Abstract
Natural killer (NK) cells mediate GVL effects after allogeneic hematopoietic cell transplantation (allo-HCT) by the production of inflammatory cytokines and by direct target lysis. The acquisition of both functions was presumed to be developmentally linked, but this linkage remained unstudied after allo-HCT. We tested the cytokine production and degranulation of reconstituting NK cells after adult unrelated donor or umbilical cord blood grafting. Recipients of T cell–depleted transplants, receiving no immune suppression, showed diminished NK cell degranulation. In contrast, degranulation was normal or increased after T-cell replete transplants given with immune suppression. Strikingly, target cell-induced IFNγ production was markedly diminished in all transplant settings, especially with T cell–depleted or naive T cell–containing umbilical cord blood grafts, suggesting a role for T cells in NK education. Although degranulation was similar in the KIR+ and KIR− populations that coexpressed NKG2A, target cell-induced IFNγ production was limited to the subset of NK cells expressing KIR inhibited by self-ligands. Thus, cytokine production and cytotoxic function do not consistently coexist in NK cells reconstituting after allo-HCT. Exposure to IL-15 rapidly increased target-inducible IFNγ production, indicative of IL-15's potential as a therapeutic tool to enhance NK cell function to protect against infection and relapse after allo-HCT.
Introduction
Natural killer (NK) cells are innate immune effectors that directly lyse virally infected or malignant cells. They also release cytokines (IFNγ and GM-CSF) and chemokines (MIP-1α, MIP-1β, IL-8, and RANTES) that modulate the adaptive immune system and hematopoiesis. NK cells directly activate antigen-presenting cells, which provide reciprocal activation of NK cells. As the first donor-derived lymphocyte subset to reconstitute after hematopoietic cell transplantation (HCT), NK cells may play a pivotal role in the GVL effect, especially in myeloid leukemia.1,2 However, it is not known which function (killing or cytokine production) is physiologically most important to mediate clinical responses, or whether these functions recover with different kinetics early after transplantation.
NK cells express a variety of surface receptors that either positively or negatively modulate their function. NK cell activation is determined by the net balance of both inhibitory and activating signals it receives through these surface receptors.3-5 The inhibitory receptor families include the killer cell immunoglobulin-like receptors (KIRs) that recognize allelic epitopes present on the classic class I human leukocyte antigen (HLA) molecules HLA-A, HLA-B, and HLA-C; and CD94/NKG2A that recognizes the nonclassic class I HLA molecule HLA-E.6,7 Interaction with target cells that lack “self” HLA molecules to signal via inhibitory receptors results in NK cell activation.8 Activating signals, which can potentially override inhibitory signaling, are mediated by receptor families such as activating KIR, CD94/NKG2C, and NKG2D; the natural cytotoxicity receptors NKp30, NKp44, and NKp46; and CD16 and CD244.4
The clinical application of NK cell–mediated therapy has focused on the role of the inhibitory KIR family and on ways to increase the frequency of alloreactive NK cells after HCT. In the setting of a potently T cell–depleted haploidentical HCT, grafts from donors with NK cells expressing KIR that are not inhibited by recipient HLA ligands are associated with decreased relapse and prolonged survival.1 In addition, non-T cell–depleted grafts from adult unrelated donors (URDs) with favorable KIR genotypes can confer similar beneficial clinical effects, with less relapse and increased survival,9 supporting the importance of NK cells in mediating outcome of HCT.
The acquisition of both cytokine-producing and cytotoxic functions occurs during NK cell development through a process commonly referred to as licensing or NK cell education.10,11 Although the exact timing and location of NK cell education is unknown, it is generally believed that NK cells acquire function after engagement of inhibitory receptors with self-ligand after their differentiation from hematopoietic progenitors.10,12 NK cells lacking inhibitory receptors for self do exist, but they remain hyporesponsive and are considered “uneducated.”12-14 In the early stages of the NK cell developmental pathway, stage III cells, which are defined in part by the absence of MHC-specific receptors, lack both cytotoxicity and cytokine production. On acquisition of the CD94/NKG2A heterodimer, stage III cells transition to stage IV, or CD56bright NK cells, at which time they acquire the capacity to produce IFNγ after stimulation with IL-12, IL-15, and IL-18.15 Still, they display low cytotoxic potential.16 Only on further development and emigration from the lymph node to the periphery do NK cells acquire CD16 and KIR and become highly cytotoxic.17
Allo-HCT provides a unique environment in which to study human NK cell education because it recapitulates NK cell development from hematopoietic stem cells in a short interval. It is unknown whether the acquisition of the cytotoxic and cytokine-producing functions occurs in parallel through interactions with inhibitory receptors or whether distinct signals are required to generate each effector function. To understand the acquisition of NK cell function early after allo-HCT, we developed a 9-color flow cytometric-based assay to simultaneously measure both degranulation by CD107a expression (as a surrogate marker for cytotoxicity) and IFNγ production in NK cell subsets with or without inhibitory receptor expression for self-class I HLA.
Methods
Patients and samples
We analyzed peripheral blood mononuclear cells (PBMCs) from 46 donor and recipient pairs from allogeneic URD transplants facilitated by the National Marrow Donor Program. Thirty-two patients with hematologic malignancies received adult donor HLA-matched unmanipulated (T-cell replete) bone marrow or peripheral blood stem cells (enrolled in the Blood and Marrow Transplant Clinical Trials Network Protocol 0201) using standard cyclosporine or tacrolimus-based GVHD prophylaxis (https://web.emmes.com/study/bmt2/protocol/0201_protocol/0201_protocol.html).
Fourteen acute myelogenous leukemia patients received adult donor HLA-partially matched T cell–depleted (CD34+-selected) grafts with no posttransplant immunosuppression. Additional samples from 10 patients who received double unit umbilical cord blood (UCB) transplants using cyclosporine and mycophenolate mofetil GVHD prophylaxis were analyzed.18 We collected pre-HCT donor samples and recipient samples at 1 month (T-cell replete and UCB only), 3 months (± 10 days), and 6 months (± 10 days) after HCT. Samples were obtained after informed consent in accordance with the Declaration of Helsinki and approval from the National Marrow Donor Program and University of Minnesota Institutional Review Boards. High-resolution HLA typing was performed and NK ligand status was assigned to Bw4, HLA-C1, and HLA-C2 group ligands.
PBMCs were isolated from each sample by density centrifugation and cryopreserved. Before analysis with the CD107a and IFNγ assay, the thawed cells were incubated overnight at 37°C in complete media without exogenous cytokines (DMEM; Cellgro; Mediatech) supplemented with 20% human AB serum (Valley Biomedical), 30% Ham's F-12 medium (Cellgro Mediatech), 100 U/mL penicillin (Invitrogen), 100 U/mL streptomycin (Invitrogen), 24μM 2-β-mercaptoethanol, 50μM ethanolamine, 20 mg/L ascorbic acid, and 50 μg/L sodium selenate.
Target cells and cytokine stimulation
The human erythroleukemia cell line K562 was maintained in Iscove modified Dulbecco medium (Cellgro Mediatech) supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin (all from Invitrogen). For cytokine stimulation, recombinant human IL-12 (10 ng/mL) and recombinant human IL-18 (100 ng/mL) both from R&D Systems, was added to 0.5 to 1 × 106 PBMCs and incubated overnight before being used in the CD107a and IFNγ assay. For low-dose IL-15 stimulation, 1 × 106/mL PBMCs were incubated overnight with 1 ng/mL recombinant human IL-15 (R&D Systems).
CD107a and IFNγ assay
PBMCs (4 × 106/mL) were mixed with K562 cells at a ratio of 2:1 in a final volume of 200 μL in V-bottomed 96-well plates in the absence of exogenous cytokines. Anti-CD107a-PerCP-Cy5.5 (clone H4A3; BioLegend) was added to each well and incubated for 1 hour at 37°C and 5% CO2. After 1 hour, brefeldin A (Golgi Plug; BD Biosciences) and monensin (Golgi Stop; BD Biosciences) were added to each well, and the cells were incubated for an additional 3 hours. Although CD107a degranulation can be detected after 2 hours, we selected a 4-hour time point readout for this assay to optimally detect simultaneous IFNγ production, which takes longer to develop. Cells were then centrifuged and resuspended in staining buffer (PBS supplemented with 0.5% human AB serum and 2mM EDTA) containing biotin-conjugated anti-CD158e (clone DX9; BioLegend). After 30-minute incubation at 4°C, cells were washed and resuspended in staining buffer containing anti-CD45–Qdot 605 (clone HI130; Invitrogen), anti-CD3-ECD (clone UCHT1; Beckman Coulter), anti-CD56-PeCy7 (NCAM16.2; BD Biosciences), anti-CD158a/h-PE (clone EB6B; Beckman Coulter), anti-CD158b/j-FITC (clone CH-L; BD Biosciences), anti-CD159a-APC (clone z199; Beckman Coulter), and streptavidin-conjugated APC–Alexa Fluor 750 (Invitrogen). After 30-minute incubation at 4°C, cells were washed and fixed in PBS containing 2% paraformaldehyde and then permeabilized and stained intracellularly with anti–IFNγ-Pacific Blue (clone 4S.B3; BioLegend). Finally, cells were washed and analyzed on an LSRII 11-color flow cytometer (BD Biosciences). Data were analyzed with FlowJo 8.8.6 software (TreeStar). All aggregate data are shown for the target-induced or cytokine-stimulated conditions after subtracting the baseline level of CD107a or IFNγ expression by unstimulated NK cells. The analysis excluded samples with < 100 total NK cell events or data from NK subsets with < 25 events.
Statistical analysis
For comparisons between independent groups, the Wilcoxon rank sum test was used. For comparisons of matched samples, the Wilcoxon signed rank test was used unless otherwise indicated in the figure legend. For comparisons of multiple groups where multiple measurements were taken on the same sample, a linear mixed model was used, assuming an exchangeable correlation structure for the correlated measurement errors among repeated measures. Linear correlation was analyzed using the Pearson correlation coefficient. Variables are summarized with mean and standard error (mean ± SEM). Statistical significance was indicated as *P < .05, **P < .01, ***P < .0001, or ns for P ≥ .05. Statistical analyses were performed using SAS Version 9.2 (SAS Institute).
Results
Target cell–induced IFNγ production is decreased after allogeneic transplantation
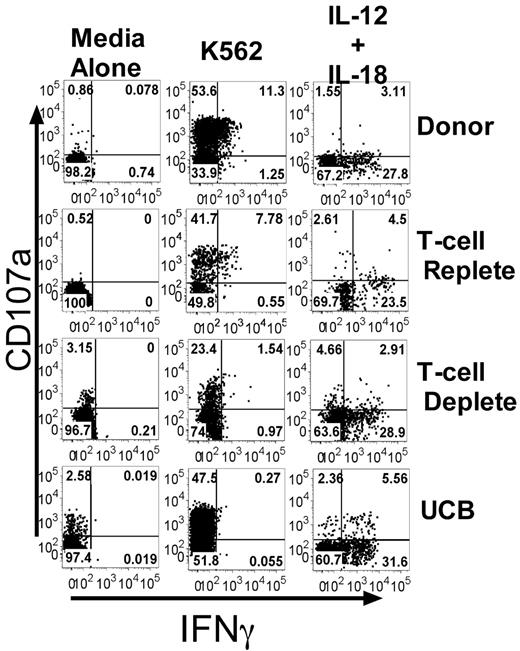
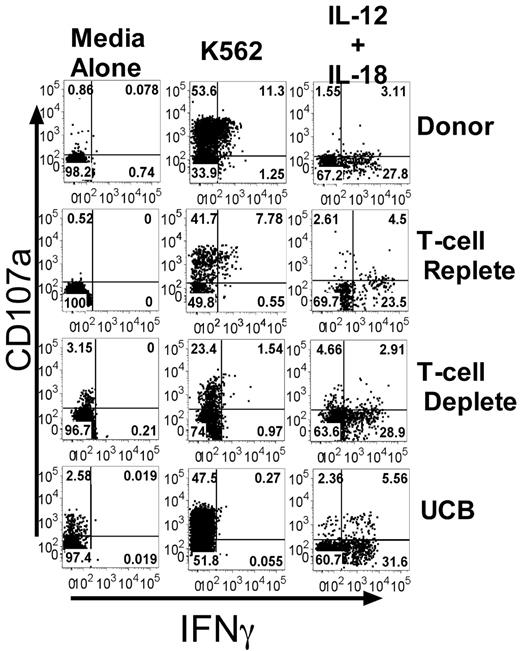
To assess NK cell function, we developed a novel assay to simultaneously evaluate degranulation of CD56+CD3− NK cells (using CD107a expression as a surrogate marker for cytotoxicity19 ) and cytokine production (intracellular IFNγ expression). Healthy donor samples were compared with samples from patients receiving adult URD unmanipulated (T-cell replete), adult URD T cell–depleted (T-cell deplete), or UCB grafts. These dual NK cell functions were tested after incubation with or without HLA class-I deficient K562 target cells in the absence of exogenous cytokines, or after cytokine stimulation with IL-12 and IL-18 (Figure 1). After target cell stimulation the percentage of NK cells expressing CD107a was always greater than the percentage producing IFNγ. Essentially all IFNγ-producing NK cells degranulated, but a large proportion of CD107a-expressing cells did not produce IFNγ. We detected a low frequency of NK cells in normal donors that produced IFNγ but did not express CD107a (0.8% ± 0.3%), as has been reported previously.12 Stimulation with IL-12 and IL-18, known potent inducers of IFNγ production,15 did not trigger degranulation.
Target cell–induced IFNγ production, but not CD107a expression, is decreased after allogeneic transplant. PBMCs from healthy donors and recipients 3 months after T-cell replete, T-cell deplete, and UCB HCT were incubated with media alone, K562 cells, or IL-12/IL-18. CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. Representative FACS plots are shown. The numbers in each quadrant are the percentages based on total CD56+CD3− NK cells.
Target cell–induced IFNγ production, but not CD107a expression, is decreased after allogeneic transplant. PBMCs from healthy donors and recipients 3 months after T-cell replete, T-cell deplete, and UCB HCT were incubated with media alone, K562 cells, or IL-12/IL-18. CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. Representative FACS plots are shown. The numbers in each quadrant are the percentages based on total CD56+CD3− NK cells.
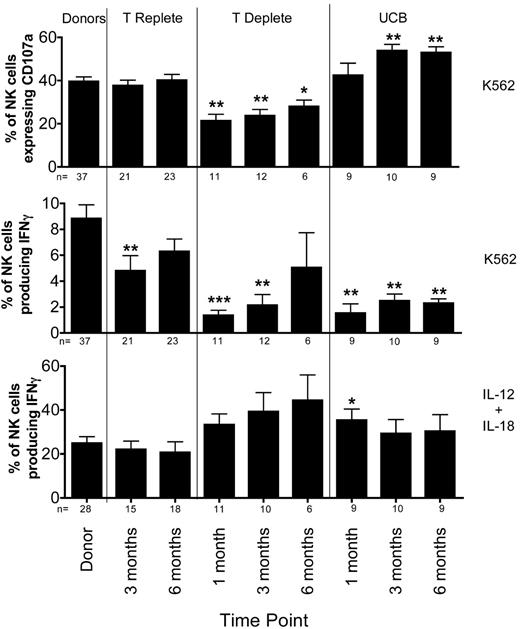
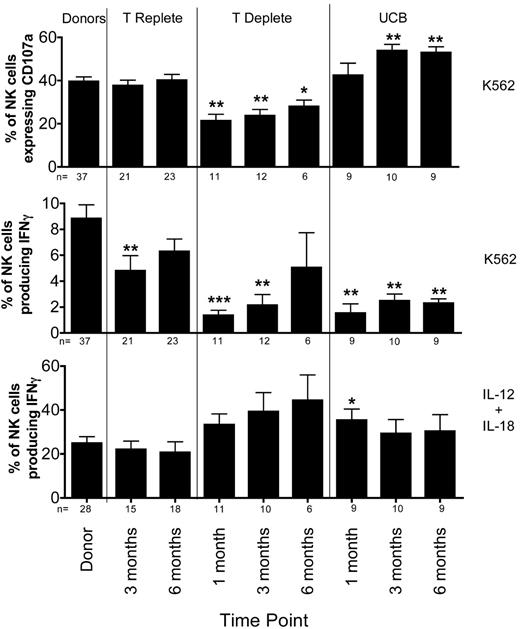
Surprisingly, K562-induced CD107a expression in reconstituting NK cells differed significantly between each type of HCT (Figure 2 top panel). There was no difference in CD107a expression between healthy donors and recipients of T-cell replete grafts who received post-HCT immune suppression. In contrast, CD107a expression was lower in NK cells from recipients of T cell–depleted grafts, who received no post-HCT immune suppression. Compared with healthy donors, the percentage of cells expressing CD107a was lower at 1 month (40% ± 2% vs. 21% ± 3%; P = .0006) and at 3 months (24% ± 3%; P = .0009) after HCT. By 6 months after T cell–deplete transplants, the CD107a expression had recovered slightly (28% ± 3%; P = .019). Although some have proposed that NK cell function may be more robust in the absence of T-cell competition for cytokines,20 our data suggest a T cell–dependent effect on the acquisition of degranulating capacity. Interestingly, compared with normal donors, the degranulation response to K562 targets was significantly higher at 3 months after UCB transplant (54% ± 3% vs 40% ± 2%; P = .004). This difference persisted at 6 months after HCT.
Target cell-induced IFNγ production is decreased after T-cell replete, T-cell deplete, and UCB transplantation. PBMCs from healthy donors and recipients at 1 (T-cell deplete and UCB only), 3, and 6 months post-HCT were incubated in either media alone (not shown), with K562 cells for 4 hours (top and middle panels), or with IL-12 and IL-18 overnight (bottom panel). After incubation, CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. Bars represent the mean ± SEM. Donors were compared with recipient samples using the Wilcoxon rank sum test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
Target cell-induced IFNγ production is decreased after T-cell replete, T-cell deplete, and UCB transplantation. PBMCs from healthy donors and recipients at 1 (T-cell deplete and UCB only), 3, and 6 months post-HCT were incubated in either media alone (not shown), with K562 cells for 4 hours (top and middle panels), or with IL-12 and IL-18 overnight (bottom panel). After incubation, CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. Bars represent the mean ± SEM. Donors were compared with recipient samples using the Wilcoxon rank sum test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
In marked contrast to degranulation activity, the target cell-induced IFNγ production by NK cells was significantly diminished in all 3 transplant cohorts compared with healthy donors (Figure 2 middle panel). At 3 months after T cell–replete HCT, NK cells produced significantly less IFNγ compared with NK cells from donors (5% ± 1% vs 9% ± 1%; P = .005), with the level of IFNγ production approaching that of normal donors by 6 months. Defects in IFNγ production were even more pronounced in settings where T cells were either removed from the graft (T-cell deplete) or when the majority of T cells were naive (UCB). Compared with their donors, recipients of T-cell deplete grafts had lower IFNγ production at 1 month (1% ± 0.4%, P < .0001) and 3 months (2% ± 0.8%, P = .0003), with partial recovery by 6 months after HCT (5% ± 3%, P = .118). After UCB grafts, where increased degranulation capacity was observed, target cell-induced IFNγ production was severely diminished at 1 (2% ± 0.7%; P = .0003), 3 (2% ± 0.5%; P = .001), and 6 (2% ± 0.3%; P = .001) months after HCT. Collectively, these results show that NK effector functions develop via distinct processes after HCT, with degranulation (cytotoxic function) appearing more rapidly than cytokine production. These results also highlight fundamental differences in the function of reconstituting NK cells based on the donor stem cell source (adult URD vs UCB), different graft processing methods (T-cell depletion), and use of post-HCT immune suppression.
To investigate whether lack of target cell induced IFNγ production represented a global defect in IFNγ production, NK cells were stimulated overnight with IL-12 and IL-18 (Figure 2 bottom panel). NK cells from both donors and recipients produced significantly more IFNγ after exposure to IL-12 and IL-18. Thus, the defect in IFNγ production by NK cells post-HCT is specific to the target cell stimulus.
KIR expression distinguishes NK cells capable of producing IFNγ
NK cell function develops after engagement of inhibitory receptors (KIR, NKG2A, or both) with “self” HLA,12 and the small KIR−NKG2A− cell subset remains functionally hyporesponsive.12-14 We tested whether KIR expression could distinguish between functional and hyporesponsive NK cell subsets post-HCT. NK cells from both donors and recipients were categorized as KIR+ if they expressed at least one of the following KIRs: CD158a/h (KIR2DL1/KIR2DS1), CD158b/j (KIR2DL2/KIR2DL3/KIR2DS2), or CD158e (KIR3DL1). All other cells were considered KIR− (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
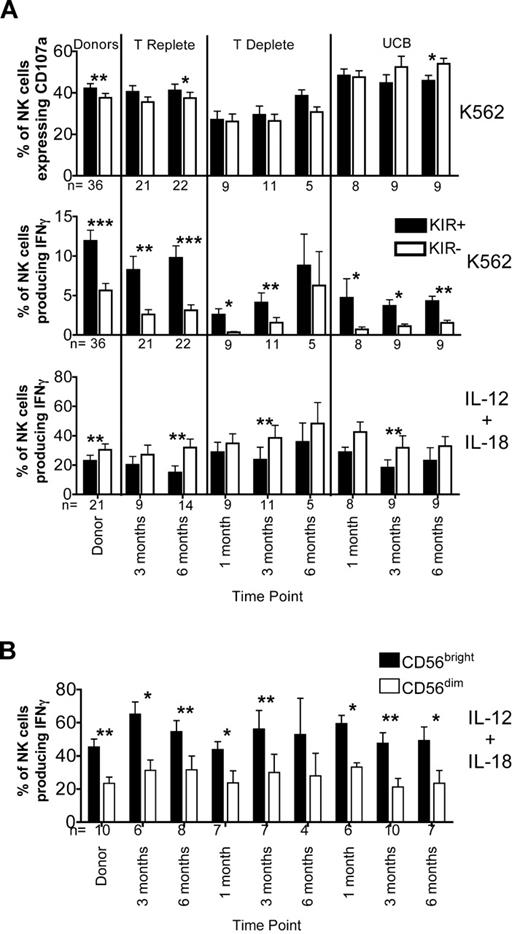
There was minimal difference in CD107a expression between the KIR+ and KIR− NK cell subsets (Figure 3A top panel), supporting the premise that receptors other than KIR can educate NK cells for cytotoxicity.14,21-23 Conversely, we found that expression of inhibitory KIR reliably distinguishes NK cells capable of producing IFNγ after target cell stimulation (Figure 3A middle panel). KIR+ NK cells from both donors and recipients in all 3 transplant cohorts produced significantly more IFNγ than did their KIR− NK cells. However, after cytokine stimulation, it was the KIR− subset of NK cells that produced more IFNγ compared with the KIR+ cells from both donors and post-HCT recipients (Figure 3A bottom panel). This result reflects the activity of the predominantly KIR− CD56bright NK cells, which produce significantly more IFNγ than do CD56dim populations in both donors and post-HCT recipients (Figure 3B).
KIR+ NK cells produce higher levels of IFNγ after target cell exposure than do KIR− NK cells. (A) CD56+CD3−–gated NK cells from donors and recipient post-HCT samples at the indicated time points were further gated as KIR+ (black bars) or KIR− (white bars) based on staining with a cocktail of antibodies containing CD158a/h, CD158b/j, and CD158e. Expression of CD107a and production of IFNγ were measured after incubation with K562 cells for 4 hours. IFNγ production also was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. KIR+ NK cells were compared with KIR− NK cells at each time point using the Wilcoxon signed rank test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001. (B) CD56+CD3−–gated NK cells from donors and recipient post-HCT samples from the indicated time points were further gated as CD56bright or CD56dim. Production of IFNγ was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. CD56bright NK cells were compared with CD56dim NK cells at each time point using the Wilcoxon signed rank test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
KIR+ NK cells produce higher levels of IFNγ after target cell exposure than do KIR− NK cells. (A) CD56+CD3−–gated NK cells from donors and recipient post-HCT samples at the indicated time points were further gated as KIR+ (black bars) or KIR− (white bars) based on staining with a cocktail of antibodies containing CD158a/h, CD158b/j, and CD158e. Expression of CD107a and production of IFNγ were measured after incubation with K562 cells for 4 hours. IFNγ production also was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. KIR+ NK cells were compared with KIR− NK cells at each time point using the Wilcoxon signed rank test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001. (B) CD56+CD3−–gated NK cells from donors and recipient post-HCT samples from the indicated time points were further gated as CD56bright or CD56dim. Production of IFNγ was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. CD56bright NK cells were compared with CD56dim NK cells at each time point using the Wilcoxon signed rank test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
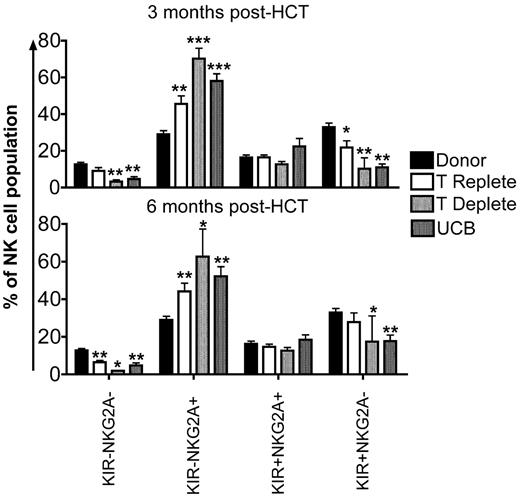
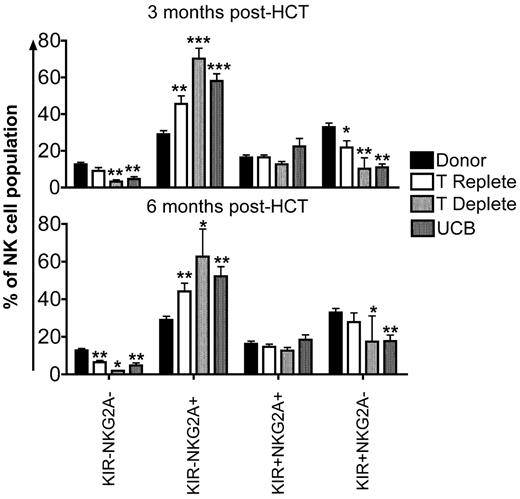
Early after HCT, the majority of NK cells express NKG2A with a proportionate decrease in KIR expression.24,25 This is consistent with current models of NK cell development, where NK cells acquire NKG2A first, followed by KIR.26,27 To investigate the sequence of developmental events in reconstituting NK cells from the 3 transplant cohorts, we evaluated the following 4 subsets: KIR−NKG2A−, KIR−NKG2A+, KIR+NKG2A+, or KIR+NKG2A− (supplemental Figure 1B). Compared with healthy donors, the KIR−NKG2A+ subset of NK cells was significantly increased at 3 and 6 months after HCT in all transplant cohorts (Figure 4). Corresponding decreases in the KIR−NKG2A− and KIR+NKG2A− populations were observed. In addition, the more mature KIR+NKG2A− subset also was decreased at 3 and 6 months after HCT compared with healthy donors.
KIR+NKG2A− NK cells are decreased and KIR−NKG2A+ NK cells are increased after HCT. CD56+CD3−–gated NK cells from donors (black bars) and post-HCT recipients (white bars indicate T-cell replete; light gray bars indicate T-cell deplete, and dark gray bars indicate UCB) at 3 and 6 months were further gated based on staining with a cocktail of KIR antibodies and NKG2A into KIR−NKG2A−, KIR−NKG2A+, KIR+NKG2A+, or KIR+NKG2A− populations. Bars represent the mean ± SEM frequency of each NK cell population. Donors were compared with recipient samples using the Wilcoxon rank sum test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
KIR+NKG2A− NK cells are decreased and KIR−NKG2A+ NK cells are increased after HCT. CD56+CD3−–gated NK cells from donors (black bars) and post-HCT recipients (white bars indicate T-cell replete; light gray bars indicate T-cell deplete, and dark gray bars indicate UCB) at 3 and 6 months were further gated based on staining with a cocktail of KIR antibodies and NKG2A into KIR−NKG2A−, KIR−NKG2A+, KIR+NKG2A+, or KIR+NKG2A− populations. Bars represent the mean ± SEM frequency of each NK cell population. Donors were compared with recipient samples using the Wilcoxon rank sum test; statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001.
NKG2A educates NK cells for degranulation but not cytokine production
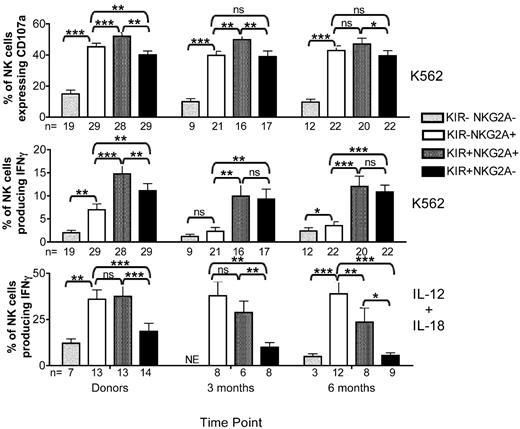
NK cell education is determined by class I-recognizing inhibitory NK cell receptors, including KIR and NKG2A.10,12 We have shown that KIR expression identifies NK cells capable of producing IFNγ (Figure 3) but that it is not associated with degranulation or cytotoxic capacity. Because the KIR− population is predominantly NKG2A+, we further explored the ability of NKG2A alone to educate NK cells. With the same gating strategy used in Figure 4, CD107a expression and IFNγ production were measured simultaneously in KIR−NKG2A−, KIR−NKG2A+, KIR+NKG2A+, or KIR+NKG2A− NK cell subsets after stimulation with K562 or cytokines. Only recipient NK cells from the T-cell replete cohort, which had higher KIR and lower NKG2A expression than the other cohorts, provided sufficient events for specific evaluation of each of the 4 subsets (Figure 5). Donor KIR−NKG2A− NK cells had low CD107a expression and were poor producers of IFNγ after stimulation by either target cells or cytokines, consistent with the known hyporesponsive state of NK cells lacking inhibitory receptors.12-14 The KIR−NKG2A+ donor NK cells expressed significantly more CD107a compared with the KIR+NKG2A− subset (Figure 5 left panels). Furthermore, NK cells expressing both NKG2A and KIR had the highest expression of CD107a. This suggests that NK cells expressing more than one self-recognizing inhibitory receptor are more responsive to activation for degranulation.22,28-30 Cytokine-induced IFNγ production was predominantly confined to NKG2A-expressing cells, which includes the CD56bright subset.
NKG2A educates reconstituting post-HCT NK cells for degranulation but not for cytokine production. CD56+CD3−–gated NK cells from donor and post-HCT recipient pairs receiving T-cell replete transplants were further gated as KIR−NKG2A− (light gray bars), KIR−NKG2A+ (white bars), KIR+NKG2A+ (dark gray bars), or KIR+NKG2A− (black bars) based on staining with a cocktail of KIR antibodies and NKG2A. CD107a expression and IFNγ production were measured on all 4 subsets after incubation with K562 cells for 4 hours. IFNγ also was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. Pairwise comparisons were based on a linear mixed model. Statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001. ns = not significant. NE = not evaluable.
NKG2A educates reconstituting post-HCT NK cells for degranulation but not for cytokine production. CD56+CD3−–gated NK cells from donor and post-HCT recipient pairs receiving T-cell replete transplants were further gated as KIR−NKG2A− (light gray bars), KIR−NKG2A+ (white bars), KIR+NKG2A+ (dark gray bars), or KIR+NKG2A− (black bars) based on staining with a cocktail of KIR antibodies and NKG2A. CD107a expression and IFNγ production were measured on all 4 subsets after incubation with K562 cells for 4 hours. IFNγ also was measured after overnight stimulation with IL-12 and IL-18. Bars represent the mean ± SEM. Pairwise comparisons were based on a linear mixed model. Statistical significance is indicated as *P < .05, **P < .01, and ***P < .0001. ns = not significant. NE = not evaluable.
As was seen with donor cells, NK cells from post-HCT recipients lacking both KIR and NKG2A had low CD107a expression and were poor producers of IFNγ (Figure 5 middle and right panels). Degranulation was observed in a majority of NK cells expressing either NKG2A or KIR. Cells coexpressing NKG2A and KIR had the highest expression of CD107a at both 3 and 6 months after HCT. In marked contrast to the donors, NK cells from post-HCT recipients that expressed NKG2A as their only inhibitory receptor were poor producers of IFNγ after target cell stimulation, with activity similar to that seen in the hyporesponsive NKG2A−KIR− subset. Only the KIR+ NK cells were capable of producing IFNγ in response to target cells. There was no additive effect if NK cells expressed both KIR and NKG2A. Importantly, after stimulation with IL-12 and IL-18, it was only the NKG2A-expressing cells that produced IFNγ. This demonstrates that IFNγ production by the NKG2A+KIR− NK cell subset depends on the mode of stimulation (target cell vs cytokine). Collectively, these data are consistent with a model where NK cells reconstituting after HCT can be educated by NKG2A to degranulate but not to produce IFNγ, whereas KIR can educate for both functions.
KIR expression correlates with IFNγ production
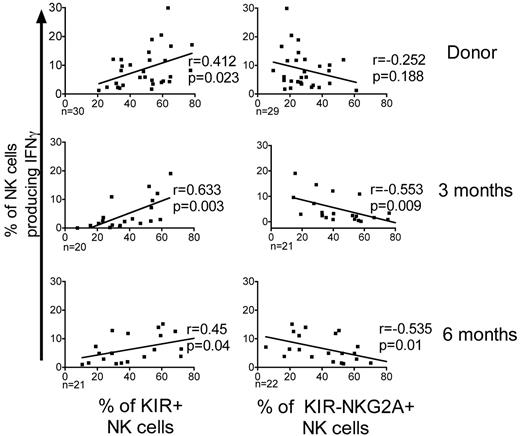
After allo-HCT the expression of KIR is decreased and expression of NKG2A is increased; the inverse of what is seen in healthy donors.24,25 To test whether KIR expression correlates with target-induced IFNγ production, we compared the K562 target-induced IFNγ production in NK cells from donors and from the T-cell replete graft recipients (Figure 6). Donors with higher frequencies of KIR-expressing NK cells had a significantly higher proportion of NK cells producing IFNγ (r = 0.412; P = .023). Similarly, at 3 and 6 months after HCT, recipients with higher frequencies of KIR+ NK cells had significantly more IFNγ-producing cells (3 months, r = 0.632, P = .003; 6 months, r = 0.45, P = .04). In the recipients, the NKG2A-expressing NK cells produced significantly less IFNγ. This explains the correlation between the increased frequency of KIR−NKG2A+ NK cells and the severely defective IFNγ production observed early after HCT.
KIR expression positively correlates with IFNγ production. The percentages of CD56+CD3−–gated NK cells producing IFNγ following incubation with K562 cells were plotted against the percentages of KIR+ NK cells or NKG2A+KIR− NK cells from both donors and post-HCT recipients of T-cell replete grafts. The estimated regression line is shown with the r value and significance based on the Pearson correlation coefficient. Significance is calculated as P < .05.
KIR expression positively correlates with IFNγ production. The percentages of CD56+CD3−–gated NK cells producing IFNγ following incubation with K562 cells were plotted against the percentages of KIR+ NK cells or NKG2A+KIR− NK cells from both donors and post-HCT recipients of T-cell replete grafts. The estimated regression line is shown with the r value and significance based on the Pearson correlation coefficient. Significance is calculated as P < .05.
Only NK cells expressing self-KIR are educated after HCT
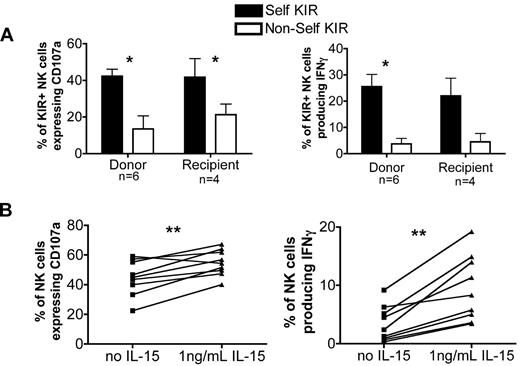
It has been demonstrated in both mice and humans that the mere expression of an inhibitory receptor is not enough to result in NK cell function. Indeed, the inhibitory receptors must specifically recognize self class I ligands.10,12 KIR2DL1, KIR2DL2/3, and KIR3DL1 recognize allelic epitopes present on certain HLA-A, HLA-B, and HLA-C alleles. Thus, we designated NK cells from donor and recipient pairs receiving HLA-matched T-cell replete grafts as expressing either self-KIR (ie, the ligands for their inhibitory KIR were present in both the donor and recipient) or non-self KIR (ie, both lacked the ligands for their inhibitory KIR). The frequency of NK cells that expressed a single KIR for self did not differ significantly between paired donors (12% ± 2%) and their recipients at both 3 months (13% ± 4%) and 6 months (11% ± 3%). Next, we examined the function of NK cells that expressed a single KIR either for self or non-self. Because NKG2A educates NK cells for degranulation (Figure 5), we excluded NKG2A+ cells from this analysis. CD107a expression and IFNγ production were significantly higher in NK cells expressing self versus non-self KIR from both donors and recipients (Figure 7A). This demonstrates that the requirement for signaling via self KIR to educate NK cells to produce IFNγ is maintained after transplant.
Self-KIR educate for cytotoxicity and IFNγ production, and low-dose IL-15 exposure restores the post-HCT defect in IFNγ response. (A) Single KIR+NKG2A− NK cells from both donors and post-HCT recipients of T-cell replete grafts were grouped as expressing either self-KIR (black bars) or non-self KIR (white bars) based on the donor or recipient KIR ligand status. Bars represent the mean ± SEM. NK cells expressing self-KIR were compared with NK cells expressing non-self KIR using the paired Student t test. Statistical significance is indicated as *P < .05. (B) PBMCs from recipients 3 months after transplantation with T-cell replete grafts were incubated overnight in media alone (squares) or media containing 1 ng/mL IL-15 (triangles). They were then tested for response induced by K562 exposure; CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. The paired Student t test was used to compare the function of cells incubated in media versus media containing 1 ng/mL IL-15. Statistical significance is indicated as *P < .05 and **P < .01.
Self-KIR educate for cytotoxicity and IFNγ production, and low-dose IL-15 exposure restores the post-HCT defect in IFNγ response. (A) Single KIR+NKG2A− NK cells from both donors and post-HCT recipients of T-cell replete grafts were grouped as expressing either self-KIR (black bars) or non-self KIR (white bars) based on the donor or recipient KIR ligand status. Bars represent the mean ± SEM. NK cells expressing self-KIR were compared with NK cells expressing non-self KIR using the paired Student t test. Statistical significance is indicated as *P < .05. (B) PBMCs from recipients 3 months after transplantation with T-cell replete grafts were incubated overnight in media alone (squares) or media containing 1 ng/mL IL-15 (triangles). They were then tested for response induced by K562 exposure; CD107a expression and IFNγ production were measured on CD56+CD3−–gated NK cells. The paired Student t test was used to compare the function of cells incubated in media versus media containing 1 ng/mL IL-15. Statistical significance is indicated as *P < .05 and **P < .01.
Exposure to low-dose IL-15 restores the defect in IFNγ response early after HCT
IL-15 plays a critical role in NK cell development, proliferation, and activation.15,16,31 We hypothesized that low-dose IL-15 stimulation might correct the defective IFNγ production seen in NK cells reconstituting early after HCT. PBMCs collected at 3 months after HCT were stimulated overnight in media with or without IL-15 (1 ng/mL). This short incubation did not alter KIR expression (data not shown). CD107a expression and IFNγ production were then measured after exposure to K562 target cells (Figure 7B). Exposure to low-dose IL-15 promoted significant increases in CD107a expression and IFNγ production to approximately normal levels.
Discussion
We have identified marked differences in the recovery of target cell-induced functions after allo-HCT from 3 cohorts with variable T-cell graft content. IFNγ production was diminished after all types of HCT, and degranulation was impaired only after T-cell deplete HCT. We have shown that both KIR expression and education via cognate KIR ligand are required for target cell–induced IFNγ production by NK cells reconstituting after HCT. Target-induced degranulation function, however, only requires NKG2A expression. NK cells reconstituting early after HCT are phenotypically immature, expressing increased NKG2A and diminished KIR.26,32 In normal development, the ability of NK cells to produce IFNγ or degranulate in response to stimulation by target cells correlates with the transition from CD56bright (immature) to CD56dim (mature) NK cell phenotype.12,33 Recently, it has been shown that immature cells, which are more responsive to cytokine stimulation, gradually decrease expression of IL-12 receptor β2 and IL-18 receptor as they acquire CD57, a marker of maturity.34,35 Thus, stimulation with IL-12 and IL-18 predominantly affects immature NK cell subsets, including CD56bright cells. IL-12 and IL-18 induce cytokine production, but not degranulation, via mechanisms that do not require KIR expression.
The most novel data from our study characterizes the different functional populations of reconstituting NK cells based on their ability to degranulate or to produce cytokines after target cell stimulus. NK cells expressing NKG2A as their only inhibitory receptor had high CD107a expression in both healthy donors and after HCT. This result supports a role for NKG2A in educating NK cells to degranulate and kill targets. In stark contrast, in the post-HCT setting the same KIR−NKG2A+ NK cell population produced minimal IFNγ after target cell stimulation. Not only was KIR expression necessary for IFNγ production but also specifically the expression of an inhibitory KIR recognizing a cognate host (“self”) ligand was required. Reconstituting NK cells lacking inhibitory receptors or expressing KIR that only recognized non-self were hyporesponsive. Similar analyses have been reported in HLA identical sibling transplant cohorts. Consistent with our findings, Björklund et al23 demonstrated that NK cells expressing non-self KIR remain uneducated and self-tolerant after both T-cell replete and deplete sibling transplants. In contrast, Yu et al36 reported that reconstituting NK cells expressing non-self KIR are hyperresponsive based on IFNγ production early after T-cell deplete HCT. Differences between these reports may be because of variability in their patient cohorts or their functional assays. Neither group used the simultaneous functional readout developed here.
It is unclear when or how during development NK cells are educated to become functional. Although it is presumed that the acquisition of NK cell function occurs through engagement of inhibitory receptors, it is becoming increasingly apparent that the education status of a NK cell is not “fixed” and that NK cell responsiveness can be influenced by the inhibitory input from the environment.37 Furthermore, studies in both mice28,37,38 and humans22,30 suggest that not all MHC alleles are equally efficient at educating NK cells and that higher affinity alleles provide stronger inhibitory signals. Increased responsiveness by NK cells to loss of self correlates with their exposure to more inhibitory input, because of either the strength of the ligand signal or to the expression of more than one inhibitory receptor. Brodin et al28 demonstrated in mice that stronger inhibitory input during education allowed cells to reach a functional threshold where they were likely to exhibit both degranulation and cytokine production in response to targets. They proposed that the threshold for inhibitory signaling required for NK cells to acquire degranulation function is much lower than the threshold required for NK cells to become cytokine producers. They observed that all cytokine-producing NK cells were able to degranulate, as we found in the human donor and post-HCT samples. We postulate that in the post-HCT setting, the interaction between NKG2A and its ligand HLA-E supports the education of NK cells to degranulate but that the signal is too weak to meet the required threshold to educate for cytokine production.
It has been proposed that T cells present in grafts compete with NK cells for cytokines, thus inhibiting the emergence of the alloreactive NK cells that mediate GVL.20 Strikingly, we found that recipients of T-cell replete (unmanipulated) grafts had better overall NK cell function than did recipients of T cell–depleted grafts or UCB grafts, which contain naive T cells. Not only was NK cell function increased early after HCT with T-cell replete grafts but also NKG2A expression was decreased and KIR expression was increased. Although these results differ from our previous report showing that KIR expression was more suppressed after T-cell replete HCT,25 they can be explained by the differences in the settings tested. In the cohort reported here, the T-cell depletion was performed using CD34 selection, which eliminates nearly all contaminating T cells from the graft. In contrast, less efficient T-cell depletion techniques such as elutriation, antithymocyte globulin, and campath were used in the earlier cohort. This concept is supported by the findings of Nguyen et al39 and Vago et al,40 who showed that early after haploidentical HCT with potent T-cell depletion, reconstituting NK cells were arrested at an immature state with higher NKG2A expression and lower overall function. A subsequent study by Nguyen et al41 comparing partial versus complete T-cell depletion suggested that grafts containing some T cells may enhance rather then suppress NK cell function, which is in agreement with our findings. Activated T cells produce IL-2, which has been shown to stimulate CD56bright NK cells in lymph nodes to produce IFNγ,42 and to contribute to NK cell maturation.17 The maturity of the T cells in the graft also may influence NK cell reconstitution and function. For example, NK cell degranulation and IFNγ production are both impaired after grafting with UCB, which contains more naive than mature T cells. Thus, both quantitative and qualitative differences in the T-cell content of the graft may affect the kinetics of recovery of NK cell function and receptor acquisition. In addition, unmanipulated grafts also differ from CD34+-selected or UCB grafts because they contain mature donor NK cells. It is possible that infused mature NK cells may contribute to the increased function seen after T-cell replete HCT. Data generated from a murine model of cytomegalovirus infection suggest that the life span of NK cells may be longer than previously assumed.43 Therefore, the improved global NK cell function observed after T-cell replete HCT may be due in part to the persistence of mature donor NK cells.
Allo-HCT affords us a unique opportunity to study the development, maturation, and acquisition of function in human NK cells. We have demonstrated that although their numbers are high, the NK cells circulating early after transplant are less functional than previously believed, depending on the T-cell content of the graft. Our results clearly demonstrate that NK cells reconstituting after HCT do not acquire cytotoxic and IFNγ-producing functions simultaneously. NK cells acquire degranulation capacity before they can produce cytokines, and different inhibitory receptors are required to educate for these functions. Prior studies have supported a role for alloreactive NK cells in reducing post-HCT relapse, and IFNγ has been shown to play a critical role in tumor suppression,44 control of viral infections45 and in mediating GVHD.46 Barker et al47 looked at the incidence of serious infections after transplantation with either T-cell replete bone marrow, T-cell deplete bone marrow, or UCB grafts. Although serious infections where seen in all groups, viral infections were more common after transplants using T-cell deplete and UCB grafts, the 2 cohorts in which we demonstrated decreased IFNγ production by reconstituting NK cells. Our data suggest NK cells reconstituting after HCT may not mediate beneficial effects until they have become educated and have acquired full effector function. Because NK cells play such an important role in the control of leukemia and infection, methods to enhance NK cell function early after transplant are warranted. Based on these studies, IL-15 may be an ideal candidate to achieve this goal.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Claudio Anasetti and the protocol committee and participants for collection of samples on the CTN 0201 clinical transplant study. They also acknowledge sample procurement and cell processing services from the Translational Therapy Core supported from the National Cancer Institute (P30-CA77598) and Minnesota Masonic Charities.
This work was supported in part by National Institutes of Health grants P01-CA65493 (J.S.M., S.C., and M.R.V.) and P01-CA111412-01 (J.S.M., S.C., M.R.V., E.K.W., and D.J.W.).
National Institutes of Health
Authorship
Contribution: B.F. designed and performed the research, analyzed data, and wrote the paper; S.C., M.R.V., E.K.W., and D.J.W. helped interpret data and helped with manuscript preparation; J.C. contributed to assay development, sample procurement, and manuscript preparation; X.L was responsible for statistical analysis and manuscript preparation; and J.S.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, Division of Hematology, Oncology, and Transplantation, University of Minnesota Cancer Center, MMC 806, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail: mille011@umn.edu.