To the editor:
Leukemia in human infants often begins with an in utero chromosomal translocation resulting in an MLL fusion oncogene that is most frequently MLL-AF4 or MLL-AF9.1 Recent gene expression studies suggest that human infant MLL fusion gene leukemia originates in cells that differ from both infant leukemia without the fusion gene and non-infant childhood leukemia. However, the cell of origin remains undefined.2,3
Mouse models of human MLL leukemia have proven to be informative. An example is the MLL-AF9 knock-in model, in which the fusion gene is expressed at physiologic levels.4-6 In this model, hematopoietic stem cells (HSCs) from postnatal murine marrow are readily transformed and leukemias develop that resemble human myeloid post natal MLL leukemias.6
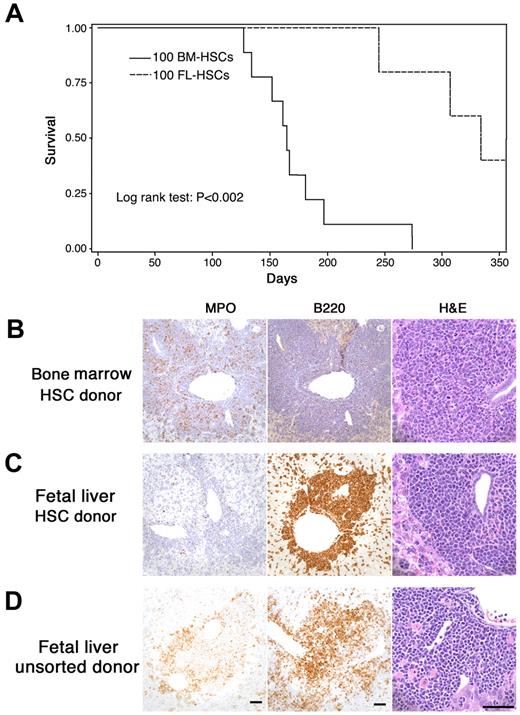
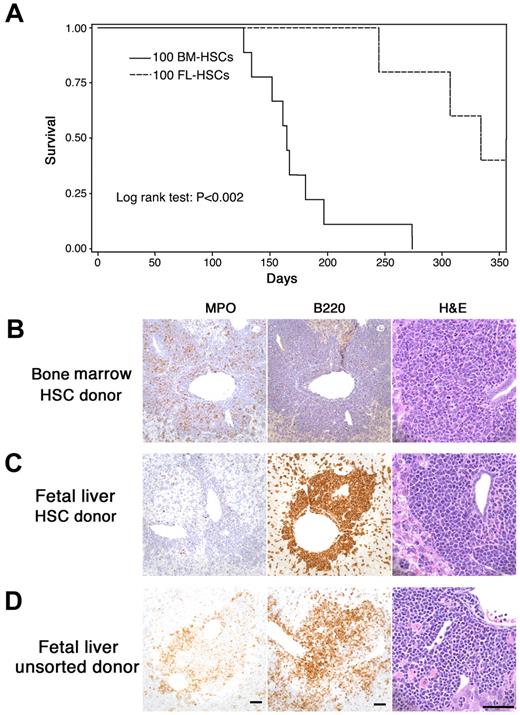
The current study was designed to model human fetal–originated infant MLL leukemia. We compared the leukemia that developed from MLL-AF9 fetal liver cells with that arising from adult marrow cells. Mice were transplanted with fetal MLL-AF9 liver cells (sorted HSCs or unsorted cells) or HSC cells from adult MLL-AF9 bone marrow. We found that the time to development of leukemia was significantly longer after transplantation of fetal liver HSCs than that from marrow HSCs (Figure 1A). Extensive analysis of the leukemias by histopathology, immunohistochemistry, and flow cytometry showed important differences in recipients of adult marrow HSCs (Figure 1B) compared with recipients of fetal liver HSCs (Figure 1C) and unsorted fetal liver cells (Figure 1D). When donor cells were from adult marrow, the leukemia was always myeloid in character with myeloperoxidase (MPO) positivity but negative for CD45R/B220, a phosphatase that appears early in hematopoietic cellular differentiation. However, when fetal liver cells (either sorted HSCs in Figure 1C or unsorted in Figure 1D) were used as donor cells, the leukemia often showed more undifferentiated cells. In some cases fetal liver–derived leukemia cells were positive for CD45R/ B220 (Figure 1C-D) and either positive or negative for MPO.
MLL-AF9 leukemia from fetal liver differs from that originating in postnatal marrow. (A) The latency of leukemia in recipient mice that received 100 hematopoietic MLL-AF9 stem cells (HSCs) from either embryonic day (E) 14.5 fetal liver or adult bone marrow. (B-D) Immunohistochemisty for myeloperoxidase (MPO), CD45R/ B220, and morphology of hematoxylin and eosin–stained cells on the sections from liver of leukemic mice showing leukemia that developed in recipients of fetal liver or bone marrow. All vertical panels are at the same magnification; bars represent 50 μm. Images were taken with a Spot Insight Wide-field 4 MP CCD Scientific Color Digital camera (Diagnostic Instrument) mounted on a Nikon Eclipse 80i microscope (Nikon Plan Apo 20×/0.75 or Plan Fluor 60×/0.85); image size was adjusted in Photoshop CS3 Version 10.0.1 (Adobe Systems). (B) Recipient of HSCs from adult marrow. Leukemia cells are MPO-positive, CD45R/B220-negative and show nuclear lobulation and indentation consistent with granulocytic differentiation. (C) Recipient of HSCs from fetal liver. Leukemia cells are MPO-negative, B220-positive and are large with large round, blastlike nuclei consistent without evidence of lineage-specific differentiation. (D) Recipient of unsorted cells from fetal liver. Leukemia cells are both MPO- and B220-positive and show no evidence of lineage-specific differentiation.
MLL-AF9 leukemia from fetal liver differs from that originating in postnatal marrow. (A) The latency of leukemia in recipient mice that received 100 hematopoietic MLL-AF9 stem cells (HSCs) from either embryonic day (E) 14.5 fetal liver or adult bone marrow. (B-D) Immunohistochemisty for myeloperoxidase (MPO), CD45R/ B220, and morphology of hematoxylin and eosin–stained cells on the sections from liver of leukemic mice showing leukemia that developed in recipients of fetal liver or bone marrow. All vertical panels are at the same magnification; bars represent 50 μm. Images were taken with a Spot Insight Wide-field 4 MP CCD Scientific Color Digital camera (Diagnostic Instrument) mounted on a Nikon Eclipse 80i microscope (Nikon Plan Apo 20×/0.75 or Plan Fluor 60×/0.85); image size was adjusted in Photoshop CS3 Version 10.0.1 (Adobe Systems). (B) Recipient of HSCs from adult marrow. Leukemia cells are MPO-positive, CD45R/B220-negative and show nuclear lobulation and indentation consistent with granulocytic differentiation. (C) Recipient of HSCs from fetal liver. Leukemia cells are MPO-negative, B220-positive and are large with large round, blastlike nuclei consistent without evidence of lineage-specific differentiation. (D) Recipient of unsorted cells from fetal liver. Leukemia cells are both MPO- and B220-positive and show no evidence of lineage-specific differentiation.
The delay in overt murine MLL leukemia from both fetal liver and bone marrow compared with many human infants with MLL fusion gene leukemia suggests that secondary cooperating events (genetic or epigenetic) may differ between species. The shorter latency of onset of leukemia in bone marrow compared with fetal liver cells suggests that by the time the targeted cells have progressed in maturity to the marrow stem/progenitor stage secondary changes have already developed. Our previous observation that MLL-AF9–transformed fetal liver had limited replating capacity compared with MLL-AF9 marrow is consistent with this hypothesis.5
The MLL fusion partner, AF9, may also be important in the timing of the onset of leukemia. Human infant MLL fusion gene leukemia involving an AF9 partner occurs with greater delay than with other partners as another group7 has observed.
Overall, these results provide further impetus for the study of the cellular origins of human and murine MLL leukemia.
Authorship
Contribution: W.C. conducted cell experiments; M.G.O. studied histopathology and immunohistochemistry; W.H. conducted experiments in mice; and J.K. planned and provided oversight for all of the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr John Kersey, 554E Cancer Center Research Bldg, 425 E River Rd, Minneapolis, MN 55455; e-mail: kerse001@umn.edu.
References
National Institutes of Health