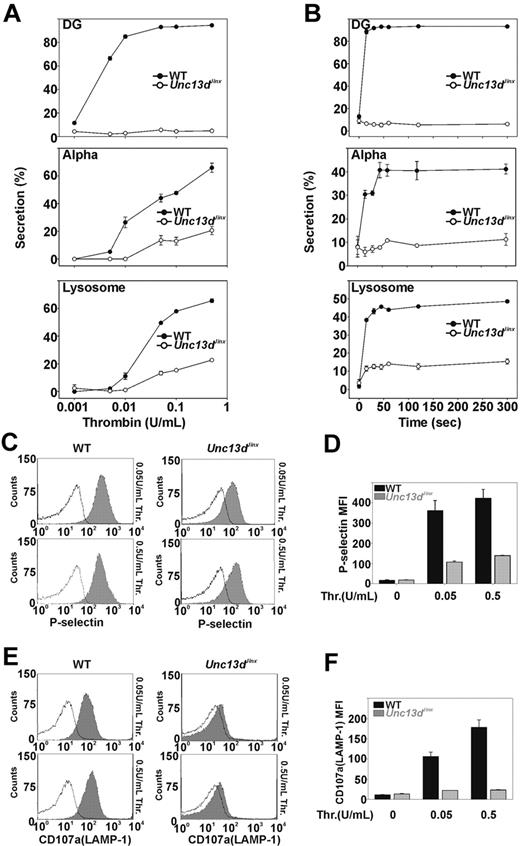
Previous studies, using permeabilized human platelets treated with recombinant Munc13-4 protein, suggested that Munc13-4 plays a positive role in platelet dense granule release.32 Whether Munc13-4 is involved in α-granule or lysosomal secretion was not addressed. The Unc13dJinx platelets provide a unique model to determine whether Munc13-4 is required. Release from all 3 granules was measured in agonist-titration (Figure 2A) and time-course experiments (Figure 2B). Thrombin was chosen as the agonist because its action can be quickly blocked with hirudin, thus improving the ability to measure secretion kinetics. In Figure 2A, various concentrations of thrombin were used to stimulate platelet secretion and the reactions were terminated after 1 minute. For wild-type platelets (Figure 2A closed circles), dense granule secretion increased as thrombin concentrations were increased and was maximal at approximately 0.05 U/mL. For Unc13dJinx platelets (Figure 2A open circles), there was almost no dense granule secretion, even in the highest concentration of thrombin (0.5 U/mL). For wild-type platelets, α-granule and lysosomal secretion also increased with increasing thrombin concentrations. At 0.5 U/mL, more than 60% of the total PF4 and β-hexosaminidase were released. For Unc13dJinx platelets, α-granule and lysosomal secretion were significantly decreased relative to wild type; at 0.5 U/mL thrombin, secretion was approximately 70% lower in the Unc13dJinx platelets compared with wild type. Similar results were observed in the time-course experiments (Figure 2B). Kinetic analysis was inconclusive for α-granule release (due to the low release); however, analysis of lysosome release suggested that only the extent of secretion was defective. The initial rates for lysosome release were similar between wild-type and Unc13dJinx platelets.
Platelets from Unc13dJinx mice show severe defects in thrombin-stimulated granule secretion. (A) Platelets from wild-type (WT) and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (final concentration 0.7mM) followed by addition of thrombin (final concentration is indicated). After a 1-minute incubation (RT), reactions were stopped by the addition of excess hirudin, and the extent of secretion from dense granules (DG), α-granules (Alpha), and lysosomes was analyzed and percent secretion was calculated as described.12 (B) Experiments were carried out as in panel A using 0.05 U/mL thrombin; reactions were stopped at the indicated time points and analyzed. (C-D) Five microliters of FITC-conjugated P-selectin antibody was added to equal amounts of platelets from wild-type and Unc13dJinx mice. Thrombin was added (gray fill) or not (black lines) and the samples were incubated for 1 minute (RT). The reactions were stopped with excess hirudin and analyzed by flow cytometry. (E-F) Experiments in panel C were repeated using a PE-conjugated CD107a/LAMP-1 antibody. Representative traces were shown in panels C and E. Data in panels D and F represent the mean ± SD from triplicates.
Platelets from Unc13dJinx mice show severe defects in thrombin-stimulated granule secretion. (A) Platelets from wild-type (WT) and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (final concentration 0.7mM) followed by addition of thrombin (final concentration is indicated). After a 1-minute incubation (RT), reactions were stopped by the addition of excess hirudin, and the extent of secretion from dense granules (DG), α-granules (Alpha), and lysosomes was analyzed and percent secretion was calculated as described.12 (B) Experiments were carried out as in panel A using 0.05 U/mL thrombin; reactions were stopped at the indicated time points and analyzed. (C-D) Five microliters of FITC-conjugated P-selectin antibody was added to equal amounts of platelets from wild-type and Unc13dJinx mice. Thrombin was added (gray fill) or not (black lines) and the samples were incubated for 1 minute (RT). The reactions were stopped with excess hirudin and analyzed by flow cytometry. (E-F) Experiments in panel C were repeated using a PE-conjugated CD107a/LAMP-1 antibody. Representative traces were shown in panels C and E. Data in panels D and F represent the mean ± SD from triplicates.
As confirmation, we measured α-granule and lysosome release using flow cytometry. P-selectin and CD107a/LAMP-1 are both integral membrane proteins in α-granules and lysosomes, respectively, and their exposure at the platelet surface is a metric of granule exocytosis. The potential effects of ADP, released from dense granules, were lessened by inclusion of apyrase (0.02 U/mL).39 For wild-type platelets, the mean fluorescence intensity of staining for P-selectin increased upon stimulation with thrombin (0.05 or 0.5 U/mL; Figure 2C). Unc13dJinx platelets showed a much smaller increase in P-selectin staining. When quantified (Figure 2D), P-selectin staining on Unc13dJinx platelets was less than 30% of wild type. Similar results were observed when staining for CD107a/LAMP-1 (Figure 2E-F). Using these 2 types of assays, one that measures release of soluble cargo and the second that measures exposure of granule membrane proteins, it is clear that Munc13-4 is required for platelet exocytosis.
Signaling is unaffected in platelets from Unc13dJinx mice
One possible reason for the observed secretion defects could be that some aspect of platelet activation is deficient due to a loss of Munc13-4. To address this we examined several important processes that occur during platelet activation: platelet cytoskeletal changes, calcium mobilization, total protein tyrosine phosphorylation, and integrin αIIbβ3 activation.
Activated platelets undergo significant cytoskeletal reorganization, forming filopodia and centralizing their granules. These rearrangements are important for secretion.42 It is formally possible that Unc13dJinx platelets could undergo faulty cytoskeletal rearrangements. To address this question, we performed transmission electron microscopy on resting and thrombin-activated platelets. In Figure 3Ai and ii, under resting conditions, wild-type and Unc13dJinx platelets were discoid with similar arrays of granules, mitochondria, and an open canalicular system. When stimulated with 0.1 U/mL thrombin for 2 minutes, wild-type platelets showed a more irregular appearance with protruding filopodia and some pale round structures in the central area (Figure 3Aiii,v,vii). This is consistent with activation-dependent cytoskeletal rearrangements and release of granule cargo (pale round structures are spent granules). Most of the thrombin-stimulated, Unc13dJinx platelets had protruding filopodia with some lamellipodia (Figure 3Aiv,vi,viii). Almost all had identifiable granules with opaque centers that were clustered in the center of the platelets. Of note, dense granules with their unique electron-dense centers were obvious in almost every section examined. These results are consistent with the data shown in Figure 2 because the granules had not fused or released their cargo. However, the Unc13dJinx platelets did show the expected activation-dependent changes in morphology that are associated with cytoskeletal rearrangements. This suggests that the processes leading to cytoskeletal rearrangements are not defective in Unc13dJinx platelets.
The morphology and response to activation are similar in platelets from wild-type and Unc13dJinx mice. (A) Platelets from wild-type and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (0.7mM final). Panels i and ii represent platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels iii through viii. After 2 minutes at RT, samples were fixed and analyzed by transmission electron microscopy. Scale bars represent 1 μm in panels iii and iv, and 200 nm for all others. (B) Washed platelets from wild-type (black trace) or Unc13dJinx mice (gray trace) were loaded with Fura-2/AM to measure intracellular [Ca2+]i upon activation with thrombin (0.1 U/mL). Arrows indicate the addition of extracellular Ca2+ (0.7mM final) and thrombin. (C) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were mixed with 5 μL of PE-conjugated-Jon/A antibody and 5 μL of FITC-conjugated CD61 antibody in the presence of 1.25mM CaCl2 and 0.02 U/mL apyrase. Samples were incubated without thrombin (resting) or with thrombin (0.1 U/mL) for 5 minutes at RT. Reactions were stopped with excess hirudin and analyzed by flow cytometry. Data were plotted as 2-color dot plots (Jon/A and CD61). The percentage of platelets in each quadrant was indicated. (D) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were either kept in the resting state or stimulated with the indicated agonists for 2 minutes at RT. The reactions were stopped by adding SDS sample buffer and the samples were analyzed by Western blot for phosphotyrosine (pY). Ponceau S red staining of actin serves as loading control (bottom panel).
The morphology and response to activation are similar in platelets from wild-type and Unc13dJinx mice. (A) Platelets from wild-type and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (0.7mM final). Panels i and ii represent platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels iii through viii. After 2 minutes at RT, samples were fixed and analyzed by transmission electron microscopy. Scale bars represent 1 μm in panels iii and iv, and 200 nm for all others. (B) Washed platelets from wild-type (black trace) or Unc13dJinx mice (gray trace) were loaded with Fura-2/AM to measure intracellular [Ca2+]i upon activation with thrombin (0.1 U/mL). Arrows indicate the addition of extracellular Ca2+ (0.7mM final) and thrombin. (C) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were mixed with 5 μL of PE-conjugated-Jon/A antibody and 5 μL of FITC-conjugated CD61 antibody in the presence of 1.25mM CaCl2 and 0.02 U/mL apyrase. Samples were incubated without thrombin (resting) or with thrombin (0.1 U/mL) for 5 minutes at RT. Reactions were stopped with excess hirudin and analyzed by flow cytometry. Data were plotted as 2-color dot plots (Jon/A and CD61). The percentage of platelets in each quadrant was indicated. (D) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were either kept in the resting state or stimulated with the indicated agonists for 2 minutes at RT. The reactions were stopped by adding SDS sample buffer and the samples were analyzed by Western blot for phosphotyrosine (pY). Ponceau S red staining of actin serves as loading control (bottom panel).
The stimulation-dependent increase in intra-platelet calcium and the activation of tyrosine kinases are important for platelet activation. Activation-dependent changes in intra-platelet calcium were measured using the ratio fluorescence of Fura-2. The [Ca2+]i traces of wild-type and Unc13dJinx platelets, in response to thrombin, were almost identical (Figure 3B, supplemental Figure 1; available on the Blood Web site, see the Supplemental Materials link at the top of the online article) suggesting that there is no difference in calcium mobilization between Unc13dJinx and wild-type platelets. In addition, there was no obvious difference between the array of tyrosine-phosphorylated proteins seen in the 2 preparations (Figure 3D). These data indicate that activation of tyrosine kinases in Unc13dJinx platelets is not defective.
Integrin αIIbβ3 is activated through inside-out signaling43 and in mice, this can be measured with the Jon/A antibody. We performed flow cytometric analysis of integrin αIIbβ3 with the Jon/A antibody (active integrin αIIbβ3) and CD61 (total surface integrin αIIbβ3; Figure 3C, supplemental Figure 2). In the resting state, total surface integrin αIIbβ3 was similar in platelets from wild-type and Unc13dJinx mice. Less than 3% of total αIIbβ3 was activated in both wild-type and Unc13dJinx platelets. Upon stimulation, 42% of αIIbβ3 molecules on wild-type platelets were activated which is comparable with 41% on Unc13dJinx platelets, indicating that αIIbβ3 activation was the same in Unc13dJinx and wild-type platelets.
Unc13dJinx mice showed no signs of thrombocytopenia consistent with previous studies33 and the mean volumes of wild-type (4.53 ± 0.32 fL) and Unc13dJinx platelets (4.25 ± 0.17 fL) were not statistically different (n = 6, p = .09). These data suggest that there is no defect in platelet biosynthesis. In summary, of the platelet activation processes examined, we found no obvious differences between Unc13dJinx and wild-type platelets. These results show that loss of Munc13-4 does not cause any gross abnormality in platelet activation or biosynthesis.
Ex vivo aggregation of Unc13dJinx platelets and in vivo hemostasis in Unc13dJinx mice
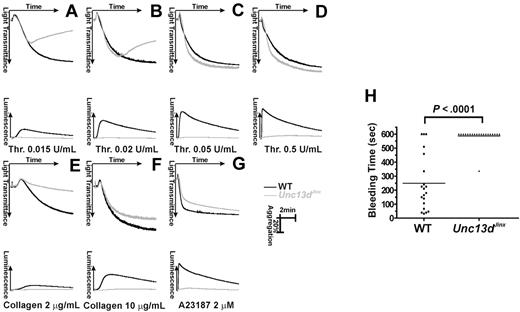
Next, we examined whether the defects in platelet secretion affected platelet function. We measured platelet function, ex vivo, by aggregometry with simultaneous measurements of ATP release. Figure 4 panels A through D shows aggregation (indicated by increased light transmission) and ATP release (detected by luciferin/luciferase luminescence) in platelets stimulated with various concentrations of thrombin. At higher concentrations (0.05 U/mL, Figure 4C; 0.5 U/mL, Figure 4D), aggregation was almost identical between wild-type (black) and Unc13dJinx platelets (gray). At these higher agonist concentrations, defective secretion is not detrimental to aggregation, especially given there is no defect in αIIbβ3 activation. Reversible aggregation traces were observed when Unc13dJinx platelets were stimulated with lower concentrations of thrombin (0.015 U/mL, Figure 4A; 0.02 U/mL, Figure 4B). Wild-type platelets aggregated normally at these doses. As previously observed, secretion is most important when lower levels of agonist are used.3 This phenotype was also obvious when collagen was used as an agonist. At 10 μg/mL (Figure 4F), there was a slight aggregation defect in Unc13dJinx platelets but when the agonist was decreased to 2 μg/mL, only shape change was observed while wild-type platelets aggregated normally (Figure 4E). When stimulated with a nonphysiologic strong agonist, A23187 (2μM), aggregation was unaffected in Unc13dJinx platelets (Figure 4G). It should be noted that in all of these experiments, release of ATP from dense granules was completely ablated (gray lines in the bottom portion of each panel), consistent with the data in Figure 2. These data underline the importance of dense granule release for full platelet activation and suggest that the secretion defect in Unc13dJinx platelets is not agonist-specific.
Platelet aggregation and hemostasis are defective in platelets from Unc13dJinx mice. (A-G) Platelets from wild-type (WT, black) and Unc13dJinx mice (Unc13dJinx, gray) were prepared as described and diluted to approximately 3 × 108/mL. CaCl2 was added to each sample (0.7mM final) and platelets were activated with the indicated agonists. Increase in light transmittance (aggregation) and luminescence (ATP release) were monitored and plotted as a function of time. (H) The tails of wild-type (■) and Unc13dJinx (▴) mice were clipped and the duration of bleeding was measured (Bleeding Time) in seconds. The individual bleeding times (points) and averages (line) from n = 20 mice were plotted. A Student t test (SigmaPlot 9.0) was used to assess the statistical significance of the differences between groups.
Platelet aggregation and hemostasis are defective in platelets from Unc13dJinx mice. (A-G) Platelets from wild-type (WT, black) and Unc13dJinx mice (Unc13dJinx, gray) were prepared as described and diluted to approximately 3 × 108/mL. CaCl2 was added to each sample (0.7mM final) and platelets were activated with the indicated agonists. Increase in light transmittance (aggregation) and luminescence (ATP release) were monitored and plotted as a function of time. (H) The tails of wild-type (■) and Unc13dJinx (▴) mice were clipped and the duration of bleeding was measured (Bleeding Time) in seconds. The individual bleeding times (points) and averages (line) from n = 20 mice were plotted. A Student t test (SigmaPlot 9.0) was used to assess the statistical significance of the differences between groups.
To further assay the role of secretion in hemostasis, bleeding time measurements were recorded for wild-type and Unc13dJinx mice (Figure 4H). The average bleeding time for wild-type mice was 248.7 plus or minus 44.5 seconds (n = 20), while that for Unc13dJinx mice was 587.85 plus or minus 13.2 seconds (n = 20; P < .001). Nineteen of the 20 Unc13dJinx mice tested did not stop bleeding after 10 minutes. This prolonged bleeding phenotype in the Unc13dJinx mice further supports the role of platelet secretion in hemostasis in vivo.
Munc13-4 is a limiting factor for platelet exocytosis
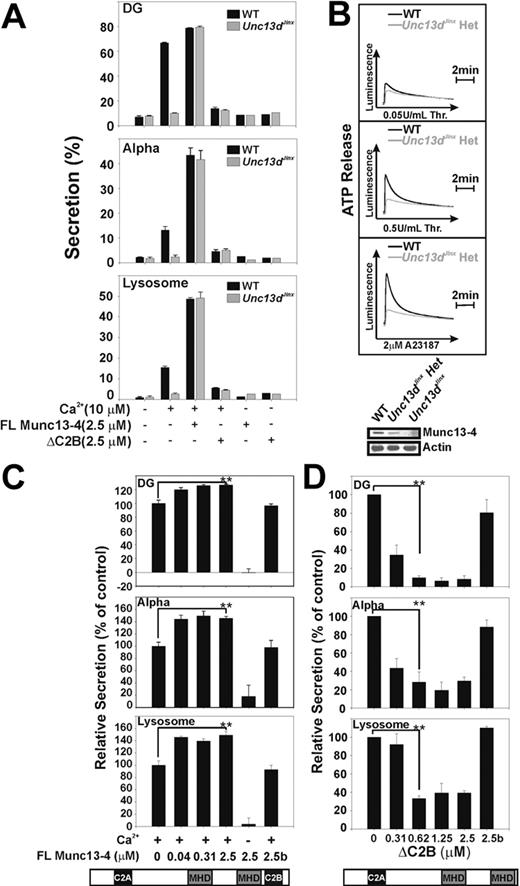
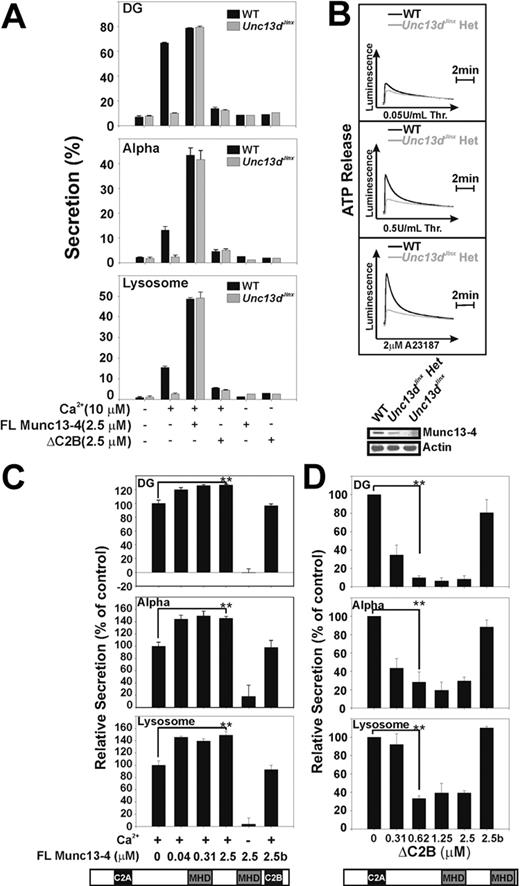
Previous studies of CTLs and NK cells from FHL3 patients showed that the secretion defects could be rescued by transfecting the cells with a cDNA encoding full-length Munc13-4. To confirm that the secretion defect in Unc13dJinx platelets was due to the loss of Munc13-4, we performed rescue experiments using recombinant Munc13-4 and permeabilized Unc13dJinx platelets. As shown in Figure 5A, calcium-triggered cargo release from all 3 granules was detected when permeabilized wild-type platelets were used (Figure 5A black bars) but not when Unc13dJinx platelets were tested (Figure 5A gray bars). When recombinant Munc13-4 was added to wild-type platelets, secretion from all 3 granules was significantly enhanced. For Unc13dJinx platelets, addition of Munc13-4 protein completely rescued secretion, reaching almost the identical maxima observed for wild-type platelets that had received recombinant Munc13-4. These results suggest that the Munc13-4 deficiency directly accounts for the secretion defect in Unc13dJinx platelets.
Munc13-4 is a limiting factor for platelet exocytosis. (A) Platelets were permeabilized by incubation with Streptolysin-O (SLO). Platelets from wild-type (WT) and Unc13dJinx mice were incubated with recombinant full-length (FL) Munc13-4 or ΔC2B as indicated. Samples in which Munc13-4 was omitted were included as controls. CaCl2 (10μM final) was added as indicated and secretion from dense granules (DG), α-granules (Alpha), and lysosomes was monitored and plotted as in Figure 2. Data are represented as the mean ± SD, n = 3. (B) Platelets from wild-type (WT, black traces) or Unc13dJinx heterozygous mice (Unc13dJinx Het, gray traces) were prepared as previously described in the legend of Figure 4. Secretion of ATP from dense granules (Luminescence) was monitored after addition of the indicated agonists. (Inset) The expression levels of Munc13-4 in platelets from wild-type (WT), Unc13dJinx, and Unc13dJinx heterozygous mice as determined by Western blotting with actin serving as loading control. (C-D) Human platelets were permeabilized with SLO as describe above and incubated with the indicated concentrations of Munc13-4 (C), ΔC2B (D), or with proteins that had been heat-inactivated (2.5b). Secretion from dense granules (DG), α-granules (Alpha), and lysosomes was monitored and normalized to controls in which no exogenous Munc13-4 was added. A Student t test (SigmaPlot 9.0) was used to assess the statistical significance of the differences between groups (**P < .01).
Munc13-4 is a limiting factor for platelet exocytosis. (A) Platelets were permeabilized by incubation with Streptolysin-O (SLO). Platelets from wild-type (WT) and Unc13dJinx mice were incubated with recombinant full-length (FL) Munc13-4 or ΔC2B as indicated. Samples in which Munc13-4 was omitted were included as controls. CaCl2 (10μM final) was added as indicated and secretion from dense granules (DG), α-granules (Alpha), and lysosomes was monitored and plotted as in Figure 2. Data are represented as the mean ± SD, n = 3. (B) Platelets from wild-type (WT, black traces) or Unc13dJinx heterozygous mice (Unc13dJinx Het, gray traces) were prepared as previously described in the legend of Figure 4. Secretion of ATP from dense granules (Luminescence) was monitored after addition of the indicated agonists. (Inset) The expression levels of Munc13-4 in platelets from wild-type (WT), Unc13dJinx, and Unc13dJinx heterozygous mice as determined by Western blotting with actin serving as loading control. (C-D) Human platelets were permeabilized with SLO as describe above and incubated with the indicated concentrations of Munc13-4 (C), ΔC2B (D), or with proteins that had been heat-inactivated (2.5b). Secretion from dense granules (DG), α-granules (Alpha), and lysosomes was monitored and normalized to controls in which no exogenous Munc13-4 was added. A Student t test (SigmaPlot 9.0) was used to assess the statistical significance of the differences between groups (**P < .01).
To examine the domain requirements for Munc13-4 function, we attempted to produce truncations of Munc13-4 in the insect cells. Only one of these constructs (ΔC2B, 1-910 aa) could be produced in sufficient quantities (Y.R. and F.M.H., unpublished observation, February 2009). When added to Unc13dJinx platelets, ΔC2B did not rescue the secretion defect (Figure 5A). However, when added to permeabilized wild-type murine platelets (Figure 5A) and human platelet (Figure 5D), secretion from all 3 granules was significantly inhibited even at the lowest concentrations added. This suggests that ΔC2B has a dominant-negative effect and is competing with endogenous Munc13-4 for some key interactions. In addition, this result indicates that the C-terminal C2 domain of Munc13-4 is important for function.
The enhanced secretion seen when Munc13-4 is added to wild-type platelets is consistent with previous work using human platelets.32 This suggested a positive role for Munc13-4 and led to the hypothesis that Munc13-4 might be a limiting factor in platelet secretion. To first determine whether this was plausible, we measured Munc13-4 levels in wild-type, Unc13dJinx heterozygotes, and homozygote platelets using quantitative Western blotting. There were approximately 320 molecules of Munc13-4 per wild-type platelet and only approximately 230 molecules per Unc13dJinx heterozygous platelet (Table 1). None was detected in the homozygote (Figure 5B). Compared with the endobrevin/VAMP-8 levels measured previously (∼ 8400 molecules per platelet)3 and 2 t-SNAREs: syntaxin 2 (1770 molecules per platelet) and syntaxin 4 (1625 molecules per platelet), there is approximately 28-fold less (mole per mole) Munc13-4 in mouse platelets than there is primary v-SNARE, and 5-fold less (mole per mole) than the 2 syntaxins. Thus Munc13-4 could be a limiting factor in the secretory pathway.
To address this question in vivo, secretion from heterozygous Unc13dJinx platelets was measured. In Figure 5B, there was a partial secretion defect, from dense granules compared with wild-type, consistent with the reduced levels of Munc13-4. As a further test, recombinant Munc13-4 protein was titrated into permeabilized human platelets (Figure 5C). A dose-dependent enhancement (by 20%-50%) of secretion from the 3 granule types was observed. This result is consistent with Figure 5A and with published results.32 Given that endogenous Munc13-4 can diffuse through the pores formed by Streptolysin-O (SLO) and equilibrate with the surrounding media,32 these data imply that in permeabilized wild-type platelets, endogenous Munc13-4 levels fall below the concentration that is optimal for secretion. Upon addition of recombinant Munc13-4, the requirement is met or surpassed and secretion is enhanced. This is another indication that Munc13-4 is limiting. Taken together, these data show that loss of Munc13-4 unequivocally leads to the secretion defect observed in Unc13dJinx platelets and that the levels of Munc13-4 directly correlate with the extent of this defect.
Discussion
Unc13dJinx mice which lack Munc13-4 provide an ideal system for studying the role of Munc13-4 in platelets. Loss of Munc13-4 did not affect granule biogenesis (Figure 1B) or platelet activation (Figure 3); however, thrombin-stimulated secretion from Unc13dJinx platelets was significantly compromised. Cargo release from dense granules was ablated and that from α-granules and lysosomes was severely impaired (Figure 2). This secretion defect was seen regardless of agonist used (Figures 4–5). Secretion from permeabilized Unc13dJinx platelets could be rescued by addition of recombinant Munc13-4 (Figure 5), suggesting that the lack of Munc13-4 directly caused the secretion defect. The degree of rescue correlated with the amount of added protein, consistent with Munc13-4 being a limiting element of the platelet secretory machinery. This was further supported by the fact that heterozygous Unc13dJinx platelets displayed an intermediate secretion defect. Platelets from Unc13dJinx mice also displayed an incomplete aggregation phenotype; aggregation of Unc13dJinx platelets was defective at lower concentrations of agonist, where full activation requires additional stimulation by the secondary agonists in dense granules, for example, ADP (Figure 4). Hemostasis was also defective; Unc13dJinx mice had significantly longer bleeding times. In summary, these data support the conclusion that Munc13-4 mediates an essential control step for platelet secretion and thus for hemostasis.
The role of Munc13-4 in platelet exocytosis
Vesicle priming is loosely defined as a maturation process that enhances SNARE-mediated vesicle fusion. In CTLs and NK cells, Munc13-4 is required for the steps upstream of membrane fusion.22 Studies of other secretory cell types also support a priming function for Munc13-4.22,31,33,34 In platelets, Munc13-4 is much more important for dense granule release than for α-granule and lysosome release, which suggests that Munc13-4 mediates at least one step that is uniquely important for efficient and rapid dense granule release. Given the bleeding diathesis of the Unc13dJinx mice, this step must also be critical for hemostasis.
Munc13-4 is also important for release from α-granules and lysosomes. This is consistent with studies of neutrophils and mast cells where multiple granule classes are released in a Munc13-4–dependent manner.34,35 However, unlike dense granule release, secretion from α-granules and lysosomes was not completely eliminated in intact Unc13dJinx platelets (Figure 2). Thus, Munc13-4 is important, but may not be essential for these 2 secretion events. Residual release from α-granules and lysosomes indicates that there could be other factors in platelets which substitute for Munc13-4, albeit less efficiently. These factors could diffuse out of the permeabilized platelets and thus the residual secretion would be less in that assay (Figure 5). A potential candidate is Munc13-1, which was detected in platelets (Figure 1B).
Alternatively, the inefficient release may be indicative of a process that does not require vesicle priming by a Munc13. SNARE-mediated, in vitro, liposome fusion does occur in the absence of a Munc13, though addition of Munc13-4 increases its efficacy (M.C.C., Q.R., and S.W.W., manuscript in preparation). Activation-dependent cytoskeletal rearrangements consolidate granules into the platelet center in association with the Open Canalicular System (this is less efficient in permeabilized platelets). It is possible that, because of this concentration, some level of SNARE complex formation and SNARE-mediated fusion occurs through a mass-action mechanism, analogous to the in vitro, liposome fusion experiments. Such a mass-action mechanism would not be as effective for dense granules which are outnumbered approximately 10 to 1 (compared with α-granules) in an average platelet. If such an alternate fusion mechanism is used, it would be very interesting to determine how biologically relevant it would be. Based on the bleeding diathesis seen in Unc13dJinx mice, such a mechanism is insufficient for dense granule release and thus normal hemostasis. However, it could play a role in subsequent secretion steps that occur once a platelet has been incorporated into a thrombus.
An unexpected finding came from the experiments with the ΔC2B truncation mutant. Munc13-4 is a multidomain protein with C2 domains at both termini and a central tandem repeat of Munc13 homology domains (MHD; see Figure 1A).20 We expected that the ΔC2B would not rescue secretion because previous studies indicated that the C-terminal C2 domain is required for function.29 It was surprising to see that ΔC2B so potently inhibited secretion. The dose-dependent, dominant-negative effect of ΔC2B indicates that it competes for other key Munc13-4-interacting factors in platelets.32,44 The inhibitory effect of the ΔC2B in wild-type platelets suggests that Munc13-4 likely functions as a multivalent molecule. The N-terminal domains may interact with a distinct set of important factors while the C-terminal C2 domain may be required for some other calcium-dependent roles. Such a bivalent feature is common to other tethering factors45 and the multivalent functional modules of Munc13-4 may explain its priming functions and its ability to promote secretion in a calcium-dependent manner.44
The functions of Rab27s and Munc13-4 are separate
Another interesting result from this study is that Unc13dJinx platelets do not have a defect in granule biogenesis. This phenotype is remarkably different from that of platelets lacking functional Rab27b.40 In Rab27b−/− and Rab27a−/−/b−/− mice, platelet dense granules are reduced and cargo levels are severely decreased. This has been taken as proof that Rab27b is important for dense granule biogenesis. Several groups have shown that Rab27a/b interacts with Munc13-4.31,32,44 Based on our report, these interactions must not be essential for dense granule biogenesis because Unc13dJinx platelets show no cargo defect. Interestingly, activation-dependent secretion from Rab27a−/−/b−/− platelets does occur, albeit to a reduced extent for dense granules.42 This is distinct from the complete ablation of dense granule secretion and the attenuated release from α-granules and lysosomes seen in Unc13dJinx platelets. These results indicate that Rab27a/b/Munc13-4 interactions may not be essential for secretion either. The data are in agreement with previous studies of CTLs and NK cells that suggest independent roles for Munc13-4 and Rab27.44,46 Further analysis will be required to determine whether/how the interactions between Munc13-4 and Rab27 are required for membrane trafficking.
Potential utility for FHL diagnosis
Because Unc13dJinx mice carry a defect in the same gene as humans with FHL3,33 our data suggest that FHL3 patients could have platelet defects. Humans with FHL3 present with fever, hepatosplenomegaly, and cytopenia, but bruising and bleeding have generally not been associated with the disease.47 Because the median survival of FHL3 patients is less than 2 months if untreated,47 it is possible that such bleeding diatheses have not manifested themselves. Functional assays for CD107a/LAMP-1 exposure have been adapted for clinical diagnosis of FHL3 using CTLs and NK cells and have shown a secretory defect in FHL3 patients.48 The work presented here suggests that abnormalities in platelet secretion could also be indicative of FHL3. Further data are required to justify this impression but given the ease of clinical assays for platelet function such future studies are warranted.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Acknowledgments
We thank the Mutant Mice Regional Resource Center at University of California Davis for the Unc13dJinx mice and the Kentucky Blood Center. We thank Dr Greg Bauman and Ms Jennifer Strange for their help with flow cytometry, and Dr Bruce Maley and Ms Mary Gail Engels for their assistance with electron microscopy. We thank the members of the Whiteheart laboratory for their comments on this manuscript.
This work was supported by grants HL56652 and HL091893 from the National Institutes of Health (S.W.W.) and fellowships from the American Heart Association Great Rivers Affiliate (0615238B, Q.R.; and 2240048, M.C.C.).
National Institutes of Health
Authorship
Contribution: Q.R., S.Y., and S.W.W. designed and performed experiments, analyzed results, and wrote the manuscript; C.W., Y.R., and F.M.H. provided critical reagents; and M.C.C. helped in the writing of the manuscript and in experiment design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.W. is European Commission, Brussels, Belgium.
Correspondence: Sidney W. Whiteheart, PhD, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, 741 South Limestone, Biomedical Biological Sciences Research Bldg, Lexington, KY 40536-0509; e-mail: whitehe@uky.edu.

![Figure 3. The morphology and response to activation are similar in platelets from wild-type and Unc13dJinx mice. (A) Platelets from wild-type and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (0.7mM final). Panels i and ii represent platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels iii through viii. After 2 minutes at RT, samples were fixed and analyzed by transmission electron microscopy. Scale bars represent 1 μm in panels iii and iv, and 200 nm for all others. (B) Washed platelets from wild-type (black trace) or Unc13dJinx mice (gray trace) were loaded with Fura-2/AM to measure intracellular [Ca2+]i upon activation with thrombin (0.1 U/mL). Arrows indicate the addition of extracellular Ca2+ (0.7mM final) and thrombin. (C) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were mixed with 5 μL of PE-conjugated-Jon/A antibody and 5 μL of FITC-conjugated CD61 antibody in the presence of 1.25mM CaCl2 and 0.02 U/mL apyrase. Samples were incubated without thrombin (resting) or with thrombin (0.1 U/mL) for 5 minutes at RT. Reactions were stopped with excess hirudin and analyzed by flow cytometry. Data were plotted as 2-color dot plots (Jon/A and CD61). The percentage of platelets in each quadrant was indicated. (D) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were either kept in the resting state or stimulated with the indicated agonists for 2 minutes at RT. The reactions were stopped by adding SDS sample buffer and the samples were analyzed by Western blot for phosphotyrosine (pY). Ponceau S red staining of actin serves as loading control (bottom panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/6/10.1182_blood-2010-02-270934/4/m_zh89991055200003.jpeg?Expires=1768604353&Signature=5FKxkAp1pgLr6xqnq3j055rn7A9lL0Fl8HOIcLA1zDUI0HljGkTRJCQ-4nM-YKj7B0Uee1LB~2O3dqyH0GPAE6DfOxJZSyDzvms9XPNdpQ-2vnvu9dvewXu3wBc5fBMuIVDNfNoI261qfFdA0NKrw9lRxyK5iFiJW-gTOuPLfJIt1NNIR6G1D2S35KyD4TXUqNc1Zvj~JmlxuiJul6xXjG~lDvIPr~D9t27ZTnwHrDJTrFvVIq1VUuH7BdYuz9HqMqIeEKi-v96vwIUYkX0OjDFRSrjwSl2dNI988p8RE2FhghKOwMWql-jJ4TFS-~bgHtElJUKOOyJxIuYZTsJJdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. The morphology and response to activation are similar in platelets from wild-type and Unc13dJinx mice. (A) Platelets from wild-type and Unc13dJinx mice were prepared as described in “Platelet preparation” and diluted to approximately 2.5 × 108/mL. CaCl2 was added to each sample (0.7mM final). Panels i and ii represent platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels iii through viii. After 2 minutes at RT, samples were fixed and analyzed by transmission electron microscopy. Scale bars represent 1 μm in panels iii and iv, and 200 nm for all others. (B) Washed platelets from wild-type (black trace) or Unc13dJinx mice (gray trace) were loaded with Fura-2/AM to measure intracellular [Ca2+]i upon activation with thrombin (0.1 U/mL). Arrows indicate the addition of extracellular Ca2+ (0.7mM final) and thrombin. (C) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were mixed with 5 μL of PE-conjugated-Jon/A antibody and 5 μL of FITC-conjugated CD61 antibody in the presence of 1.25mM CaCl2 and 0.02 U/mL apyrase. Samples were incubated without thrombin (resting) or with thrombin (0.1 U/mL) for 5 minutes at RT. Reactions were stopped with excess hirudin and analyzed by flow cytometry. Data were plotted as 2-color dot plots (Jon/A and CD61). The percentage of platelets in each quadrant was indicated. (D) Equal amounts of washed platelets from wild-type or Unc13dJinx mice were either kept in the resting state or stimulated with the indicated agonists for 2 minutes at RT. The reactions were stopped by adding SDS sample buffer and the samples were analyzed by Western blot for phosphotyrosine (pY). Ponceau S red staining of actin serves as loading control (bottom panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/6/10.1182_blood-2010-02-270934/4/m_zh89991055200003.jpeg?Expires=1768687588&Signature=lSVm80dH88BBAVZCXApBDgEGSggaxg60o1qwtO7Mrnw-3l9Xuw81C1KSpqhR4DKQg5x84VkjKfryJ~r~LcA2cORG4GDHn9hfU23MsfRLjWOQdHlkkyJM-KfccPoY81FCBXPZKsrCNTn7YqmxWS0u6m9bWQJQ23TK9ZRE5~XWa3LMEMJLZSh2GYDG6yCNf1Nmv~aAJk124qOr30vr7DkzYq~X-W2EYurZ36Xmjow-7DwK9dzNYLm3yG~hSzT9J63cKDDWdpL2MiAgHFUI26gtlOJLXOnImXi7AfBZYt-eQjo1QHSkdPEHoEsoS3XGYgBQ6n62OHNp3dOusZAUgEvh9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)