Abstract
p90 ribosomal S6 kinase 2 (p90RSK2) is important in diverse cellular processes including gene expression, cell proliferation, and survival. We found that p90RSK2 is commonly activated in diverse leukemia cell lines expressing different leukemogenic tyrosine kinases, including BCR-ABL and FMS-like tyrosine kinase 3–internal tandem duplication (FLT3-ITD). Interestingly, in a murine BM transplantation (BMT) model, genetic deficiency of RSK2 did not affect the pathogenesis or disease progression of BCR-ABL–induced myeloproliferative neoplasm (PN). In contrast, FLT3-ITD induced a T-cell acute lymphoblastic leukemia in BMT mice receiving RSK2 knockout (KO) BM cells, phenotypically distinct from the myeloproliferative neoplasm induced by FLT3-ITD using wild-type BM cells. In consonance with these results, inhibition of RSK2 by an RSK inhibitor, fmk, did not effectively induce apoptosis in BCR-ABL–expressing murine Ba/F3 cells, human K562 cells or primary tissue samples from CML patients, whereas fmk treatment induced significant apoptotic cell death not only in FLT3-ITD–positive Ba/F3 cells, human Molm14 and Mv(4;11) leukemia cells, but also in primary tissue samples from AML patients. These results suggest that RSK2 is dispensable for BCR-ABL–induced myeloid leukemia, but may be required for pathogenesis and lineage determination in FLT3-ITD–induced hematopoietic transformation. RSK2 may thus represent an alternative therapeutic target in the treatment of FLT3-ITD–positive leukemia.
Introduction
RSK2 is a Ser/Thr kinase that belongs to a family containing 4 members, RSK1 to 4, all of which are downstream substrates of ERK and play a role in various cellular processes including gene expression, cell cycle, survival, and proliferation. RSK family members share both structural and functional similarities, and are uniquely characterized by the presence of 2 distinct kinase domains, both of which are catalytically functional1-3 (reviewed in Blenis,4 Frodin and Gammeltoft,5 and Anjum and Blenis6 ). The carboxyl-terminal kinase (CTK) domain is responsible for autophosphorylation at Ser386 (numbering based on the murine RSK2 amino acid sequence), which is critical for RSK activation, while the N-terminal kinase (NTK) domain phosphorylates RSK substrates.2 We recently reported that tyrosine phosphorylation of RSK2 facilitates inactive ERK binding to RSK2 in the initial activation step, and disrupts an autoinhibitory region of RSK2 to achieve full activation.7-9
RSK2 phosphorylates multiple signaling effectors that possess RRXS/T or RXRXXS/T motifs.10 These RSK2 phosphorylation targets include transcriptional regulators such as cAMP-response element-binding protein (CREB),11 c-Fos,12,13 NFATc4,14 NFAT3,15 ATF4,16 and Nur77.17 Phosphorylation and activation of these transcription factors are important for regulation of gene expression. RSK2 also phosphorylates histone H3, which contributes to chromatin remodeling during mitosis and transcriptional activation.18 In addition, RSK2 promotes cell survival by phosphorylating and inhibiting proapoptotic protein factors including BAD,19 Bim,20 and death-associated protein kinase (DAPK).21 Moreover, RSK2 promotes proliferation by phosphorylating GSK3β,22 NHE-1,23 and p27kip1.24 Therefore, RSK2 may serve as a key regulator by activating multiple signaling effectors in a signaling network that promotes cell survival and proliferation.
Defects in the human RSK2 gene are associated with Coffin-Lowry syndrome (CLS), an X-linked mental retardation.25,26 Although there is no evidence that RSK2 is mutated in human cancers, RSK2 signaling has been demonstrated to play a key role in the pathogenesis and disease progression of some human malignancies, including metastatic head and neck cancer,27 FGFR1-expressing prostate cancer,28,29 and osteosarcoma.16,30 We recently found that oncogenic FGFR3 phosphorylates and activates RSK2 to induce hematopoietic transformation.7,9 Targeting RSK2 but not RSK1 by siRNA or treatment with a specific RSK inhibitor fmk31,32 effectively induced apoptosis in FGFR3-expressing human t(4;14)-positive myeloma cells and primary patient myeloma cells. These findings suggest a critical role for RSK2 in FGFR3-induced hematopoietic transformation.
In this report, we focus on the role of RSK2 in other hematopoietic malignancies induced by different leukemogenic tyrosine kinases (LTKs) including BCR-ABL and FMS-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) mutant. BCR-ABL is a constitutively active fusion tyrosine kinase that is associated with the Philadelphia chromosome translocation t(9;22)(q34;q11), and clinically presents as either chronic myeloid leukemia (CML) characterized by excessive proliferation of differentiated myeloid cells, or B-cell acute lymphoblastic leukemia (B-ALL), a rapidly fatal disease of undifferentiated lymphoid B cells. FLT3, also known as fetal liver kinase-2 (FLK-2), is a member of the class III receptor tyrosine kinase (RTK) family. FLT3 is expressed in approximately 70% or greater cases of acute myelogenous leukemia (AML) of all French-American-British (FAB) subtypes, a small fraction of T-cell ALLs, and chronic myelogenous leukemia (CML) in blast crisis.33 The frequency of FLT3-ITD mutations is reported to be 24% in adult AML and 15% in secondary AML. The pathogenic role of BCR-ABL and FLT3 has made them important therapeutic targets for treatment of related leukemias. However, the signaling properties of these leukemogenic tyrosine kinases that contribute to hematopoietic transformation remain unclear.
Here we report that RSK2 is dispensable in BCR-ABL–induced myeloproliferative neoplasm, but is required for FLT3-ITD induced hematopoietic transformation, where it is likely involved in pathogenesis and lineage determination. Our findings suggest that the role of RSK2 in hematopoietic transformation may depend on distinct upstream oncogenic signals mediated by different leukemogenic tyrosine kinases.
Methods
Cell culture
Ba/F3 cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1.0 ng/mL IL-3 (R&D Systems). Ba/F3 cells expressing various leukemogenic tyrosine kinases were cultured in RPMI 1640 with 10% FBS and 1 mg/mL G418 (Invitrogen). Leukemia cell lines including EOL-1. HEL, KARPAS, K562, Molm14, Mv(4,11), Mo91, and FGFR3-positive multiple myeloma cell line OPM1 were cultured in RPMI 1640 medium supplemented with 10% FBS and penicillin/streptomycin. ANBL6 cells were cultured in RPMI 1640 medium supplemented with 10% FBS and IL-6 (R&D Systems).
Immunoprecipitation and Western blot
Cells (∼ 1 × 107) were lysed and cell extracts were clarified by centrifugation and used for immunoprecipitation or immunoblotting as described.34 Phospho-Tyr Ab pY99, c-ABL, FLT3, RSK1, and RSK2 Abs were from Santa Cruz Biotechnology; phospho-RSK (Ser380) Ab was from Cell Signaling Technology (CST). Ab against β-actin was from Sigma-Aldrich.
Mice
Balb/C RSK2 knockout (RSK2 KO) mice were developed as described previously.35 Murine BMT assays were performed as described previously.36 In brief, retroviral supernatants were generated by transient cotransfection of 293T cells with the MSCV2.2-Gateway-IRES-GFP-BCR-ABL37 or FLT3-ITD/5138 constructs and packaging construct using Superfect (QIAGEN). The viral titer was estimated by infecting Ba/F3 cells with serial dilutions of viral supernatant and percentage of GFP-positive cells was determined by flow cytometry 48 hours postinfection. Viral titers of constructs used for each round of murine BMT were 24.56 × 106/mL, 32.55 × 106/mL, and 14.56 × 106/mL for BCR-ABL, 11.57 × 106/mL, 43.27 × 106/mL, and 20.03 × 106/mL for FLT3-ITD. The wild-type (WT) and RSK2 KO donor mice used for each BMT experiment were littermates of the same age. Four- to 6-week-old WT or RSK2 KO Balb/C donor mice were treated with 150 mg/kg 5-Fluorouracil (5-FU; Sigma-Aldrich) 6 days before harvest of BM cells. Two days before the BMT, the BM cells were collected from the femurs and tibias of the donor mice. The same batch of retroviral supernatant with the same titer was used to infect WT or RSK2 KO Balb/C donor BM cells by spin infection in RPMI 1640 media containing recombinant murine IL-3 (rmIL-3; 6 ng/mL; R&D Systems), rmSCF (10 ng/mL; R&D Systems), recombinant human IL-6 (10 ng/mL; R&D Systems), and 10% FBS. Cells (1 × 106) in 0.5 mL of HBSS were injected into the lateral tail veins of lethally irradiated (2 × 450 cGy) syngeneic Balb/C recipient mice. Animals were carefully monitored under the auspices of Emory University institutional review board–approved protocols for the humane care of animals.
Diseased BMT mice were examined each day, and killed at first signs of morbidity, including scruffy coat, lethargy, weight loss, leukocytosis, and splenomegaly palpable beyond the midline. White blood cell (WBC) counts and weights of organs including spleen and liver were recorded at time of necropsy.
Histopathology and flow cytometric immunophenotyping
Histopathologic analyses were performed as described previously.39 Sections of mouse tissue were stained with hematoxylin and eosin and slides were digitally scanned at 20× magnification using a ScanScope XT from Aperio Technologies Inc. Images were analyzed and captured using ImageScope software (Aperio Technologies Inc) without any additional or subsequent image processing. Before flow cytometric analysis, cell samples of single-cell suspensions were washed in the staining buffer (PBS with 0.1% NaN3 and 0.1% BSA) and stained for 20 minutes on ice with combinations of labeled mAbs recognizing Gr-1/Mac-1 and CD4/CD8 (BD Biosciences). After washing, the cells were resuspended in staining buffer containing 0.5 μg/mL 7-amino-actinomycin D (7-AAD; BD Biosciences) to allow discrimination of nonviable cells, and flow cytometric analysis was done on a FACSCalibur cytometer (BD Biosciences). At least 10 000 events were acquired, and the data were analyzed using CellQuest software (Version 3.3). The results are presented as dot plots of viable cells selected on the basis of scatter and 7-AAD staining.
Cell viability assay and apoptosis assay
For cell viability assays, 1 × 105 cells were cultured in 24-well plates with increasing concentrations of fmk. The relative cell viability at each experimental time point up to 72 hours was determined using a CellTiter96AQueous One solution proliferation kit (Promega). For apoptosis assays, 1 × 106 cells were treated with increasing concentrations of fmk for up to 48 hours. Cells were collected and stained using FITC-conjugated annexin V labeling reagent and propidium iodide (PI; BD Pharmingen) as per the recommendations of the manufacturers, followed by FACS analysis for apoptotic cell population.
Primary tissue samples from CML and AML patients
The primary patient samples were analyzed as previously described.40 Briefly, mononuclear cells (MNCs) were ficolled from blood samples of CMLPh+ and AML patients. Cells (1 × 106 /mL) were cultured in RPMI 1640 medium supplemented with 10% FBS and penicillin/streptomycin and incubated with increasing concentrations of fmk for up to 72 hours. Cell viability assay and apoptosis assay were performed as described in “Cell viability assay and apoptosis assay.” All clinical samples were obtained with informed consent with approval by the Emory University Institutional Review Board.
Results
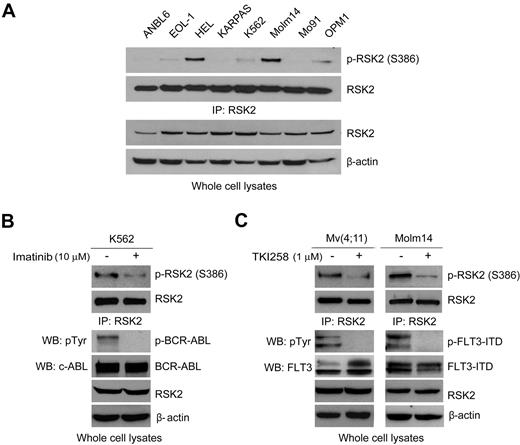
RSK2 is activated in diverse human leukemia cells transformed by different leukemogenic tyrosine kinases including BCR-ABL and FLT3-ITD
To better understand whether RSK2 is a common signaling pathway responsible for cell proliferation and survival in hematopoietic transformation induced by different leukemogenic tyrosine kinases, we examined RSK2 activation as assessed by phosphorylation levels of S386 in diverse human hematopoietic cancer cell lines. These cell lines include: EOL-1 that is associated with idiopathic hypereosinophilic syndrome (HES) and expresses FIP1L1-PDGFRA fusion tyrosine kinase; HEL that is associated with myeloproliferative neoplasms and expresses the constitutively activated JAK2 V617F mutant; KARPAS that is associated with t(2;5)(p23;q35) advanced-stage anaplastic large-cell lymphoma (ALCL) and expresses NPM-ALK fusion tyrosine kinase; K562 that are associated with t(9,22) Ph+ CML and expresses BCR-ABL fusion tyrosine kinase; Molm14 that is associated with acute myelogenous leukemia (AML) and expresses the constitutively activated FLT3-ITD (internal tandem duplication) mutant; and Mo91 that is associated with t(12;15)(p13;q25) AML and expresses TEL-TrkC fusion tyrosine kinase. We also included t(4;14) positive, FGFR3-expressing human myeloma OPM1 cells, in which RSK2 is activated by FGFR37 as a positive control, and the t(4;14)–negative myeloma ANBL6 cells without dysregulated expression of tyrosine kinase, in which activation of RSK2 was not detectable,7 was included as a negative control. As shown in Figure 1A, RSK2 is activated in leukemia cells expressing FIP1L1-PDGFRA (EOL-1), JAK2 V617F mutant (HEL), BCR-ABL (K562), and FLT3-ITD (Molm14), but not in cells expressing NPM-ALK (KARPAS) or TEL-TrkC (Mo91). RSK2 activation was assessed by phosphorylation levels of RSK2 at S386. This result suggests that RSK2 signaling may represent a common proliferative and prosurvival pathway activated in leukemia cells transformed by different leukemogenic tyrosine kinases that are associated with various myeloid malignancy subtypes.
RSK2 is activated in diverse human leukemia cells transformed by different leukemogenic tyrosine kinases including BCR-ABL and FLT3-ITD. (A) Immunoblotting detects S386 phosphorylation of RSK2 in diverse hematopoietic cancer cell lines expressing diverse leukemogenic tyrosine kinases, including EOL-1 (HIP1L1-PDGFRA), HEL (JAK2 V617F), KARPAS (NPM-ALK), K562 (BCR-ABL), Molm14 (FLT3-ITD), Mo91 (TEL-TrkC), OPM1 (FGFR3). ANBL6 is a human myeloma cell line without dysregulated expression of tyrosine kinase and included as a negative control. (B-C) Immunoblotting shows that targeting BCR-ABL by imatinib in K562 cells (B) and FLT3 by TKI258 in Mv(4;11) and Molm14 cells (C) decreases phosphorylation of RSK2 S386.
RSK2 is activated in diverse human leukemia cells transformed by different leukemogenic tyrosine kinases including BCR-ABL and FLT3-ITD. (A) Immunoblotting detects S386 phosphorylation of RSK2 in diverse hematopoietic cancer cell lines expressing diverse leukemogenic tyrosine kinases, including EOL-1 (HIP1L1-PDGFRA), HEL (JAK2 V617F), KARPAS (NPM-ALK), K562 (BCR-ABL), Molm14 (FLT3-ITD), Mo91 (TEL-TrkC), OPM1 (FGFR3). ANBL6 is a human myeloma cell line without dysregulated expression of tyrosine kinase and included as a negative control. (B-C) Immunoblotting shows that targeting BCR-ABL by imatinib in K562 cells (B) and FLT3 by TKI258 in Mv(4;11) and Molm14 cells (C) decreases phosphorylation of RSK2 S386.
We next focused on BCR-ABL– and FLT3-ITD–induced hematopoietic transformation. Immunoblotting results show that RSK2 is activated and phosphorylated at S386 in BCR-ABL positive human leukemia K562 cells. Inhibition of BCR-ABL by a small molecule tyrosine kinase inhibitor imatinib resulted in decreased S386 phosphorylation levels of RSK2 in K562 cells (Figure 1B, supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition, RSK2 is activated in FLT3-ITD–positive human leukemia Mv(4;11) and Molm14 cells. We first targeted FLT3-ITD in these cells by using TKI258 (4-amino-5-fluor-3-[5-(4-metylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one, formerly known as CHIR258), which is an adenosine triphosphate-competitive inhibitor with activities against class III or IV receptor kinases including FGFR, VEGFR, PDGFR, FLT3, and c-kit receptor tyrosine kinase.41,42 Targeting FLT3-ITD by TKI258 led to markedly reduced S386 phosphorylation levels of RSK2 in these cells (Figure 1C, supplemental Figure 1B). In addition, inhibition of FLT3-ITD by 2 alternative FLT3 inhibitors, CEP701 and MLN518, also resulted in decreased RSK2 activation (supplemental Figure 2). These data suggest that leukemogenic tyrosine kinases BCR-ABL and FLT3-ITD activate RSK2 in transformed leukemia cells.
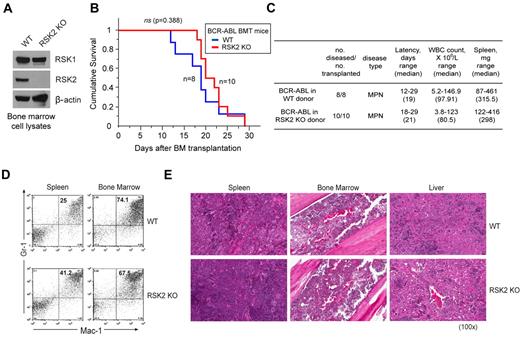
Genetic deficiency of RSK2 does not affect BCR-ABL–induced myeloproliferative neoplasm in a murine BMT assay
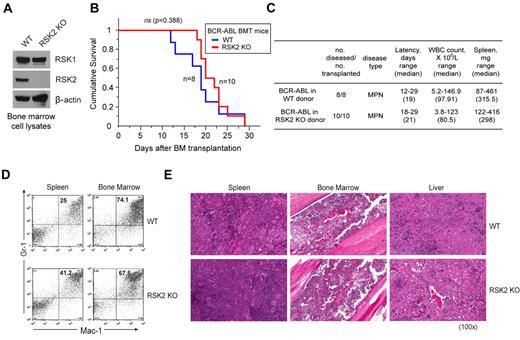
To further decipher the role of RSK2 in BCR-ABL– or FLT3-ITD–induced hematopoietic transformation, we performed a murine BMT assay to examine whether RSK2 is required for the in vivo transforming activity of BCR-ABL or FLT3-ITD in primary hematopoietic cells. BCR-ABL or FLT3-ITD were retrovirally transduced into donor BM cells from either WT Balb/C mice or mice that are genetically deficient of RSK2 (RSK2 KO)35 (Figure 2A). The transduced cells were subsequently injected into lethally irradiated syngeneic WT Balb/C recipient mice. Our pilot BMT experiments demonstrated that RSK2 KO BM cells are comparable with WT BM cells in regard to retroviral infection efficiency, homing efficiency in the BMT recipient mice, and capacity to reconstitute the hematopoietic properties in lethally irradiated recipient mice (supplemental Figure 3).
Genetic deficiency of RSK2 does not affect BCR-ABL induced myeloproliferative neoplasm in a murine BMT assay. (A) Immunoblotting shows protein expression of RSK1 and RSK2 in BM cells from Balb/C WT and RSK2-deficient (RSK2 KO) mice. (B) Kaplan-Meier survival plot of mice receiving either WT or RSK2 KO BM cells retrovirally transduced by BCR-ABL. All BMT mice in the WT group (n = 8) and RSK2 KO group (n = 10) developed an aggressive and rapidly fatal myeloproliferative neoplasm with a comparable latency. The statistical significance for survival was assessed by log-rank. (C) Analyses of mice transplanted with either WT or RSK2 KO BM cells expressing BCR-ABL. (D) Flow cytometric analysis demonstrates an expansion of Gr-1/Mac-1 double-positive mature myeloid cells in spleen (left) and BM (right) consistent with a myeloproliferative neoplasm in representative BMT mice receiving either WT or RSK2 KO BM cells expressing BCR-ABL, with comparable percentages that are supportive of similar disease burdens. (E) Tissue sections of spleen (left), BM (middle), and liver (right) demonstrate evidence of a marked myeloproliferative neoplasm with an expansion of maturing myeloid cells observed in representative BMT mice receiving either WT or RSK2 KO BM cells expressing BCR-ABL. Magnifications are as indicated (H&E).
Genetic deficiency of RSK2 does not affect BCR-ABL induced myeloproliferative neoplasm in a murine BMT assay. (A) Immunoblotting shows protein expression of RSK1 and RSK2 in BM cells from Balb/C WT and RSK2-deficient (RSK2 KO) mice. (B) Kaplan-Meier survival plot of mice receiving either WT or RSK2 KO BM cells retrovirally transduced by BCR-ABL. All BMT mice in the WT group (n = 8) and RSK2 KO group (n = 10) developed an aggressive and rapidly fatal myeloproliferative neoplasm with a comparable latency. The statistical significance for survival was assessed by log-rank. (C) Analyses of mice transplanted with either WT or RSK2 KO BM cells expressing BCR-ABL. (D) Flow cytometric analysis demonstrates an expansion of Gr-1/Mac-1 double-positive mature myeloid cells in spleen (left) and BM (right) consistent with a myeloproliferative neoplasm in representative BMT mice receiving either WT or RSK2 KO BM cells expressing BCR-ABL, with comparable percentages that are supportive of similar disease burdens. (E) Tissue sections of spleen (left), BM (middle), and liver (right) demonstrate evidence of a marked myeloproliferative neoplasm with an expansion of maturing myeloid cells observed in representative BMT mice receiving either WT or RSK2 KO BM cells expressing BCR-ABL. Magnifications are as indicated (H&E).
As shown in Figure 2B, mice receiving either WT or RSK2 KO BM cells transduced by BCR-ABL developed a fatal myeloproliferative neoplasm with no significant difference in survival latency (P = .388). The disease phenotype in both WT and RSK2 KO BMT mice was similarly characterized by marked splenomegaly and a peripheral blood leukocytosis comprised predominantly of mature granulocytes, with no significant difference in white blood counts (WBC) and spleen weights (Figure 2C). These results indicate a comparable myeloproliferative neoplasm state in both WT and RSK2 KO BMT mice. This notion was further confirmed by flow cytometric analysis, which showed similar numbers of mature neutrophils that were positive for late myeloid markers Gr-1 and Mac-1 in spleen and BM samples of representative mice transplanted with BCR-ABL–transformed RSK2 KO BM cells, compared with BCR-ABL–expressing WT BM-transplanted animals (Figure 2D).
In consonance with these observations, histopathologic examination of tissue samples from representative BCR-ABL–expressing WT BM-transplanted mice demonstrated a perturbation of normal splenic architecture with loss of white pulp and expansion of red pulp by a prominent population of maturing myeloid forms, markedly hypercellular BM with a predominance of mature myeloid forms and frequent number of admixed histiocytes and macrophages, and extensive myeloid cell infiltration in the liver (Figure 2E). Similar histologic evidence of myeloproliferation was also evident in the spleen, BM, and liver from representative BCR-ABL–expressing RSK2 KO BM-transplanted mice with a comparable extent and degree of a myeloproliferative neoplasm. These data together suggest that RSK2 is dispensable for BCR-ABL–induced myeloproliferative neoplasm in mice.
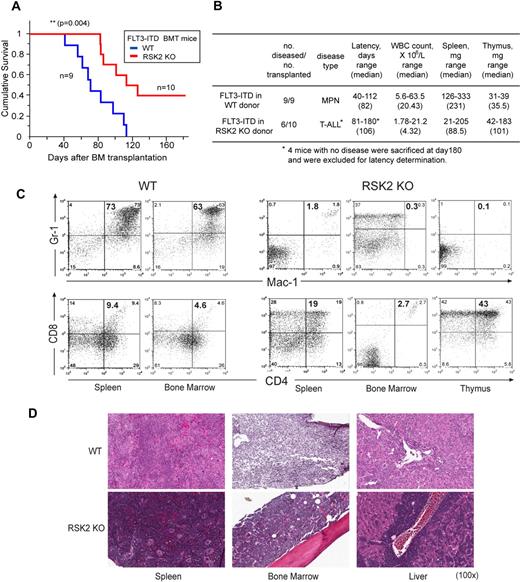
FLT3-ITD induces a T-ALL in BMT mice receiving RSK2 KO BM cells, phenotypically distinct from the myeloproliferative neoplasm induced by FLT3-ITD using WT BM cells
Next we sought to explore the role of RSK2 in FLT3-ITD–induced hematopoietic transformation in vivo. As shown in Figure 3A, mice transplanted with FLT3-ITD expressing WT BM cells from Balb/C mice developed a myeloproliferative neoplasm with a median latency of 82 days. In contrast, mice transplanted with RSK2 KO BM cells expressing FLT3-ITD showed a significant delay in disease latency (median latency = 106 days; Figure 3A). Moreover, 6 of 10 mice in this group developed T-ALL characterized by thymic enlargement with a much longer latency, while the remaining 4 mice in this group did not develop any discernable disease by the experimental end point (180 days). Those mice with T-ALL had significantly lower WBC and spleen weight compared with BMT mice receiving WT BM cells expressing FLT3-ITD (Figure 3B).
FLT3-ITD induced T-ALL in BMT mice using RSK2 KO BM cells, phenotypically distinct from the myeloproliferative neoplasm induced by FLT3-ITD using WT BM cells. (A) Kaplan-Meier survival plot of mice receiving either WT or RSK2 KO BM cells retrovirally transduced by FLT3-ITD. All BMT mice in the WT group (n = 9) developed an aggressive and fatal myeloproliferative neoplasm. In contrast, 6 of 10 mice in the group receiving RSK2 KO BM cells transformed by FLT3-ITD developed T-ALL characterized by thymic enlargement, with a significantly longer latency, while 4 mice in this group did not develop any discernable disease by the experimental end point (180 day). The statistical significance for survival was assessed by log-rank. (B) Analyses of mice transplanted with either WT or RSK2 KO BM cells expressing FLT3-ITD. (C) Flow cytometric analysis demonstrates an expansion of Gr-1/Mac-1 double-positive mature myeloid cells in spleen and BM consistent with a myeloproliferative neoplasm in a representative BMT mouse receiving WT BM cells expressing FLT3-ITD (top left), whereas such myeloid expansion was absent in the representative FLT3-ITD mouse receiving RSK2 KO BM cells (top right). Instead, an expansion of CD4/CD8 double-positive T cells in spleen and thymus consistent with T-ALL was detected in the representative FLT3-ITD BMT mouse receiving RSK2 KO BM cells (bottom right), compared with the FLT3-ITD mouse receiving WT BM cells (bottom left). (D) Tissue sections of spleen, BM, and liver demonstrate evidence of a marked myeloproliferative neoplasm with an expansion of maturing myeloid cells observed in the representative FLT3-ITD BMT mouse receiving WT cells, and a T-ALL disease in the FLT3 mouse receiving RSK2 KO BM cells. Magnifications are as indicated (H&E).
FLT3-ITD induced T-ALL in BMT mice using RSK2 KO BM cells, phenotypically distinct from the myeloproliferative neoplasm induced by FLT3-ITD using WT BM cells. (A) Kaplan-Meier survival plot of mice receiving either WT or RSK2 KO BM cells retrovirally transduced by FLT3-ITD. All BMT mice in the WT group (n = 9) developed an aggressive and fatal myeloproliferative neoplasm. In contrast, 6 of 10 mice in the group receiving RSK2 KO BM cells transformed by FLT3-ITD developed T-ALL characterized by thymic enlargement, with a significantly longer latency, while 4 mice in this group did not develop any discernable disease by the experimental end point (180 day). The statistical significance for survival was assessed by log-rank. (B) Analyses of mice transplanted with either WT or RSK2 KO BM cells expressing FLT3-ITD. (C) Flow cytometric analysis demonstrates an expansion of Gr-1/Mac-1 double-positive mature myeloid cells in spleen and BM consistent with a myeloproliferative neoplasm in a representative BMT mouse receiving WT BM cells expressing FLT3-ITD (top left), whereas such myeloid expansion was absent in the representative FLT3-ITD mouse receiving RSK2 KO BM cells (top right). Instead, an expansion of CD4/CD8 double-positive T cells in spleen and thymus consistent with T-ALL was detected in the representative FLT3-ITD BMT mouse receiving RSK2 KO BM cells (bottom right), compared with the FLT3-ITD mouse receiving WT BM cells (bottom left). (D) Tissue sections of spleen, BM, and liver demonstrate evidence of a marked myeloproliferative neoplasm with an expansion of maturing myeloid cells observed in the representative FLT3-ITD BMT mouse receiving WT cells, and a T-ALL disease in the FLT3 mouse receiving RSK2 KO BM cells. Magnifications are as indicated (H&E).
Flow cytometry–based immunophenotypical analysis confirmed that the majority of leukemic cells in the spleen and BM of representative FLT3-ITD WT BMT mice were Gr-1/Mac-1 double-positive neutrophils, while such myeloid expansion was absent from the spleen and BM of FLT3-ITD RSK2 KO BMT mice (Figure 3C top panels). Instead, CD4/CD8 double-positive T-cell expansion in spleen and thymus consistent with a T-ALL disease was detected in the representative FLT3-ITD RSK2 KO BMT mice (Figure 3C bottom panels).
Histopathologic examination of spleen, BM, and liver tissue samples from FLT3-ITD WT BMT mice demonstrated evidence of a marked myeloproliferative neoplasm with an expansion of maturing myeloid cells, characterized by effacement of splenic architecture with expansion of red pulp by a prominent population of maturing myeloid elements, hypercellularity composed primarily of mature myeloid elements in BM, and extramedullary hematopoiesis with predominantly maturing myeloid elements in liver (Figure 3D). In contrast, the tissue samples from mice receiving RSK2 KO BM cells transformed by FLT3-ITD revealed extensive cell infiltration in spleen, BM, and liver consistent with T-ALL (Figure 3D). This was essentially characterized by splenic red pulp expansion with infiltration of immature lymphoid blasts with some admixed maturing erythroid elements, marked hypercellularity in BM with prominent infiltration of immature lymphoid blasts, infiltration of immature lymphoid blasts in liver, and effacement of normal thymic architecture by diffuse infiltration of immature lymphoid blasts.
Thus, these data together suggest that RSK2 is required for FLT3-ITD–induced myeloproliferative neoplasm in mice
Targeting RSK2 by a small molecule RSK inhibitor fmk attenuates cell viability and induces apoptosis in FLT3-ITD–, but not BCR-ABL–expressing, murine Ba/F3 cells and human leukemia cells
Next we tested whether RSK2 is a critical signaling effector in FLT3-ITD but not BCR-ABL-mediated hematopoietic transformation. We used a specific RSK inhibitor, fmk, which is a fluoromethylketone molecule that was designed to specifically exploit 2 selectivity filters of RSK and has been shown to potently inactivate the CTD autokinase activity of RSK.31 We found that fmk treatment effectively inhibited RSK2 activation, as assessed by phosphorylation levels of RSK2 S386, in Ba/F3 cells stably expressing BCR-ABL or FLT3-ITD (Figure 4A), as well as in human leukemia cells including BCR-ABL–expressing K562 cells and FLT3-expressing Molm14 cells and Mv(4;11) cells (Figure 4B). We then performed a dose-response analysis of Ba/F3 cells stably expressing BCR-ABL or FLT3-ITD (Figure 4C). Ba/F3 cells depend on IL-3 to proliferate, while expression of active, leukemogenic tyrosine kinases such as BCR-ABL and FLT3-ITD confers IL-3–independent proliferation to Ba/F3 cells. Treatment of fmk did not affect cell viability (Figure 4C left) or induce apoptotic cell death (Figure 4C right) in control parental Ba/F3 cells, Ba/F3 cells with FLT3-ITD in the presence of IL-3, or cells transformed by BCR-ABL in the absence of IL-3. In contrast, fmk treatment effectively inhibited growth of the FLT3-ITD–expressing Ba/F3 cells in the absence of IL-3 with a cellular IC50 of 4.19μM, and also induced apoptosis in a dose-dependent manner (Figure 4C, left and right, respectively).
Targeting RSK2 by fmk attenuates cell viability and induces apoptosis in FLT3-ITD– but not BCR-ABL–expressing Ba/F3 cells and human leukemia cells. (A) Left: Immunoblotting results show stable expression of BCR-ABL and FLT3-ITD in Ba/F3 cells. Right: A total of 3μM Fmk treatment inhibits RSK2 in Ba/F3-expressing BCR-ABL or FLT3-ITD. (B) A total of 3μM Fmk treatment inhibits RSK2 in BCR-ABL–positive K562 (top) and FLT3-ITD positive Molm14 and Mv(4;11) human leukemia cells (bottom). (C) Treatment with increasing concentrations of fmk resulted in decreased cell viability (left) and increased apoptosis (right) in FLT3-ITD–positive Ba/F3 cells in the absence of IL3 (-IL3), but not in cells expressing BCR-ABL (-IL3), control Ba/F3 cells cultured in the presence of IL3 (+IL3), or FLT3-ITD–positive cells in the presence of IL3 (+IL3). (D-F) Targeting RSK2 by fmk does not affect cell viability nor induce apoptosis in K562 cells expressing BCR-ABL (D), but results in decreased cell viability and increased apoptosis in FLT3-ITD–expressing Molm14 (E) and Mv(4;11) cells (F). (G-H) RNAi-mediated targeted down-regulation of RSK2 (G) significantly induced apoptosis (H; left) and attenuated cell viability (H; right) in FLT3-ITD–expressing Molm14 and Mv(4;11) human leukemia cell lines, but not in K562 cells expressing BCR-ABL. Cells were transiently infected with lentivirus harboring an empty pLKO.1 vector or shRNA specific to RSK2 for 24 hours. The apoptotic population was characterized as the fraction of annexin V–positive cells of total treated cells. The relative cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide–based assay. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.
Targeting RSK2 by fmk attenuates cell viability and induces apoptosis in FLT3-ITD– but not BCR-ABL–expressing Ba/F3 cells and human leukemia cells. (A) Left: Immunoblotting results show stable expression of BCR-ABL and FLT3-ITD in Ba/F3 cells. Right: A total of 3μM Fmk treatment inhibits RSK2 in Ba/F3-expressing BCR-ABL or FLT3-ITD. (B) A total of 3μM Fmk treatment inhibits RSK2 in BCR-ABL–positive K562 (top) and FLT3-ITD positive Molm14 and Mv(4;11) human leukemia cells (bottom). (C) Treatment with increasing concentrations of fmk resulted in decreased cell viability (left) and increased apoptosis (right) in FLT3-ITD–positive Ba/F3 cells in the absence of IL3 (-IL3), but not in cells expressing BCR-ABL (-IL3), control Ba/F3 cells cultured in the presence of IL3 (+IL3), or FLT3-ITD–positive cells in the presence of IL3 (+IL3). (D-F) Targeting RSK2 by fmk does not affect cell viability nor induce apoptosis in K562 cells expressing BCR-ABL (D), but results in decreased cell viability and increased apoptosis in FLT3-ITD–expressing Molm14 (E) and Mv(4;11) cells (F). (G-H) RNAi-mediated targeted down-regulation of RSK2 (G) significantly induced apoptosis (H; left) and attenuated cell viability (H; right) in FLT3-ITD–expressing Molm14 and Mv(4;11) human leukemia cell lines, but not in K562 cells expressing BCR-ABL. Cells were transiently infected with lentivirus harboring an empty pLKO.1 vector or shRNA specific to RSK2 for 24 hours. The apoptotic population was characterized as the fraction of annexin V–positive cells of total treated cells. The relative cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide–based assay. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.
We next performed fmk treatment experiments using human leukemia cells expressing either BCR-ABL or FLT3-ITD. Consistent with the result of the BMT assays and fmk treatment experiments using Ba/F3 cells, inhibition of RSK2 by fmk treatment did not affect cell viability nor induce apoptosis in K562 cells (Figure 4D). In contrast, treatment with fmk inhibited RSK2 in FLT3-expressing human leukemia Molm14 cells and Mv(4;11) cells (Figure 4B), which resulted in decreased cell viability in Molm14 and Mv(4;11) cells with cellular IC50 values of 7.19μM and 12.94μM, respectively, and increased significant apoptosis in these cells (Figure 4E-F). Furthermore, as shown in Figure 4, panels G and H, and supplemental Figure 4, siRNA-mediated targeted down regulation of RSK2 (Figure 4G), but not RSK1 (supplemental Figure 4), significantly induced apoptosis (Figure 4H left) and attenuated cell viability (Figure 4H right) in FLT3-ITD expressing human leukemia Molm14 and Mv(4;11) cells, but not K562 cells expressing BCR-ABL. These data further support our hypothesis that RSK2 plays a critical role in cell proliferation and survival in FLT3-ITD– but not BCR-ABL–transformed leukemia cells.
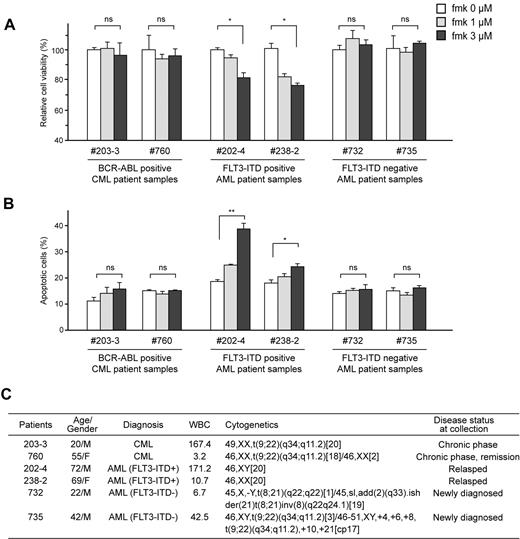
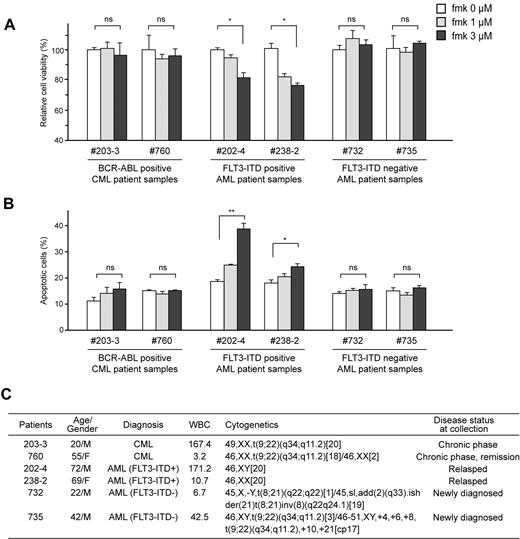
Fmk treatment induces significant apoptosis in FLT3-ITD– but not BCR-ABL–expressing primary human leukemia cells
Moreover, we observed that fmk significantly attenuated cell viability (Figure 5A) and induced apoptosis (Figure 5B) in primary FLT3-ITD–positive leukemia cells from AML patients, but not in primary BCR-ABL–expressing leukemia cells from 2 Ph+ leukemia patients, nor in primary leukemia cells from 2 FLT3-ITD–negative AML patients (Figure 5C). These data provide “proof of principle” that demonstrate the therapeutic potential of targeting RSK2 by fmk in FLT3-ITD–positive AML.
Fmk treatment induces significant apoptosis in FLT3-ITD– but not BCR-ABL–expressing primary human leukemia cells. Inhibition of RSK2 by treatment with fmk does not affect cell viability nor induce apoptosis (left, A and B, respectively) in primary BCR-ABL–positive leukemia cells from 2 CML patients, and primary FLT3-ITD-negative leukemia cells from 2 AML patients (right, A and B, respectively), whereas fmk treatment results in decreased cell viability and increased apoptosis in primary FLT3-ITD–positive leukemia cells from 2 AML patients. Apoptotic population was determined as the percentage of annexin V–positive cells of total treated cells. (C) Clinical information for the leukemia patients. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.
Fmk treatment induces significant apoptosis in FLT3-ITD– but not BCR-ABL–expressing primary human leukemia cells. Inhibition of RSK2 by treatment with fmk does not affect cell viability nor induce apoptosis (left, A and B, respectively) in primary BCR-ABL–positive leukemia cells from 2 CML patients, and primary FLT3-ITD-negative leukemia cells from 2 AML patients (right, A and B, respectively), whereas fmk treatment results in decreased cell viability and increased apoptosis in primary FLT3-ITD–positive leukemia cells from 2 AML patients. Apoptotic population was determined as the percentage of annexin V–positive cells of total treated cells. (C) Clinical information for the leukemia patients. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.
Discussion
Our data suggest that although both BCR-ABL and FLT3-ITD activate RSK2 in leukemia cells, RSK2 is differentially required for hematopoietic transformation induced by BCR-ABL and FLT3-ITD. Genetic deficiency of RSK2 did not affect the pathogenesis or progression of BCR-ABL–induced myeloproliferative neoplasm in a murine BMT model, and inhibition of RSK2 by small molecule inhibitor fmk did not effectively attenuate cell proliferation or induce apoptosis in murine Ba/F3 cells and human leukemia K562 cells expressing BCR-ABL, or in primary leukemia cells from BCR-ABL–positive CML patients. In contrast, FLT3-ITD failed to induce myeloproliferative neoplasm in BMT mice receiving RSK2 KO BM cells, and instead induced T-ALL. In consonance with these results, targeting RSK2 by fmk resulted in decreased cell viability and increased apoptosis in FLT3-ITD–positive Ba/F3 cells and human leukemia Molm14 and Mv(4;11) cells, as well as in primary leukemia cells from FLT3-ITD–positive AML patients. These findings suggest that RSK2 is dispensable for BCR-ABL–induced hematopoietic transformation, but is likely required for pathogenesis and myeloid lineage determination in FLT3-ITD–induced hematopoietic transformation, and that RSK2 plays a key role in cell proliferation and survival of FLT3-ITD–transformed myeloid cells.
Moreover, we reported previously that in a murine BMT assay, RSK2 plays an important role in leukemogenic TEL-FGFR3–induced myeloproliferative neoplasm.9 TEL-FGFR3 is a constitutively activated fusion tyrosine kinase that is associated with t(4;12)(p16;p13) AML.43 Genetic deficiency of RSK2 resulted in a significantly delayed and attenuated myeloproliferative syndrome induced by TEL-FGFR3 compared with wild type cells, suggesting that RSK2 may be required in TEL-FGFR3–induced pathogenesis and disease progression, but not lineage determination in hematopoietic transformation. Together these findings suggest that, although all 3 leukemogenic tyrosine kinases are involved in hematopoietic transformation and induce a similar myeloproliferative neoplasm characterized by expansion of Gr-1+/Mac-1+ neutrophils in a murine BMT assay, the role of RSK2 in hematopoietic transformation might depend on discrete upstream oncogenic signals mediated by different leukemogenic tyrosine kinases.
Previous studies reported that RSK2 KO mice have decreased bone mass because of the critical role of RSK2 in osteoblast differentiation.16,44 However, RSK2 KO mice have a normal lifespan and no histologic or metabolic evidence of internal organ dysfunction.16,35,44 Lin et al demonstrated that RSK2 is dispensable for normal lymphoid development and RSK2 KO animals had normal numbers of Gr-1+ and Mac-1+ cells in the spleen.35 Therefore, the change in disease phenotype and attenuation of disease burden seen in FLT3-ITD– and TEL-FGFR3–induced disease using RSK2-deficient BM cells in BMT mice, respectively, is most likely because of impairment of RSK2-mediated signal transduction rather than abnormalities in the target cell populations. It is possible that, although RSK2 may not be required for normal myelopoiesis, these 3 leukemogenic tyrosine kinases activate distinct signaling pathways to induce hematopoietic transformation. While BCR-ABL appears not to require RSK2 signaling, the role of RSK2 in FLT3-ITD–induced hematopoietic transformation is more critical and is likely associated with disease initiation, lineage determination and progression. In the case of TEL-FGFR3, RSK2 is more involved in the proliferation of TEL-FGFR3–transformed myeloid cells than the initiation of TEL-FGFR3–dependent myeloid transformation. Future studies to explore the differential involvement of RSK2 in FLT3-ITD– and TEL-FGFR3–dependent transformation of hematopoietic stem cells and/or myeloid progenitors are warranted.
Our studies cumulatively indicate a differential requirement for RSK2 in FLT3-ITD–positive AML and T-ALL. It was previously reported that BMT mice receiving BM cells expressing FLT3-ITD developed myeloproliferative disease, whereas mice transplanted with BM cells transformed by FLT3-TKD mutants harboring mutations in tyrosine kinase domain (TKD) developed a lymphoid disorder.45 Interestingly, FLT3-TKD mutants have differential signaling properties compared with FLT3-ITD. FLT3-ITD does while FLT3-TKD does not activate STAT5 and inhibit protein expression of CCAAT/enhancer binding proteinα and PU.1.46,47 However, the activation levels of STAT5 and protein expression levels of C/EBPα and PU.1 are not affected by RSK2 knockdown in Molm14 cells expressing FLT3-ITD, or in Ba/F3 cells stably expressing FLT3-ITD (data not shown). These results suggest that the RSK2 pathway is involved in FLT3-ITD–induced hematopoietic transformation independent of STAT5, C/EBPα and PU.1, which warrants future studies to decipher the signaling mechanism underlying RSK2-mediated lineage-dependent transformation by FLT3-ITD.
In addition, it appears that the other RSK family members such as RSK1 that are expressed in the RSK2 KO BM cells could not functionally compensate the genetic deficiency of RSK2 to maintain myeloproliferative neoplastic initiation and development in BMT mice receiving FLT3-ITD–transformed RSK2 KO BM cells. Moreover, targeting RSK2 by fmk effectively induced apoptosis in FLT3-ITD–positive human leukemia cell lines and primary leukemia cells from AML patients. Thus, RSK2 may represent an alternative therapeutic target in treatment of FLT3-ITD–positive AML, but not T-ALL. We recently demonstrated that fmk induced significant apoptosis in human t(4;14) primary myeloma cells from a multiple myeloma patient, but not in primary myeloma cells from a t(4;14)–negative myeloma patient.7 These data demonstrate that RSK inhibitors such as fmk may have minimal nonspecific cytotoxicity in human cells. In consonance with this finding, Dumont et al demonstrated that the triple knockout of RSK1, 2, and 3 does not affect mouse viability,48,49 further suggesting that targeting RSK may represent a promising therapeutic strategy to treat FLT3-ITD–positive AML without serious side effects in organism homeostasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the critical reading of the manuscript by Dr Jing Chen. We thank Susan Sunay (the Hematology Division Tissue Bank, Winship Cancer Institute of Emory) for providing primary tissue samples from leukemia patients. We also thank Drs Ross Levine (Memorial Sloan-Kettering Cancer Center) and Jan Cools (Center for Human Genetics, KU Leuven) for the Ba/F3-JAK2 V617F and Ba/F3-FIP1L1-PDGFRA stable cells, respectively.
This work was in part supported by American Cancer Society grant RSG-11-081-01 (S.K.), Leukemia & Lymphoma Society Career Development Program Special Fellow Award LLS 3302-09 (S.K.), National Institutes of Health (NIH)/National Cancer Institute (NCI) SPORE in Head and Neck Cancer (P50CA128613) Career Development Program Award (S.K.), and the Pharmacologic Sciences Training Grant T32 GM008602 (S.E.). J.T. acknowledges support from the NIH (GM071434).
S.E. is an NIH predoctoral fellow and an Achievement Rewards for College Scientists (ARCS) Foundation Scholar. S.K. is an American Cancer Society Basic Research Scholar, a Special Fellow of the Leukemia & Lymphoma Society, a Georgia Cancer Coalition Scholar, and a Robbins Scholar.
National Institutes of Health
Authorship
Contribution: S.E., D.B., L.J., T.-W.C., and S.K. designed research, performed research, analyzed data, and wrote the paper; I.R.W. performed immunophenotypical analysis; B.H.L. performed histopathologic analysis; and J.-X.L., W.J.L., J.T., and H.J.K. provided reagents and materials.
Conflict-of-interest disclosure: J.T. is as an inventor on a patent application filed by the University of California, which describes the RSK inhibitor, FMK. The remaining authors declare no competing financial interests.
Correspondence: Sumin Kang, PhD, Department of Hematology and Medical Oncology, Emory University School of Medicine, Winship Cancer Institute of Emory, 1365-C Clifton Rd NE, C3006, Atlanta, GA 30322; e-mail: smkang@emory.edu.


![Figure 4. Targeting RSK2 by fmk attenuates cell viability and induces apoptosis in FLT3-ITD– but not BCR-ABL–expressing Ba/F3 cells and human leukemia cells. (A) Left: Immunoblotting results show stable expression of BCR-ABL and FLT3-ITD in Ba/F3 cells. Right: A total of 3μM Fmk treatment inhibits RSK2 in Ba/F3-expressing BCR-ABL or FLT3-ITD. (B) A total of 3μM Fmk treatment inhibits RSK2 in BCR-ABL–positive K562 (top) and FLT3-ITD positive Molm14 and Mv(4;11) human leukemia cells (bottom). (C) Treatment with increasing concentrations of fmk resulted in decreased cell viability (left) and increased apoptosis (right) in FLT3-ITD–positive Ba/F3 cells in the absence of IL3 (-IL3), but not in cells expressing BCR-ABL (-IL3), control Ba/F3 cells cultured in the presence of IL3 (+IL3), or FLT3-ITD–positive cells in the presence of IL3 (+IL3). (D-F) Targeting RSK2 by fmk does not affect cell viability nor induce apoptosis in K562 cells expressing BCR-ABL (D), but results in decreased cell viability and increased apoptosis in FLT3-ITD–expressing Molm14 (E) and Mv(4;11) cells (F). (G-H) RNAi-mediated targeted down-regulation of RSK2 (G) significantly induced apoptosis (H; left) and attenuated cell viability (H; right) in FLT3-ITD–expressing Molm14 and Mv(4;11) human leukemia cell lines, but not in K562 cells expressing BCR-ABL. Cells were transiently infected with lentivirus harboring an empty pLKO.1 vector or shRNA specific to RSK2 for 24 hours. The apoptotic population was characterized as the fraction of annexin V–positive cells of total treated cells. The relative cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide–based assay. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/25/10.1182_blood-2010-10-315721/4/m_zh89991173200004.jpeg?Expires=1766339003&Signature=hxCVXfl6leTcz0NXR7yCLP32kAIMutpeub3QkMXFqyAda6EzE7YoV5GysNEx1RYkwGV74WeytViS8QF7qrLxunjJrMGCmAf67Ikyf8bS2DSXgxil6CLLXbfiMuAIMEG1U~oYUc~SD066KPAo2aXYorhUZpplmd2cU1ymrOp-xx1~W6pP~SDyFrJvfYP3zK5EuyCNyRCUKaqFuF-PI7M84hYoQil3aePXVtkmCF47LzuocTj-klpS9SzDtT~cc6FU2neY-cjsUuGStHGzFH8Psxniidde9ZFBfe4YJfJwFkrWf55AchpSzN2TuTp86XcgzTwx0lh36~kuJ9tj-9ETjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. Targeting RSK2 by fmk attenuates cell viability and induces apoptosis in FLT3-ITD– but not BCR-ABL–expressing Ba/F3 cells and human leukemia cells. (A) Left: Immunoblotting results show stable expression of BCR-ABL and FLT3-ITD in Ba/F3 cells. Right: A total of 3μM Fmk treatment inhibits RSK2 in Ba/F3-expressing BCR-ABL or FLT3-ITD. (B) A total of 3μM Fmk treatment inhibits RSK2 in BCR-ABL–positive K562 (top) and FLT3-ITD positive Molm14 and Mv(4;11) human leukemia cells (bottom). (C) Treatment with increasing concentrations of fmk resulted in decreased cell viability (left) and increased apoptosis (right) in FLT3-ITD–positive Ba/F3 cells in the absence of IL3 (-IL3), but not in cells expressing BCR-ABL (-IL3), control Ba/F3 cells cultured in the presence of IL3 (+IL3), or FLT3-ITD–positive cells in the presence of IL3 (+IL3). (D-F) Targeting RSK2 by fmk does not affect cell viability nor induce apoptosis in K562 cells expressing BCR-ABL (D), but results in decreased cell viability and increased apoptosis in FLT3-ITD–expressing Molm14 (E) and Mv(4;11) cells (F). (G-H) RNAi-mediated targeted down-regulation of RSK2 (G) significantly induced apoptosis (H; left) and attenuated cell viability (H; right) in FLT3-ITD–expressing Molm14 and Mv(4;11) human leukemia cell lines, but not in K562 cells expressing BCR-ABL. Cells were transiently infected with lentivirus harboring an empty pLKO.1 vector or shRNA specific to RSK2 for 24 hours. The apoptotic population was characterized as the fraction of annexin V–positive cells of total treated cells. The relative cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide–based assay. P value was determined by 2-tailed Student t test. ns indicates not significant.* P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/25/10.1182_blood-2010-10-315721/4/m_zh89991173200004.jpeg?Expires=1766394113&Signature=yxDRue62paWB1awk7LSARFvn9D0PxKPtfdySrj4zC7e23tZHMAO9Z6WMsV-7LO6HjiQEWkg6ehRh1cCxXB4AzGGAmmm0Y6FE2Q1DMNlwxL2aOfO1YUODyHsnH24Xz2UeJZG5Ye9SdDqsF0JpKrR~vuSxYhk000SDgn8vLSS6KSti25bRr8Ti2ysdT6vlZ~BTtLb1PaW5l6daK8n29xdQ233AUX9qHD9FnrnKhE81frhXRUIox~3IxpSz57QMSKnWeZfexqmIknjTEWB9K6Zas7~sogzbbGZF7UPq6OfxSqUMGvLipGbHkaR3dZvAxBLfby~eNVPFMPFnoPABzwFFrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)