Abstract
In the bone marrow (BM), stromal cells constitute a supportive tissue indispensable for the generation of pro-B/pre-BI, pre-BII, and immature B lymphocytes. IL-7–producing stromal cells constitute a cellular niche for pro-B/pre-BI cells, but no specific stromal cell microenvironment was identified for pre-BII cells expressing a functional pre-B cell receptor (pre-BCR). However expression of the pre-BCR represents a crucial checkpoint during B-cell development. We recently demonstrated that the stromal cell derived-galectin1 (GAL1) is a ligand for the pre-BCR, involved in the proliferation and differentiation of normal mouse pre-BII cells. Here we show that nonhematopoietic osteoblasts and reticular cells in the BM express GAL1. We observed that pre-BII cells, unlike the other B-cell subsets, were specifically localized in close contact with GAL1+ reticular cells. We also determined that IL-7+ and GAL1+ cells represent 2 distinct mesenchymal populations with different BM localization. These results demonstrate the existence of a pre-BII specific stromal cell niche and indicate that early B cells move from IL-7+ to GAL1+ supportive BM niches during their development.
Introduction
The bone marrow (BM) is the primary site of hematopoiesis after birth. Hematopoietic stem cells (HSCs) and hematopoietic progenitors are located in close contact with a special stromal microenvironment, known as niches, composed of mesenchymal cells, osteoblasts, endothelial cells, and adipocytes, indispensable for their differentiation.1,2 Recent reports indicate that the spatial repartition of BM stromal cells and hematopoietic progenitors follows a coordinated regulation.3 HSCs are in contact with osteoblasts on bone surfaces and endothelial cells lining sinusoids, and move near CXCL12+ reticular stromal cells while they differentiate. In this tissue, B-cell differentiation is also a highly regulated process, which generates widely diversified immature B cells tolerant to self. Stage-specific cellular niches have been identified for the first steps of the B lymphopoiesis.2 The earliest B220+cKit+ pro-B/pre-BI cells undergoing immunoglobulin H (IgH) gene rearrangements locate near IL-7–producing stromal cells. At the next step, when a functional Igμ chain is produced and associated with the surrogate light chain (SLC) composed of λ5 and VpreB and with the CD79a/CD79b signaling complex, the pre-B-cell receptor (pre-BCR) is formed and expressed at the surface of pre-BII cells. At this stage, cells leave IL-7-expressing stromal cells, but no specific pre-BII cellular niche has yet been identified.3
Expression of the pre-BCR represents a crucial checkpoint during B-cell development as this receptor controls pre-BII-cell development and the selection of the Igμ repertoire.4 The galectin-1 (GAL1) lectin and heparan sulfates, produced by the BM microenvironment, have been reported to bind to the pre-BCR,5-7 and GAL1 was shown to promote pre-BCR clustering and signaling.5 GAL1 binds to the SLC by a direct protein/protein interaction and to glycosylated integrins via the recognition of β-galactosides.8 During coculture between pre-BII and stromal cell lines, pre-BCRs, GAL1, and glycosylated integrins form complexes, which relocalize at the contact zone between the 2 cells. This immune “developmental synapse” is accompanied by the initiation of intracellular tyrosine kinase activity and signal transduction from the pre-BCR.5,8 More recently, using normal mouse pre-BII cells, we demonstrated that pre-BCR clustering and activation also depend on specific contacts between stromal and pre-BII cells, involving pre-BCRs, GAL1, VLA4, and LFA1 integrin interactions. Finally, we showed that these interactions are required for the efficient differentiation and proliferation of normal pre-BII cells.9 Altogether, our results highlighted the importance of specific contacts between pre-BII cells and the BM microenvironment in physiologic conditions.
Deregulation of pre-BCR signaling has been implicated in the development of pre-B acute lymphoblastic leukemias (pre–B-ALLs).10 In addition, compelling evidence suggests that the reciprocal crosstalk between B-cell malignancies and stromal cells in the BM microenvironment favors disease progression by promoting malignant B-cell growth.11,12 This is indeed the case for acute B lymphoblastic leukemia (B-ALL), the most frequent pediatric malignancy, a disease that initiates and progresses in the BM. It was shown that direct contacts with stromal cells via β1 integrins are important for the survival of B-ALL13 and that secreted factors, such as CXCL12, favor pre–B-ALL engraftment in the BM.14 Thus, to determine how the BM microenvironment can influence and sustain the development of early B-ALL, it is essential to characterize stromal cell niches in physiologic conditions.
In this study, we characterized GAL1-expressing stromal cells in normal mouse BM and demonstrated that these cells constitute a specific cellular niche for pre-BII cells. Moreover, we found that GAL1-expressing stromal cells correspond to a cell population distinct from IL-7+ stromal cells, suggesting that developing B cells migrate from the IL-7+ niche to the galectin-1+ (GAL1+) niche during their differentiation.
Methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories, France. GAL1-deficient mice15 and actin–green fluorescent protein (GFP) transgenic mice16 were backcrossed to C57BL/6 background. Mice were housed under specific pathogen-free conditions and handled in accordance with European directives, with the approval of the Institutional Review Board of Inserm/CNRS. IL-7cre Rosa26-eYFP mice17 were backcrossed to C57BL/6 background. These mice were bred under specific pathogen-free conditions at the University of York with institutional ethics approval and under home office license. For the generation of chimeras, C57BL/6 actin-GFP mice were γ-irradiated with a single dose of 10 Gy from a cesium source and were reconstituted during 8 to 10 weeks with 5 to 10 × 106 C57BL/6 BM cells. For hydroxyurea treatment, C57Bl/6 and C57BL/6 actin-GFP chimera (10- to 16-week-old) received 2 intraperitoneal injections of hydroxyurea (Merck Chemicals), each of 1 mg/g of body weight and at the rate of 2 injections separated by 8 hours.
Flow cytometry and cell sorting
Sorting of pro-B/pre-BI and large pre-BII cells was performed on single BM cell suspensions, initially enriched for B cells using B220 microbeads and separated on the autoMACS Separator (Miltenyi Biotec), as described previously.9 For BM stromal cell analysis, femur and tibia from C57BL/6 mice were flushed and incubated with 100 U/mL collagenase IV and 10 U/mL DNaseI (Invitrogen) at 37°C during 15 minutes. BM stromal cells were enriched by negative selection using immunomagnetic anti–rat IgG Dynabeads (Invitrogen) following the manufacturer's recommendation. Depletion was performed using antibodies against CD45 (H129–326 supernatant, a gift from M. Pierres, Centre d'Immunologie de Marseille-Luminy) and Ter-119 (BD Biosciences). Enrichment of BM stromal cells was also eventually performed by in vivo depletion of hematopoietic cells, using hydroxyurea (HU)–treated mice with no adverse effect on the stromal cell subpopulations. In that case, BM cells were harvested 2 days after treatment.
For flow cytometry experiments, single-cell suspensions were stained using standard protocols using the antibodies listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Dead cells were excluded using either Sytox Blue or the Live/Dead Violet Dead Cell Stain Kit (Invitrogen). The anti-GAL1 antibody was conjugated to AlexaFluor-488 or AlexaFluor-647 using the monoclonal antibody labeling kit (Invitrogen). GAL1 was stained intracellularly using the BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences). For the BM stromal cell gating strategy, cells were considered as being not only CD45− but also Ter119−CD19−CD11c−CD117−Gr1−, markers excluding the main hematopoietic subpopulations. In addition, we confirmed that other lineage markers, such as B220, NK1.1, or CD11b, were not expressed in the stromal cell population (data not shown).
For cell sorting experiments, BM stromal cells were defined as described in the previous paragraph and were stained in addition with antibodies specific for CD54, CD31, CD106, and BP1. Three stromal cell subpopulations were sorted based on CD54 and CD31 expression (CD54+CD31−, CD54+CD31+, and CD54−CD31− cells). The CD54+CD31− subpopulation was further refined into CD106+BP1+ and CD106−BP1− cells. FACS analysis was performed on a FACSLSRII and cell sorting on a FACSAria (BD Biosciences) apparatus. Data were analyzed with the DiVa Version 6.1 (BD Biosciences) or FlowJo Version 9.1 software (TreeStar).
Cellular labeling and mice injection
Pro-B/pre-BI and large pre-BII cells were stained with 4μM of PKH26 (Sigma-Aldrich) during 4 minutes at room temperature as recommended. A total of 2 × 105 to 2 × 106 labeled cells were transferred to stroma-GFP mice by intravenous injection 48 hours after HU treatment. Mice were killed 15 hours after injection.
Immunohistology
Mice were killed and hind limbs excised. Femurs and tibias were prepared by following 2 different protocols after fixation in 4% paraformaldehyde. First, bones were decalcified in phosphate-buffered saline/20% ethylenediaminetetraacetic acid for 3 to 4 weeks at 4°C. After 2 days in PBS 20% sucrose, bones were embedded in OCT (Tissue-Tek OCT Compound; Sakura Finetek) and frozen at −80°C. Twenty-micrometer-thick sections were prepared and mounted on gelatin-coated glass slides. In the second protocol, bones were dehydrated by 3 successive baths of sucrose (10%, 20%, and 30%, respectively), embedded in OCT, and frozen at −80°C. Twenty-micrometer-thick sections were transferred to adhesive-coated slides with the CryoJane Tape-Transfer system according to the manufacturer's protocol (Instrumedics). Nonecalcified sections (after fixation in acetone) or decalcified ones were incubated for 1 hour at room temperature with a blocking solution (PBS, 2% BSA, 1% donkey serum, 1% FCS, 0.1% Triton X-100, 0.05% Tween 20). After washes, bone sections were incubated with primary antibodies listed in supplemental Table 1 in a humidified chamber overnight at 4°C. The anti-GFP antibody used during the study was conjugated to AlexaFluor-488 using the monoclonal antibody labeling kit (Invitrogen). After several washes, slides were incubated with the corresponding secondary antibodies. They were finally mounted in Mowiol antifade reagent and analyzed by confocal microscopy on a LSM510 Meta (Carl Zeiss) or on a Leica SP5X (Leica Microsystems). The ImageJ 1.44 software was used for image analysis.
Real-time PCR
mRNA from sorted BM stromal cells was purified using an RNeasy plus microkit (QIAGEN). cDNA was generated using MessageBOOSTER cDNA Synthesis Kit (Epicentre Biotechnology) according to the manufacturer's instructions.
Real-time PCR was performed with Power SYBR Green PCR Master Green (Applied Biosystems). Specific primers for reverse-transcribed (RT)-PCR quantification are listed in supplemental Table 2 and were designed using Primer Express Version 3.0 software (Applied Biosystems) and optimized for minimal primer dimer formation. Detection was performed using an ABI Prism 7500 Fast PCR System (Applied Biosystems). All experiments were conducted in duplicates, and expression levels for the target genes were calculated using the Δ-Δ-Ct method and normalized to the relative quantity of hypoxanthine phosphoribosyl transferase expression in each sample.
Results
Galectin-1 is expressed by BM stromal cells
It has been reported that, although pro-B/pre-BI cells are in contact with IL-7–expressing stromal cells, pre-BII cells move away from that cellular niche.3 However, in that study, the authors did not determine the existence of a specific BM niche for pre-BII cells. As normal pre-BII cell development was improved on GAL1-induced pre-BCR clustering and activation at the contact zone between stromal and pre-BII cells,9 we investigated whether GAL1-expressing cells could represent a niche for pre-BII cells in vivo.
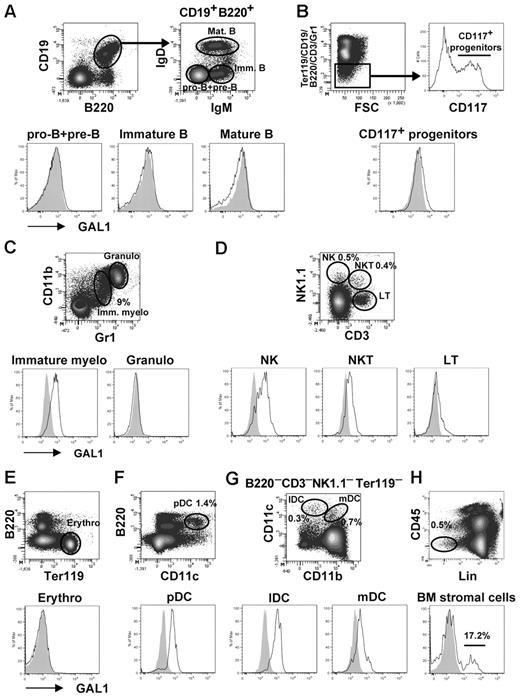
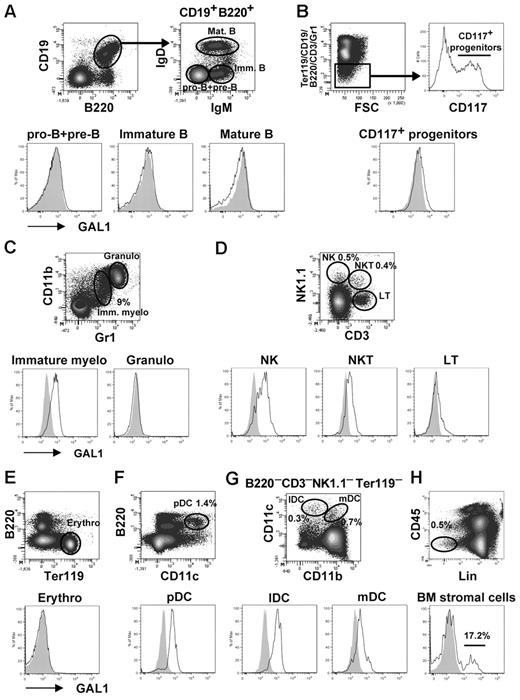
We first determined by flow cytometry the cellular subpopulations that express GAL1 in the BM. As shown previously, none of the B-cell subsets expressed GAL19 (Figure 1A), neither was GAL1 expressed on (Ter119−CD19−B220−CD3−Gr1−CD117+) hematopoietic progenitors, (CD11bhiGr1hi) granulocytes, (CD3+NK1.1−) T cells, or (Ter119+) erythrocytes (Figure 1B-E). By contrast, GAL1 was expressed by (CD11bloGr1lo) immature myeloid cells, (NK1.1+CD3−) natural killer cells, (NK; NK1.1+CD3+) NK T cells, (B220+CD11c+) plasmacytoid dendritic cells, and (CD11c+CD11b+/−) dendritic cell subsets (Figure 1C-D,F-G), representing approximately 12% of BM hematopoietic cells. We then focused on the analysis of nonhematopoietic CD45− BM stromal cells, which represent approximately 0.1% of total BM cells. These cells were enriched by depleting CD45+ and Ter119+ cells before analysis by flow cytometry, increasing their proportion up to 0.5% to 1% (Figure 1H). As expected, GAL1+ cells were detected and represented 21.4% ± 7.2% of the CD45−Lin− stromal cells (n = 5; ie, 0.02% of the total BM cells).
GAL1 expression in BM cells. Different BM subpopulations were analyzed for intracellular expression of GAL1 by flow cytometry. GAL1 intracellular staining in C57Bl/6 mice (unshaded) was compared with GAL1−/− mice as a negative control (shaded). The gating strategy is shown in the figure, and the cellular subsets were defined as follows: (A) Pro-B/pre-B (CD19+B220+IgM−IgD−), immature B (CD19+B220+IgM+IgD−), and recirculating B cells (CD19+B220+IgM+IgD+). (B) CD117+ hematopoietic progenitors (Ter119−CD19−B220−CD3−Gr1−CD117+). (C) Immature myeloid cells (CD11b+Gr1+), granulocytes (CD11bhiGr1hi). (D) NK (NK1.1+CD3−), NKT (NK1.1+CD3+), and T cells (NK1.1−CD3+). (E) Erythrocytes (Ter119+). (F) Plasmacytoid dendritic cells (CD11c+B220+). (G) Lymphoid DC (CD11c+CD11b−) and myeloid DC (CD11c+CD11b+). (H) BM stromal cells (CD45−Lin−). The lineage (Lin) staining corresponds to a mix of Ter119, CD19, CD11c, CD117, and Gr1.
GAL1 expression in BM cells. Different BM subpopulations were analyzed for intracellular expression of GAL1 by flow cytometry. GAL1 intracellular staining in C57Bl/6 mice (unshaded) was compared with GAL1−/− mice as a negative control (shaded). The gating strategy is shown in the figure, and the cellular subsets were defined as follows: (A) Pro-B/pre-B (CD19+B220+IgM−IgD−), immature B (CD19+B220+IgM+IgD−), and recirculating B cells (CD19+B220+IgM+IgD+). (B) CD117+ hematopoietic progenitors (Ter119−CD19−B220−CD3−Gr1−CD117+). (C) Immature myeloid cells (CD11b+Gr1+), granulocytes (CD11bhiGr1hi). (D) NK (NK1.1+CD3−), NKT (NK1.1+CD3+), and T cells (NK1.1−CD3+). (E) Erythrocytes (Ter119+). (F) Plasmacytoid dendritic cells (CD11c+B220+). (G) Lymphoid DC (CD11c+CD11b−) and myeloid DC (CD11c+CD11b+). (H) BM stromal cells (CD45−Lin−). The lineage (Lin) staining corresponds to a mix of Ter119, CD19, CD11c, CD117, and Gr1.
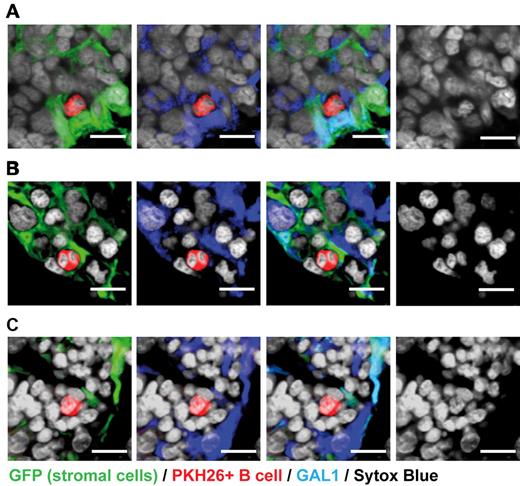
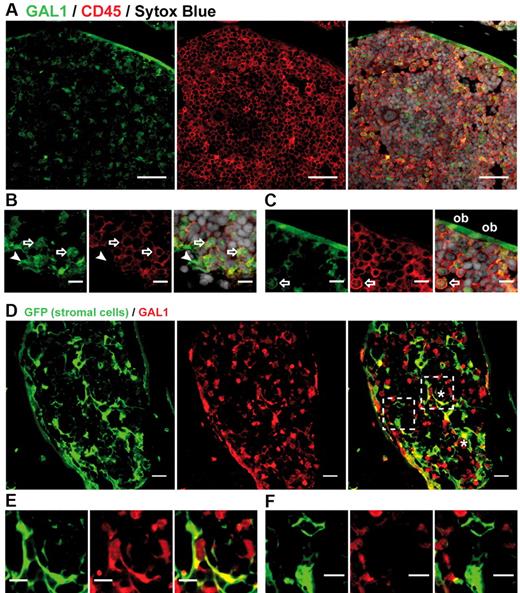
The fluorescence-activated cell sorter results were confirmed by immunohistofluorescence (IHF) performed on bone sections (Figure 2). Indeed, most of the GAL1+ cells were CD45+ hematopoietic cells, but 2 types of GAL1+CD45− cells were also identified: fibroblastic-shaped reticular cells interspersed in the BM and cells lining the bone, which correspond to osteoblasts, as we recently demonstrated for human BM18 (Figure 2A-C). To specifically visualize GAL1+ BM stromal cells, we generated chimeric mice using wild-type BM cells to reconstitute irradiated actin promoter-GFP transgenic animals in which the fluorescent protein is expressed by all nucleated cells.16 Animals were allowed to rest for a minimum of 8 weeks to ensure replacement of hematopoietic cells with nonfluorescent populations. Hereafter, these mice are called “stroma-GFP mice.” As shown in Figure 2D and E, a network of GFP+ stromal cells was clearly identifiable in bone section of stroma-GFP mice. As expected, although most hematopoietic and stromal cells do not express GAL1, small and round GAL1+GFP− hematopoietic cells and GAL1+GFP+ mesenchymal cells were clearly seen (Figure 2D-F). Among the latter population, osteoblasts lining the bones and stromal cells forming a reticular network within the BM were observed. GAL1-expressing stromal cells were morphologically heterogeneous, with either a cuboid or a fibroblast-like shape (Figure 2D asterisks). Importantly, the cuboid cells were not of myeloid origin, as GFP expression was not found by cytometry in the GAL1+ immature myeloid cell population (data not shown).
GAL1 is expressed by BM stromal cells. (A) BM sections of wild-type mice were stained with anti-GAL1, anti-CD45, and Sytox Blue (nuclei) (original magnification ×40). Bar represents 10 μm. (B-C) Representative regions of BM sections stained as in panel A (original magnification 63×/1.4 NA oil objective). Hematopoietic cells (arrow), reticular cells (arrowhead), and osteoblasts (ob) expressing GAL1 are designated. Bar represents 20 μm. (D) BM sections of stroma-GFP mice were stained with anti-GFP and anti-GAL1 antibodies (original magnification 40×/1.3 NA oil objective). *Characteristic cuboid or fibroblatic-like GAL1+ stromal cells are indicated. Bar represents 10 μm. Hatches boxes indicate the areas magnified in lower panels. GAL1+ (E) and GAL1− (F) stromal cells are shown. Bar represents 20 μm. Images acquired on Zeiss LSM510 Meta and analyzed with ImageJ 1.44.
GAL1 is expressed by BM stromal cells. (A) BM sections of wild-type mice were stained with anti-GAL1, anti-CD45, and Sytox Blue (nuclei) (original magnification ×40). Bar represents 10 μm. (B-C) Representative regions of BM sections stained as in panel A (original magnification 63×/1.4 NA oil objective). Hematopoietic cells (arrow), reticular cells (arrowhead), and osteoblasts (ob) expressing GAL1 are designated. Bar represents 20 μm. (D) BM sections of stroma-GFP mice were stained with anti-GFP and anti-GAL1 antibodies (original magnification 40×/1.3 NA oil objective). *Characteristic cuboid or fibroblatic-like GAL1+ stromal cells are indicated. Bar represents 10 μm. Hatches boxes indicate the areas magnified in lower panels. GAL1+ (E) and GAL1− (F) stromal cells are shown. Bar represents 20 μm. Images acquired on Zeiss LSM510 Meta and analyzed with ImageJ 1.44.
Altogether, these results demonstrate the presence of GAL1+ stromal cells in the BM microenvironment of normal mice.
Large pre-BII cells specifically contact stromal cells expressing GAL1
The existence of GAL1-expressing BM stromal cells leads us to determine whether large pre-BII cells, which express the pre-BCR, make specific contacts with such cells.
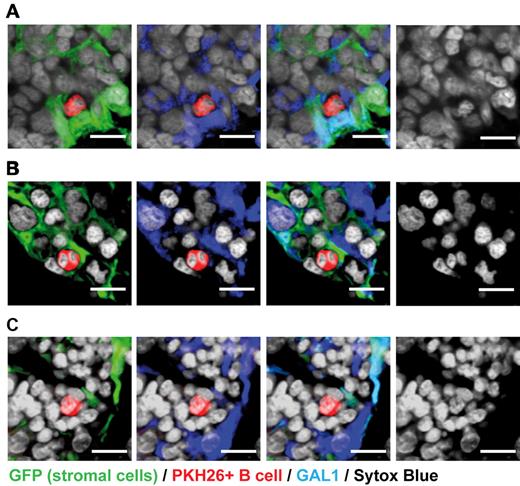
Large pre-BII cells (CD19+IgM−CD23−CD25+) were purified as previously described9 and stained with the PKH26 dye. They were injected intravenously into stroma-GFP mice treated with HU to eliminate cycling BM cells. Fifteen hours after injection, labeled pre-BII cells were shown to be present in the BM and not in lymphoid organs, such as the lymph nodes or the thymus (supplemental Figure1). We determined that PKH26 labeling did not affect the phenotype of BM-labeled pre-BII cells, as these cells maintain the expression of the CD25 pre-BII specific marker (supplemental Figure1). IHF analysis of BM sections revealed that 95% of PKH26+ large pre-BII cells were in contact with GFP+ mesenchymal cells and, more precisely, that 80% of them contacted GAL1+GFP+ stromal cells (Figure 3A; Table 1). The close contacts established by pre-BII cells with GAL1+GFP+ stromal cells were visualized by performing a 3-dimensional reconstruction of z-stack images (supplemental Video 1). Interestingly, most of the pre-BII cells were in contact with cuboid GAL1-expressing stromal cells. These interactions are highly specific as GAL1+ stromal cells are approximately 600-fold less abundant in the BM than GAL1+ hematopoietic cells (0.02% vs 12% of BM cells, respectively). Furthermore, pre-BII cells were not found in contact with GAL1+ osteoblasts from mesenchymal origin, and only 11% of them were observed in contact with GAL1+ cells from hematopoietic origin (12 cells among 112 analyzed), indicating that GAL1 expression was not sufficient to account for pre-BII cell homing in the BM. Finally, we observed that the large pre-BII cells in contact with GAL1+GFP+ stromal cells present more frequently a divided nucleus than pre-BII cells away from GAL1+ stromal cells (Figure 3B; 28% vs 10%, respectively), suggesting that pre-B/stromal cell contacts favor the development of large pre-BII cells.
Large pre-BII, but not pro-B/pre-BI, cells are found in close contact with GAL1-expressing stromal cells. Large pre-B II cells (A-B) and pro-B/pre-BI cells (C) were purified from the BM of C57Bl/6 mice by flow cytometry and stained with PKH26. Labeled cells were then injected intravenously in stroma-GFP mice treated with HU. Mice were killed 15 hours after injection. BM sections were stained with anti-GAL1, anti-GFP antibodies, and Sytox Blue (nuclei) as indicated. The presence or the absence of contacts between PKH26-labeled B cells and GAL1+GFP+ stromal cells was determined by analyzing 10-μm-thick z sections by confocal microscopy (original magnification 63×/1.4 NA oil objectives). Bar represents 10 μm. Confocal images are representative of 3 (A-B) and 2 (C) independent experiments. Cell counts are presented in Table 1. Images acquired on Zeiss LSM310 Meta and analyzed with ImageJ 1.44.
Large pre-BII, but not pro-B/pre-BI, cells are found in close contact with GAL1-expressing stromal cells. Large pre-B II cells (A-B) and pro-B/pre-BI cells (C) were purified from the BM of C57Bl/6 mice by flow cytometry and stained with PKH26. Labeled cells were then injected intravenously in stroma-GFP mice treated with HU. Mice were killed 15 hours after injection. BM sections were stained with anti-GAL1, anti-GFP antibodies, and Sytox Blue (nuclei) as indicated. The presence or the absence of contacts between PKH26-labeled B cells and GAL1+GFP+ stromal cells was determined by analyzing 10-μm-thick z sections by confocal microscopy (original magnification 63×/1.4 NA oil objectives). Bar represents 10 μm. Confocal images are representative of 3 (A-B) and 2 (C) independent experiments. Cell counts are presented in Table 1. Images acquired on Zeiss LSM310 Meta and analyzed with ImageJ 1.44.
To determine whether GAL1+ BM stromal cells constitute a stromal cell niche specific for large pre-BII cells, we performed the same experiment using PKH26+ pro-B/pre-BI cells injected to HU-treated stroma-GFP mice. Once again, pro-B/pre-BI cells home specifically to the BM (supplemental Figure 1), and 85.7% of them were found in contact with GFP+ stromal cells (Figure 3C; Table 1). However, in contrast to large pre-BII cells, only 13% of pro-B/pre-BI cells formed contact with GAL1+GFP+ cells (Table 1). Finally, we showed that IgM+IgD− immature and IgM+IgD+ recirculating B cells were mainly localized in extravascular and perivascular regions, in accordance with previous reports19,20 (supplemental Figure 2). In addition, immature and recirculating B cells found in the mesenchyme were away from GAL1+ stromal cells (17 of 65 cells, 26.1%; and 56 of 167 cells, 33.5% in contact with GAL1+GFP+ stromal cells, respectively); and in opposition to pre-BII cells, most of the contacts were observed with GAL1+ fibroblastic- but not cuboid-shaped cells.
These results demonstrate that developing B cells are mainly in contact with BM stromal cells, large pre-BII cells being preferentially in contact with stromal cells expressing GAL1, the pre-BCR ligand. Furthermore, these data strongly suggest the existence of distinct stromal cell niches for the different B-cell subsets.
IL-7 and GAL1 are expressed by distinct BM stromal cell subpopulations
Although human and mouse mesenchymal stem cells have been largely studied,21,22 little is known about the heterogeneity of the BM stroma and about the mesenchymal cells implicated in B-cell differentiation. To characterize the pro-B/pre-BI and pre-BII cellular niches without performing in vitro stromal cell cultures that could modify the initial phenotype, a multiparametric flow cytometry analysis was performed directly on nonhematopoietic BM cells. Briefly, CD45−CD117−Ter119−CD19−Gr1−CD11c− BM stromal cells were analyzed for cell surface markers implicated in pre-BCR clustering (GAL1, CD54),5,8,9 expressed by the pro-B/pre-BI cellular niche (IL-7, CD106),3 by mesenchymal stem cells (platelet-derived growth factor receptor-α [PDGFR-α]),22 and by peripheral stromal cells or endothelial cells (PDGFR-α, BP1, CD31, Tie-2, CD34).23
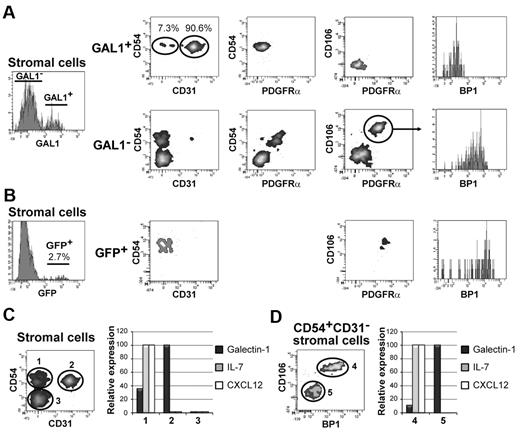
Among the GAL1+ stromal cells (Figures 1H, 4A), 2 different subpopulations were identified on the basis of CD31 expression. The main population expressed CD31 (84.8% ± 15.2%), and the minor population was negative for CD31 (9.2% ± 5.9%). Both populations were positive for CD54 and negative for PDGFR-α, CD106, and BP1. As CD31 is considered to be an endothelial cell marker, we checked whether GAL1+CD31+ cells express other characteristic endothelial cell markers, as Tie-2 and CD34.23 Interestingly, none of these markers was expressed by GAL1+ stromal cells (supplemental Figure 3). As expected, endothelial cells, which were occasionally detected in the BM stromal cell suspension, were CD31hiTie2+CD34+ and did not express GAL1 (supplemental Figure 3). Finally, similar to the GAL1+ stromal cells, CD54 is also expressed by GAL1− cells (Figure 4A). However, in contrast to GAL1+ cells, CD54+GAL1− cells expressed PDGFR-α, CD106, and BP1 but were negative for CD31.
GAL1- and IL-7–expressing stromal cells are distinct populations. (A) BM stromal cells from C57Bl/6 mice (n = 5) were analyzed by flow cytometry as described in Figure 1H and were further characterized using antibodies specific for CD54, CD31, PDGFRa, CD106, and BP1. The analysis of the stromal cells was performed on the GAL1+ and GAL1− cell populations. The gating strategy is shown in the figure. (B) CD45−Lin− stromal cells from IL-7-GFP reporter mice (n = 4) were analyzed by flow cytometry as described in panel A. The phenotypic analysis was performed on the GFP+ population. (C-D) BM stromal cells from C57Bl/6 mice were defined as shown in Figure 1H and were sorted by flow cytometry using, in addition, antibodies specific for CD54, CD31, CD106, and BP1. (C) mRNA was prepared from the CD54+CD31− (population 1), CD54+CD31+ (population 2), and CD54−CD31− (population 3) stromal cells (left panel). RT-PCR was performed using primers specific for GAL1, IL-7, and CXCL12 (right panel). (D) The CD54+CD31− population was further refined by cell sorting using anti-BP1 and anti-CD106 antibodies in 2 subpopulations: CD106+BP1+ (population 4) and CD106−BP1− (population 5). The RT-PCR was performed using primers specific for GAL1, IL-7, and CXCL12 (right panel). The results of RT-PCR are representative of 3 independent experiments.
GAL1- and IL-7–expressing stromal cells are distinct populations. (A) BM stromal cells from C57Bl/6 mice (n = 5) were analyzed by flow cytometry as described in Figure 1H and were further characterized using antibodies specific for CD54, CD31, PDGFRa, CD106, and BP1. The analysis of the stromal cells was performed on the GAL1+ and GAL1− cell populations. The gating strategy is shown in the figure. (B) CD45−Lin− stromal cells from IL-7-GFP reporter mice (n = 4) were analyzed by flow cytometry as described in panel A. The phenotypic analysis was performed on the GFP+ population. (C-D) BM stromal cells from C57Bl/6 mice were defined as shown in Figure 1H and were sorted by flow cytometry using, in addition, antibodies specific for CD54, CD31, CD106, and BP1. (C) mRNA was prepared from the CD54+CD31− (population 1), CD54+CD31+ (population 2), and CD54−CD31− (population 3) stromal cells (left panel). RT-PCR was performed using primers specific for GAL1, IL-7, and CXCL12 (right panel). (D) The CD54+CD31− population was further refined by cell sorting using anti-BP1 and anti-CD106 antibodies in 2 subpopulations: CD106+BP1+ (population 4) and CD106−BP1− (population 5). The RT-PCR was performed using primers specific for GAL1, IL-7, and CXCL12 (right panel). The results of RT-PCR are representative of 3 independent experiments.
As pre-BII cells were found in contact with GAL1+ stromal cells (Figure 3) and were not located near IL-7+ stromal cells,3 we checked whether GAL1+ and IL-7+ cells correspond to 2 distinct stromal cell populations. For this purpose, we used the IL-7cre-Rosa26-eYFP reporter mice, generated by Repass et al.17 Briefly, these mice are transgenic for a bacterial artificial chromosome in which IL-7 regulatory elements drive expression of the Cre recombinase and are backcrossed with the Rosa26-eYFP reporter mice, so that cells that have expressed IL-7 become enhanced yellow fluorescent protein+ (EYFP+). When BM stromal cells of IL-7 reporter mice were analyzed, 1.8% ± 1% of the cells (n = 4) expressed EYFP (Figure 4B). A detailed phenotypic analysis revealed that these cells expressed CD54, CD106, PDGFR-α, and BP1 but were negative for CD31 expression (Figure 4B). This result demonstrates that IL-7+ stromal cells are included within the GAL1−CD54+ population, described in Figure 4A. Thus, based on the differential expression of PDGFR-α, CD106, and BP1, IL-7+ stromal cells are distinct from that of GAL1+ subpopulations.
This conclusion was confirmed by checking GAL1 and IL-7 expression by real-time PCR performed on stromal cell subpopulations isolated from wild-type BM using a combination of CD54, CD31, CD106, and BP1 markers (Figure 4C-D). Real-time PCR indicated that GAL1 mRNA was expressed by CD31+CD54+ and by CD54+CD31− cells and that IL-7, but also CXCL12, were expressed exclusively by CD54+CD31− cells (Figure 4C). The separation of CD54+CD31− cells into CD106+BP1+ and CD106−BP1− subpopulations allowed us to determine that the IL-7 mRNA was present in the CD106+BP1+ cell fraction, whereas CD106−BP1− cells expressed GAL1 (Figure 4D), in accordance with the phenotypic characterization.
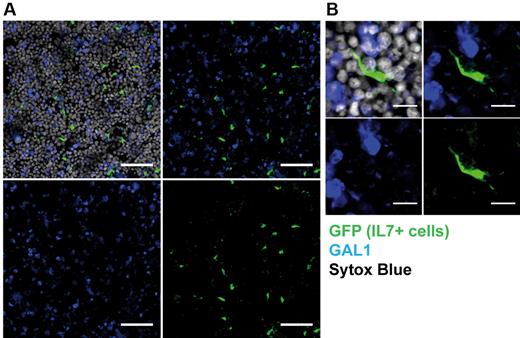
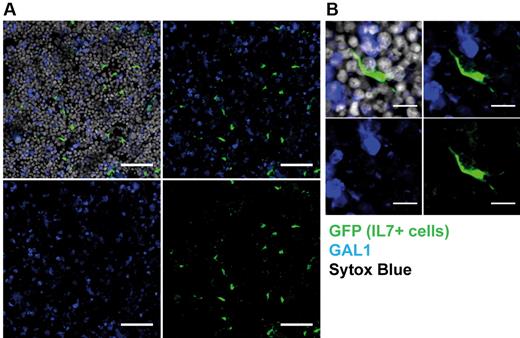
Finally, detection of IL-7+ stromal cells was performed by IHF on bone sections of IL-7 reporter mice. As shown in Figure 5A, reticular IL-7+ cells were scattered in the BM and did not express GAL1. Moreover, they exhibit a characteristic spindle shape with extended processes (Figure 5B), in contrast with GAL1+ stromal cells (Figures 2–3).
IL-7+ and GAL1+ stromal cells constitute distinct stromal cell populations. BM sections of IL-7-GFP reporter mice were stained with anti-GAL1, anti-GFP antibodies, and Sytox Blue (nuclei) as indicated and analyzed by confocal microscopy. (A) GFP+ IL-7–expressing stromal cells and GAL1+ cells are scattered in the BM (original magnification 40×/1.25 NA oil objective). Bar represents 50 μm. (B) Representative IL-7+ spindle-shaped stromal cell (original magnification 63×/1.4 NA oil objective). Bar represents 10 μm. Images acquired on Leica SP5X and analyzed with ImageJ 1.44.
IL-7+ and GAL1+ stromal cells constitute distinct stromal cell populations. BM sections of IL-7-GFP reporter mice were stained with anti-GAL1, anti-GFP antibodies, and Sytox Blue (nuclei) as indicated and analyzed by confocal microscopy. (A) GFP+ IL-7–expressing stromal cells and GAL1+ cells are scattered in the BM (original magnification 40×/1.25 NA oil objective). Bar represents 50 μm. (B) Representative IL-7+ spindle-shaped stromal cell (original magnification 63×/1.4 NA oil objective). Bar represents 10 μm. Images acquired on Leica SP5X and analyzed with ImageJ 1.44.
Altogether, these results show that the BM microenvironment is composed of several stromal cell subpopulations, including at least 2 GAL1-expressing subsets. Moreover, IL-7 and GAL1 expression in stromal cells was mutually exclusive, consistent with the existence of distinct stromal cell niches specific for pro-B/pre-BI and pre-BII cells, respectively.
Stage-specific niches are differentially localized in the BM microenvironment
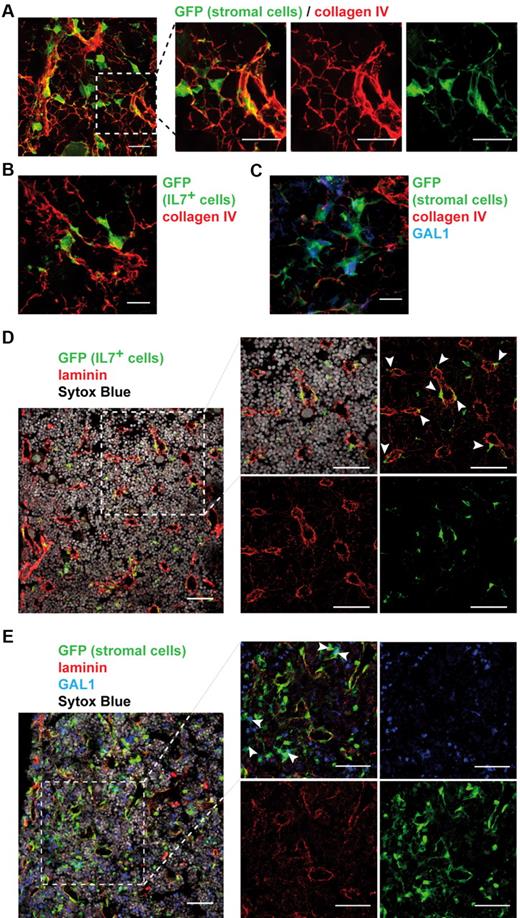
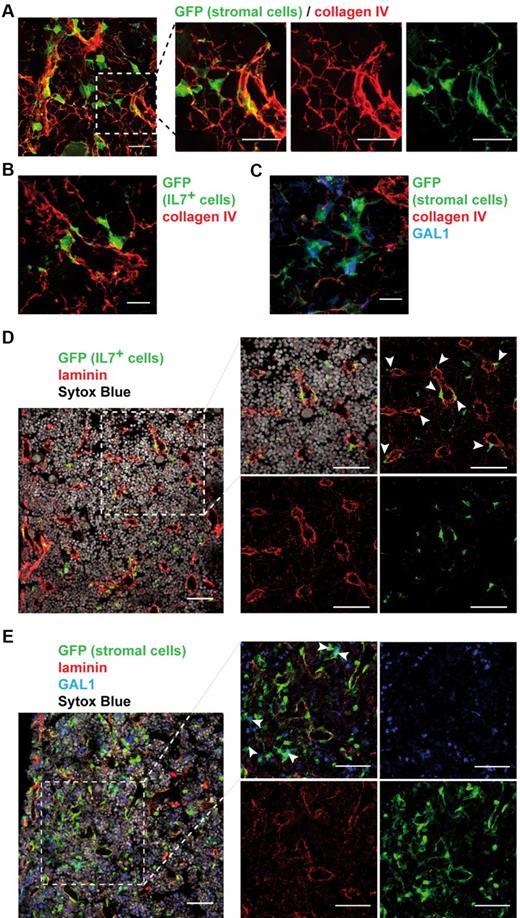
As we determined that the BM stroma is composed of distinct cellular subpopulations, we wondered how these cells were spatially arranged. Using IHF on bone sections of stroma-GFP mice, we found that the stromal network was closely associated with a framework of collagen IV fibers (Figure 6A; supplemental Video 2). GFP+ stromal cells were either entangled in dense collagen structures or spread out on thin collagen fibers. Both IL-7+ and GAL1+ stromal cells were found associated with this lattice (Figure 6B-C). IL-7+ cells, in opposition to GAL1+ cells, were often located contiguous to the dense fiber structures that suggested vessels. Indeed, pan-laminin staining, which identified vessels, confirmed that the majority of IL-7+ reticular cells were in close contact with the vasculature (Figure 6D; 65%, 72 cells among 111 counted). By contrast, GAL1+ stromal cells are more dispersed within the BM, with only 3% of the cells in contact with vessels (Figure 6E; 2 cells among 59 counted).
IL-7+ and GAL1+ stromal cells are localized in distinct regions of the BM. BM sections were stained to determine the organization of the whole stroma and of IL-7+ and GAL1+ stromal cells compared with the extracellular matrix (A-C) and the vasculature (D-E). Representative BM sections are shown. (A) Staining of BM sections of stroma-GFP mice with anti-GFP and anticollagen IV antibodies (original magnification 40×/1.25 NA oil objective). (Right panels) A magnification of the region in the dotted square of the left panel. (B) Staining of BM sections of IL-7-GFP reporter mice with anti-GFP and anticollagen IV antibodies. (C) Staining of BM sections of stroma-GFP mice with anti-GFP, anti-GAL1, and anticollagen IV antibodies. (D) Staining of BM sections of IL-7-GFP reporter mice with anti-GFP, anti-laminin antibodies, and Sytox Blue (nuclei). (Right panels) A magnification of the region in the dotted square of the left panel. (E) Staining of BM sections of stroma-GFP mice with anti-GFP, anti-GAL1, anti-laminin antibodies, and Sytox Blue (nuclei). (Right panels) A magnification of the region in the dotted square of the left panel. (A,D-E) Bar represents 50 μm. (B-C) Bar represents 10 μm. Images acquired on Leica SP5X and analyzed with ImageJ 1.44.
IL-7+ and GAL1+ stromal cells are localized in distinct regions of the BM. BM sections were stained to determine the organization of the whole stroma and of IL-7+ and GAL1+ stromal cells compared with the extracellular matrix (A-C) and the vasculature (D-E). Representative BM sections are shown. (A) Staining of BM sections of stroma-GFP mice with anti-GFP and anticollagen IV antibodies (original magnification 40×/1.25 NA oil objective). (Right panels) A magnification of the region in the dotted square of the left panel. (B) Staining of BM sections of IL-7-GFP reporter mice with anti-GFP and anticollagen IV antibodies. (C) Staining of BM sections of stroma-GFP mice with anti-GFP, anti-GAL1, and anticollagen IV antibodies. (D) Staining of BM sections of IL-7-GFP reporter mice with anti-GFP, anti-laminin antibodies, and Sytox Blue (nuclei). (Right panels) A magnification of the region in the dotted square of the left panel. (E) Staining of BM sections of stroma-GFP mice with anti-GFP, anti-GAL1, anti-laminin antibodies, and Sytox Blue (nuclei). (Right panels) A magnification of the region in the dotted square of the left panel. (A,D-E) Bar represents 50 μm. (B-C) Bar represents 10 μm. Images acquired on Leica SP5X and analyzed with ImageJ 1.44.
Therefore, IL-7+ and GAL1+ stromal cell subpopulations constitute 2 distinct cellular niches scattered throughout the BM but located in specific regions, differentiated with respect to their position toward the vasculature.
Discussion
In a previous study, we investigated the formation of the pre-B/stromal cell synapse in the case of normal pre-BII cells expressing physiologic levels of the pre-BCR.9 We demonstrated that pre-BCR/GAL1/integrin interactions are required, in vivo, for the efficient proliferation and differentiation of pre-BII cells. These results highlight the role played by the stromal cell-derived GAL1 for the development of normal pre-BII cells and suggest the existence of pre-BII specific stromal cell niches in normal BM.
Little is known about the heterogeneity of BM stromal cells supporting hematopoietic and early B-cell differentiation. Very few subpopulations of mesenchymal stromal cells have been identified in situ. Mesenchymal stem cells, with a potential to differentiate into stromal cells, osteoblasts, and adipocytes, have been characterized in the CD45−Lin− BM cell population. They express PDGFR-α and Sca-1 and produce low levels of CXCL12.22 CD106+CXCL12+ and CD106+IL-7+ reticular cells have also been characterized as niches for hematopoietic progenitors and for pro-B/pre-BI cells, respectively, leading to the notion of “stage-specific” niches.3 In the same report, the authors showed that cells differentiating into pre-B cells, identified as expressing B220 and IL-7R, leave IL-7–expressing cells. In this study, we showed the existence of a new stromal cell subpopulation, expressing GAL1, which forms specific contacts with normal pre-BII cells.
Expression of GAL1 in the BM was detected in various subtypes of hematopoietic cells and in nonhematopoietic cells, as osteoblasts and reticular cells. The GAL1+ stromal reticular network was shown to be the localization site of pre-BII cells, as 80% of pre-BII cells were found in close contact with it (Table 1). By contrast, no pre-BII cells were detected near GAL1+ osteoblasts, and only 11% of them were in the vicinity of GAL1+ hematopoietic cells, such as immature myeloid cells, showing that GAL1 was not the only prerequisite in the niche permitting pre-BII cell differentiation signals. In accordance with this observation, we found that GAL1+ stromal cells expressed CD54, which was implicated (together with its LFA1 ligand) in the formation of the pre-B/stromal cell synapse in the presence of GAL1.9 Moreover, as previously highlighted,8 pre-B-cell integrins bound to their stroma cell ligands drive pre-BCR and GAL1 at the pre-B/stromal cell interface, and inhibition of GAL1-integrin interactions by lactose treatment prevents pre-BCR relocalization, without affecting integrin movements. Therefore, GAL1 and pre-BCRs are not required to account for pre-BII cell localization in the BM, whereas integrins are essential. Finally, even if GAL1 were also considered to be an autoantigen for the polyreactive pre-BCR,24,25 our study demonstrates that normal pre-BII cells require specific contacts with specialized BM stromal cells expressing GAL1. The fact that pre-BII, but not pro-B/pre-BI, immature B, and recirculating B, cells are in close contact with GAL1+ cuboid stromal cells (80% of contacts vs 13%, 26%, and 33.5%, respectively) highlights the specificity of this niche for the pre-BII cell stage.
Recently, both the N46 glycosylation site of the Igμ chain and the unique region of λ5 were shown to be specifically required for pre-BCR expression at the cell surface and autonomous signaling. The authors proposed that these interactions put the SLC into position for trans-association with adjacent Igμ chains, leading to pre-BCR tonic signaling.26 On the other hand, we demonstrated that the binding between GAL1 and the unique region of λ5 was involved in ligand-induced pre-BCR signaling, improving pre-BII cell proliferation and differentiation.5,8,9 Thus, it remains to be determined whether the autonomous and the GAL1-induced pre-BCR signaling act in synergy or whether these events take place successively, GAL1 disrupting the N46 Igμ/SLC interaction.
During the course of this study, we identified 2 subpopulations of GAL1+ stromal cells on the basis of CD31 expression, the majority being CD31+. All the GAL1+ cells express CD54 and are negative for PDGFR-α, CD106, and BP1 (Figure 4) or for Sca-1 and Nestin,22,27 2 MSC markers (data not shown). Although most of the GAL1+ stromal cells express CD31, the absence of CD34 and Tie2 on their surface and histologic observations indicate that they do not correspond to endothelial cells. However, because of the low level of CD31 expression compared with endothelial cells (supplemental Figure 3), the available antibodies did not allow CD31 detection on stromal cells by IHF. As a consequence, we could not yet determine which of the CD31+GAL1+ or the CD31−GAL1+ cell population corresponds to the pre-BII cell niche. Nonetheless, we preferentially observed pre-BII cells in contact with the cuboid- and not the spindle-shaped GAL1+ stromal cells.
By various techniques, the GAL1+ niche was shown to be independent of the IL-7+ niche specific for the pro-B/pre-BI stage of B-cell differentiation.3 IL-7+ cells were CD54+PDGFR-α+CD106+BP1+CD31− and did not express GAL1 mRNA. In accordance with Tokoyoda et al,3 expression of the CXCL12 and IL-7 transcripts was found in the CD106+ stromal cell subpopulation. Finally, IL-7–GFP+ stromal cells do not express GAL1 and are spindle-shaped reticular cells, whereas GAL1+ stromal cells are morphologically more heterogeneous. The fact that GFP expression in the IL-7–GFP reporter mice is maintained indefinitely once acquired, and that GFP+ cells never coexpress GAL1, indicates that GAL1+ cells are not derived from IL-7+ cells but that these populations constitute 2 separate stromal cell lineages.
During this study, the IL-7– and GAL1-expressing stromal cells were also shown to be located in distinct areas of the BM, the former being closely associated with the vasculature and the latter being at a distance from these regions (Figure 6). Similarly, the localization of CXCL12-secreting cells, which support HSCs and multipotent progenitors, is not random because they are specifically found close to the endosteum and to the vasculature.28 These data are consistent with the idea that BM stroma constitutes an organized environment allowing the establishment of specialized supportive niches with an ordered distribution of cells throughout the BM to create gradients of nutrients and soluble factors. Indeed, Paige et al showed that signals from the IL-7 receptor synergize with pre-BCR signals but that pre-BII cells require a lower concentration of IL-7 than pro-B/pre-BI cells to proliferate.29,30 Therefore, the distance between the stromal cells producing IL-7 and those expressing GAL1 allows the formation of an IL-7 gradient. In addition, it has been demonstrated more recently that the most potent HSCs are located in regions of the BM with negligible blood perfusion, such as the endosteum, whereas the cycling HSCs engaged in differentiation are in more perfused locations, close to the vasculature.31
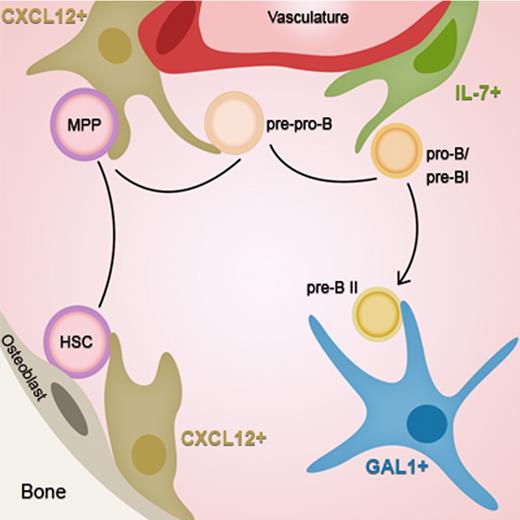
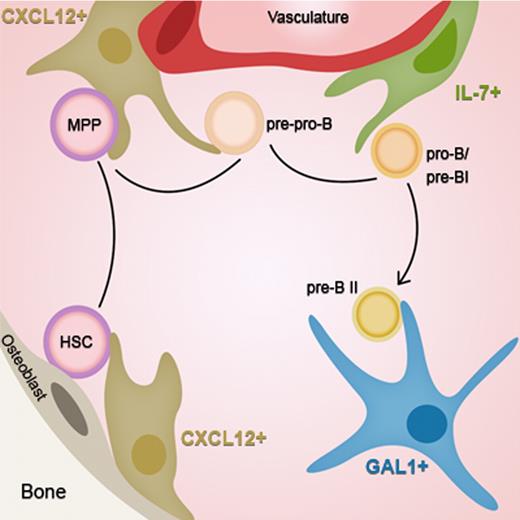
Our results extend to pre-BII cells, the notion of “stage-specific” niches that has emerged in the literature, adding a new step on the migratory route of B-cell development. As schematized in Figure 7, HSCs move from a CXCL12+ niche near the endosteum toward a CXCL12+ niche close to the vasculature while differentiating into pre-pro-B cells.28,31 Then, the cells reach the proximal IL-7+ niche in the perivascular region, entering the pro-B/pre-BI stage where IgH gene rearrangements are initiated. Next, cells expressing a functional Igμ chain leave the vasculature to enter deeper into the BM, close to GAL1+ stromal cells, allowing the efficient development of pre-BCR expressing pre-BII cells.
Stage-specific niches of early B-cell development. HSCs move from an endosteal CXCL12+ cellular niche toward a vascular CXCL12+ niche while differentiating into pre-pro-B cells. At the next step, cells reach the proximal IL-7+ niche in the perivascular region, entering the pro-B/pre-BI stage. Finally, at the pre-BCR+ pre-BII stage, cells leave the vasculature to enter deeper into the BM, close to GAL1+ stromal cells.
Stage-specific niches of early B-cell development. HSCs move from an endosteal CXCL12+ cellular niche toward a vascular CXCL12+ niche while differentiating into pre-pro-B cells. At the next step, cells reach the proximal IL-7+ niche in the perivascular region, entering the pro-B/pre-BI stage. Finally, at the pre-BCR+ pre-BII stage, cells leave the vasculature to enter deeper into the BM, close to GAL1+ stromal cells.
The distance between the different niches implies an active migration of the differentiating B cells. The attraction toward a particular niche is certainly performed by chemokines, but this aspect has been poorly studied. For human B cells, it was reported that CXCL12 attracts both pro-B/pre-BI and pre-BII cells and that CCR7 ligands allow the specific migration of pre-BII cells.32 Modulation of adhesion molecules may also be implicated in the retention/release of B cells into/from their specific niche. Indeed, the LFA-1 integrin, which binds CD54, is highly expressed in pro-B/pre-BI cells, down-modulated in pre-BCR+ large pre-BII cells, and extinguished in small pre-BII cells (S.J.C.M., unpublished results, June 2007). In contrast, CD2 (LFA-2) is up-regulated on pre-BCR activation, from the pre-BII stage onwards.33 Therefore, the contact between large pre-BII cells and the GAL1+ stromal cell niche is probably the result of a particular combination of various adhesion molecules, differentially expressed between niches. This redundancy is supported by the observation that neither LFA134 nor CD5435 deficiencies impede B lymphopoiesis in mice.
Understanding the heterogeneity and organization of stromal cells in normal BM appears to be a prerequisite to address the role of the stromal microenvironment in pathologic situations. Indeed, the BM stroma was shown to sustain survival and progression of tumor B cells.11,12 In mice deficient for Blnk or for both Blnk and Btk, key components of the pre-BCR signaling pathway, pre-BII cell leukemia expressing high levels of pre-BCR at the cell surface develop with time.10,36,37 In these cases, we may speculate that GAL1+ stromal cell niches participate in leukemogenesis by enhancing pre-BCR cross-linking and thus leukemic cell proliferation and progression.9 Finally, it was reported that resistance to anticancer treatments could be the result of the presence of residual tumor cells receiving survival signals from protective niches.38 As a consequence, understanding the inter-relationship between the BM stroma and tumor B cells is also a major challenge for the development of new anticancer therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the flow cytometry facility and the PICsL imaging core for expert technical assistance, E. Richie for the IL-7 reporter mice, L. Onder for providing essential material, and L. Leserman, M. Bajénoff, M. Aurrand-Lions, and M. L. Arcangeli for critical reading of the manuscript.
This work was supported by Institut National du Cancer, Program d'Actions Intégrées de Recherche Lymphomes (2008 contract Projet R08008AP), Association pour la Recherche contre le Cancer (contract 1089), Inserm (institutional grants), and CNRS. C.B. was supported by Ministère de l'Education Nationale et de la Recherche and Ligue Contre le Cancer.
Authorship
Contribution: F.M., C.B., J.T., L.C., A.J., and S.J.C.M. performed research; F.M., C.B., J.T., C.S., and S.J.C.M. analyzed data and designed research; C.S. and S.J.C.M. wrote the paper; and P.N. and M.C. provided essential material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphane J. C. Mancini, Centre d'Immunologie de Marseille-Luminy, Case 906, F-13288 Marseille Cedex 09, France; e-mail: mancini@ciml.univ-mrs.fr; and Claudine Schiff, Centre d'Immunologie de Marseille-Luminy, Case 906, F-13288 Marseille Cedex 09, France; e-mail: schiff@ciml.univ-mrs.fr.
References
Author notes
F.M., C.B., and J.T. contributed equally to this study.
C.S. and S.J.C.M. contributed equally to this study.