Abstract
Filaminopathies A caused by mutations in the X-linked FLNA gene are responsible for a wide spectrum of rare diseases including 2 main phenotypes, the X-linked dominant form of periventricular nodular heterotopia (FLNA-PVNH) and the otopalatodigital syndrome spectrum of disorders. In platelets, filamin A (FLNa) tethers the principal receptors ensuring the platelet–vessel wall interaction, glycoprotein Ibα and integrin αIIbβ3, to the underlying cytoskeleton. Hemorrhage, coagulopathy, and thrombocytopenia are mentioned in several reports on patients with FLNA-PVNH. Abnormal platelet morphology in 2 patients with FLNA-PVNH prompted us to examine a third patient with similar platelet morphology previously diagnosed with immunologic thrombocytopenic purpura. Her enlarged platelets showed signs of FLNa degradation in Western blotting, and a heterozygous missense mutation in FLNA was detected. An irregular distribution of FLNa within the total platelet population was shown by confocal microscopy for all 3 patients. In vitro megakaryocyte cultures showed an abnormal differentiation, including an irregular distribution of FLNa with a frayed aspect, the presence of enlarged α-granules, and an abnormal fragmentation of the cytoplasm. Mutations in FLNA may represent an unrecognized cause of macrothrombocytopenia with an altered platelet production and a modified platelet–vessel wall interaction.
Introduction
Filamin A (FLNa) is an essential component of the cell cytoskeleton; it cross-links actin filaments by an N-terminal actin-binding domain, comprised of calponin homology domains 1 and 2 and 24 immunoglobulin (Ig)–like repeats interrupted by 2 hinge regions.1 FLNa function depends on homodimerization mediated by Ig repeat 24 at the C terminus. GPIbα mediates platelet attachment under flow to surface-exposed von Willebrand factor (VWF) at injured sites in the vasculature, whereas αIIbβ3 promotes platelet-to-platelet binding in thrombus formation.2 FLNa Ig repeat 17 primarily binds the GPIbα intracellular tail and repeat 21 those of β-integrins, similar activities in other repeats allow for multiligand binding, thereby promoting receptor clustering and cellular activation.1,3-6 Loss of the GPIbα–FLNa interaction disrupts platelet plasma membrane stability under high shear.7
Mutations in the FLNA gene located on chromosome Xq28 are responsible for a wide spectrum of rare diseases. In periventricular nodular heterotopia (FLNA-PVNH), there is a neuronal migration defect that gives to the neurons the appearance of nodules lining the margins of the cerebral ventricles,8 and variable associated features may be observed extending to Ehlers-Danlos syndrome.9 The otopalatodigital spectrum encompass heterogeneous diseases characterized by skeletal dysplasia, mental retardation in some cases, and congenital malformations.10 Familial cardiac valvular dystrophy,11 congenital intestinal pseudo-obstruction,12 and terminal osseous dysplasia13 define other presentations of filaminopathies A. This diversity of phenotypes suggests that interference with the multiple functions of FLNa has as a consequence the appearance of distinct pathophysiologic manifestations without, as yet, clear phenotype–genotype correlations, except for Melnick-Needles syndrome, one of the otopalatodigital spectrum disorders. Because most studies have concentrated on FLNA-PVNH with seizures and cognitive defects, platelet counts and platelet size are not always reported. Yet, thrombocytopenia and bleeding have occasionally been described in filaminopathies A, although they have been little studied so far. For example, 20 patients with FLNA mutations in a large cohort with classic bilateral FLNA-PVNH showed vascular defects including insufficiency of the aortic valve and patent ductus arteriosus, whereas idiopathic thrombocytopenia was noted for some of them but more accurate data were lacking.14
Recently, mouse models with deficient FlnA have been reported.15 Because total FlnA deficiency is lethal, mice were generated lacking FlnA only in the megakaryocytic lineage using the Cre-Lox technology by breeding FlnAloxp/loxp females with GATA-1-Cre males. Thrombocytopenia was moderate in heterozygous FlnA female deleted mice and was more severe for the FlnAloxp/y GATA1-Cre males in which the reduction in FlnA reached > 80%, showing the important role of FlnA in maintaining a normal platelet count. It should be noted that in this second model with the near absence of FlnA, the consequences of deletion of the Flna gene were mostly studied. More recently, a study on megakaryocytes (MKs) was reported for these mice showing that FlnA-null MKs prematurely release large and fragile platelets.16
Our laboratory has characterized many patients with filaminopathies.17 We now report how platelet morphologic analyses on 2 FLNA-PVNH patients prompted us to identify FLNa defects in a patient with macrothrombocytopenia previously treated for immunologic thrombocytopenic purpura (ITP); the detection of a novel missense mutation demonstrates that FLNA mutations also can give an isolated hematologic phenotype and are an unsuspected cause of inherited thrombocytopenias. Platelets were enlarged with an abnormal distribution as well as giant granules. The study was extended to CD34+ MK cultures from the patients. The results suggested that the presence of abnormal FLNa during MK maturation influenced their differentiation and platelet production.
Methods
Patient characteristics and genetic analyses
Our report concerns 3 patients with platelet size variations, thrombocytopenia, or both (see Table 1). Only for the first 2 patients was a FLNA defect immediately suspected. Patient 1 (P1), a 50-year-old woman, has mental retardation, early onset pharmacoresistent partial epilepsy, and pyramidal and cerebellar signs related to FLNA-PVNH (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); a heterozygous frameshift point mutation (c.4573_4insA, p.Tyr1525X) has been identified previously by us in exon 27 of FLNA, and X-inactivation was random (48/52) in lymphocytes.17 Hematomas and heavy menstrual bleeding were associated with a low platelet count (80 000 platelets/μL; normal range, 150 000-400 000 platelets/μL) and slow wound healing. Patient 2 is a 49-year-old woman who associated FLNA-PVNH with Ehlers-Danlos syndrome. Brain magnetic resonance imaging showed diffuse bilateral and symmetric PVNH, megacisterna magna, and white matter hyperintensities (supplemental Figure 1). Her platelet count was normal (238 000 platelets/μL) but with the presence of giant platelets; she has a mild bleeding syndrome (hematomas, gum bleeding, and epistaxis) and problems with wound healing. No mutation was identified on direct sequencing; however, gene dosage analysis using array-comparative genomic hybridization revealed a heterozygous intragenic deletion involving FLNA exons 31-32 to 48 (see Table 1; supplemental Figure 2) that was confirmed by a quantitative multiplex fluorescent-PCR assay. X chromosome inactivation was random in lymphocytes (53/47). Patient 3 is a 75-year-old woman with a life-long history of thrombocytopenia and bleeding (gum bleeding, easy bruising, and rectal hemorrhage). A bone marrow examination showed that marrow was rich in MKs; she was considered and treated as ITP (corticotherapy and immunoglobulins) and finally underwent splenectomy when she was 65 years old. However, her platelet count was only transiently and partially improved, the diagnosis was reconsidered, and new investigations were performed. A novel heterozygous FLNA point mutation c.5407G>A, p.Glu1803Lys was detected; Glu1803 is highly conserved between species (see Table 1). This missense mutation was absent from 600 control chromosomes. X chromosome inactivation was random in lymphocytes (55/45). Her brain magnetic resonance imaging was normal (supplemental Figure 1). She has a normal intelligence and no neurologic abnormalities. Supplemental Figure 3 shows the positions of the mutations as they affect the protein. For P1, a stop codon predicts a truncated protein before hinge 1 and to the major GPIbα-interacting site within repeat 17. For P2, the deletion involves a large part of the C-terminal domain of the molecule starting before the hinge region. For P3, the highly conserved Glu1803Lys mutation occurs in repeat 16 located just after hinge 1 and close to the principal GPIbα-binding site (Ig repeat 17).
Methods used in the genetic analysis are reported in supplemental Methods.
MK in vitro culture from CD34+ peripheral cells
Blood samples were obtained after informed consent in accordance with the Declaration of Helsinki. Ethical approval was obtained from Inserm RBM 01-14 for the project “Network on the inherited diseases of platelet function and platelet production. ” CD34+ cells were isolated after a first purification step with Ficoll by cell sorting or by using an immunomagnetic purification kit (EasySep Human CD34+ selection kit; StemCell Technologies). The culture medium was supplemented with recombinant human thrombopoietin (TPO, 10 ng/mL). Cells were grown in serum-free medium as reported previously.18 Cells were cultured in an incubator at 37°C during 14 days on a 24- or 96-well plate. Fresh culture medium with TPO was supplemented in the culture every 2 or 3 days.
Proplatelet formation assay
MKs were plated from day 8 of culture in triplicate in 96-well plates at a concentration of 3000 cells/well in serum-free medium containing TPO. Four days later, MKs displaying proplatelets were quantified by enumerating 500 cells per well using an inverted microscope at a magnification of ×200 using AxioVision 4.6 software (Carl Zeiss) as described previously.18
Standard EM
Standard electron microscopy (EM) for platelet morphology was performed for each patient using venous blood taken in ACD-A (1 vol/7 vol) and centrifuged for 10 minutes at 80g; platelet-rich plasma was aspirated up to the buffy coat. Platelets from 6 healthy control donors were analyzed in parallel. After incubation for 20 minutes at 37°C, platelets were fixed in 1.25% glutaraldehyde (Fluka) in 0.1M phosphate buffer, pH 7.2, for 1 hour at room temperature. For morphometry, a minimum of 100 sections were analyzed for each subject; platelet diameters and surface area were measured using ImageJ (National Institutes of Health). To study MKs, a minimum of 50 000 cells from controls and patients were fixed as described for platelets at day 14 of culture and treated as described for platelets. Samples were processed for EM according to our standard procedures, and sections were examined using a JEM-1010 transmission electron microscope (JEOL).19,20
Confocal microscopy
For studies on platelets, smears of citrated blood from the 3 patients or control donors were prepared on slides and fixed with cold acetone, washed in PBS-0.5% albumin, and then incubated for 1 hour after simultaneous addition of predetermined optimal dilutions of a mouse monoclonal antibody (MoAb) against FLNa (Santa Cruz Biotechnology from Tebu-Bio), and a polyclonal rabbit anti-VWF antibody (Dako Denmark). VWF was detected using a species-specific polyclonal antibody to rabbit IgG conjugated to Alexa-Fluor 568 (Invitrogen, Fisher Scientific) and FLNa with an anti-IgG goat anti–mouse antibody conjugated with FITC (DakoCytomation). The slides were mounted using Fluoroshield with 4,6-diamidino-2-phenylindole (Sigma-Aldrich) and examined with an FV1000 confocal microscope (Olympus) equipped with a 60×/1.55 objective. Images were deconvoluted with AutoQuant software (Media Cybernetics). The 3-dimensional reconstruction and image processing analyses were performed with Imaris software (Bitplane).
MK suspensions (100 μL) were incubated at day 14 of culture for at least 6 hours at 37°C in a moist chamber on polylysine-coated slides (Thermo Scientific). After a gentle wash, adherent cells were fixed in 2% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton X-100 for 5 minutes, and washed with PBS-0.5% BSA for 5 minutes. After a saturation step with PBS-0.5% BSA (30 minutes at room temperature), primary antibodies directed against FLNa or VWF were added. After 1 hour at room temperature, species-specific antibodies conjugated with Alexa-Fluors were added as described for the experiments with platelets. Alexa-Fluor 488–conjugated phalloidin (Invitrogen) also was used in some experiments. Incubations were for 1 hour at room temperature. Slides were finally washed 3 times with PBS for 5 minutes before being observed under an epifluorescence microscope (Eclipse 600; Nikon) equipped with a 60× objective (Nikon). Images were processed using the Photoshop 6.0 (Adobe Systems) and LSM 5 Image Browser software (Carl Zeiss), respectively.
Western blotting
Western blotting of platelet proteins was performed using SDS-soluble lysates of washed platelets with subsequent transfer and renaturation of the proteins on nitrocellulose membrane followed by chemiluminescence detection.19 Selected MoAbs were used to assess the platelet content of GPIbα (Bx-1), αIIb (SZ22), and β3 (Y2/51).19 Also used was the same anti-FLNa MoAb as in confocal microscopy. β-actin detected using a rabbit antibody (Sigma-Aldrich) was the loading control.
Statistical analyses
Data are presented as mean ± SD. Statistical significance was determined by Student t test, for continuous variables, and by the χ2 test for categorical variables. A P value < .05 was considered as statistically significant.
Platelet aggregation and flow cytometry studies are reported in supplemental Methods.
Results
Platelet morphological characteristics and location of the mutations
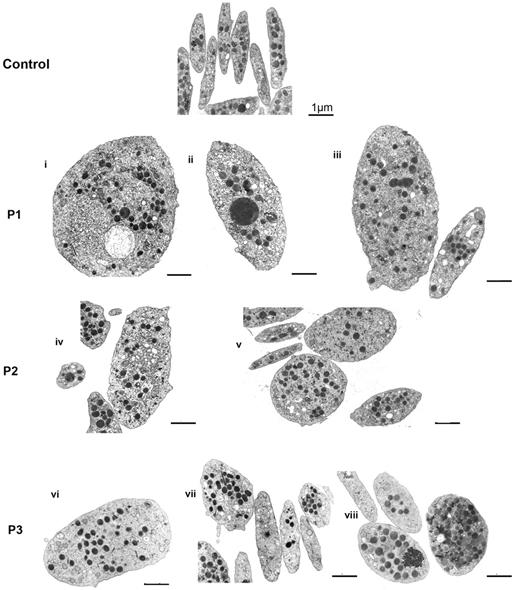
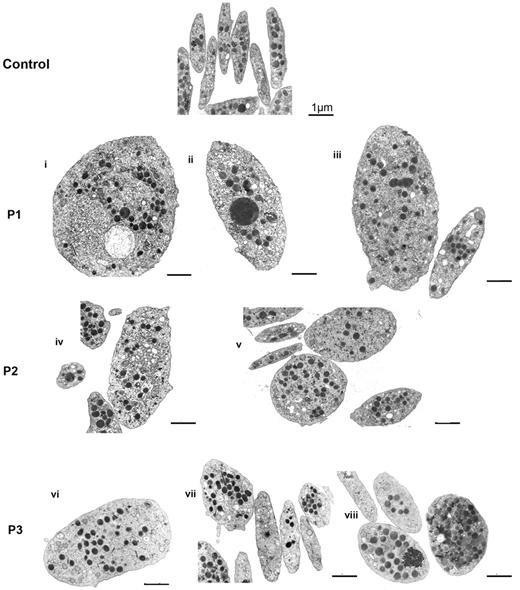
Platelets from 3 patients with defects in the FLNA gene and a bleeding tendency were examined (Table 1). Figure 1 shows a gallery of photos of platelets examined by EM for the patients. Compared with control platelets, anisotropy was a clear characteristic for all 3 patients; some platelets are large even giant, whereas others are normal or small. Another feature is the heterogeneous distribution of α-granules within platelets with the occasional presence of enlarged forms. A morphometric quantitative analysis confirmed that mean platelet size was increased for each patient (Table 2) and that enlarged platelets were more spherical because the minimum diameter/maximum diameter ratio was closer to 1. The largest heterogeneity was seen for P3.
Effect of FLNA mutations on platelet morphology. Typical illustrations of platelet morphology as seen for each patient by EM. Discoid control platelets are homogeneous in size, and the α-granules are distributed homogeneously. Several examples are shown for each patient (P1-P3). Note the presence of size heterogeneity with enlarged more rounded platelets intermingled with discoid platelets of normal size. Heterogeneity also is noted in the α-granule content (i,iii,v-vii) with some platelets having few granules (v,vii), whereas giant granules are occasionally seen (ii,iv). Zones enriched in membrane complexes are seen in some giant platelets (i,iv). Bars indicate 1 μm for all EMs.
Effect of FLNA mutations on platelet morphology. Typical illustrations of platelet morphology as seen for each patient by EM. Discoid control platelets are homogeneous in size, and the α-granules are distributed homogeneously. Several examples are shown for each patient (P1-P3). Note the presence of size heterogeneity with enlarged more rounded platelets intermingled with discoid platelets of normal size. Heterogeneity also is noted in the α-granule content (i,iii,v-vii) with some platelets having few granules (v,vii), whereas giant granules are occasionally seen (ii,iv). Zones enriched in membrane complexes are seen in some giant platelets (i,iv). Bars indicate 1 μm for all EMs.
Platelet function studies
The expression of GPIb-IX complexes and αIIbβ3 integrin was normal in platelets of P1 and P2 in flow cytometry, whereas GPIb was selectively increased for P3 (typical mean fluorescence intensities are given in supplemental Table 1A). There were no signs that the platelets were spontaneously activated (PAC-1 binding or P-selectin expression). Platelets aggregated well to different agonists in citrated platelet-rich plasma under standard conditions, although some responses were reduced for P3 (supplemental Table 1B).
Platelet content of FLNa
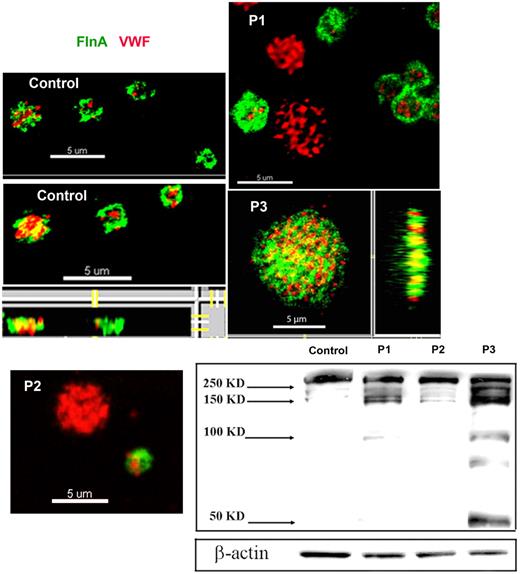
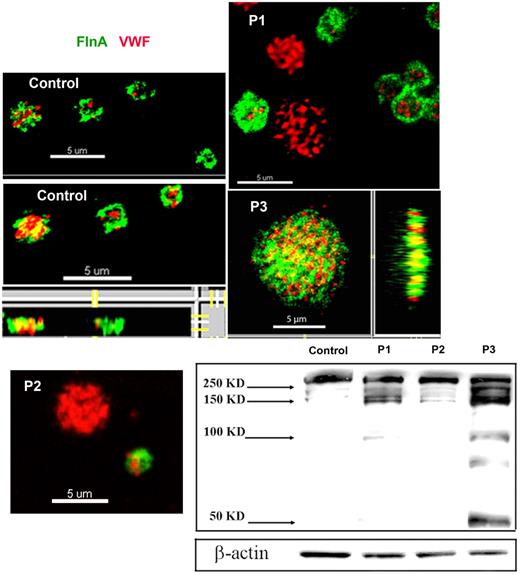
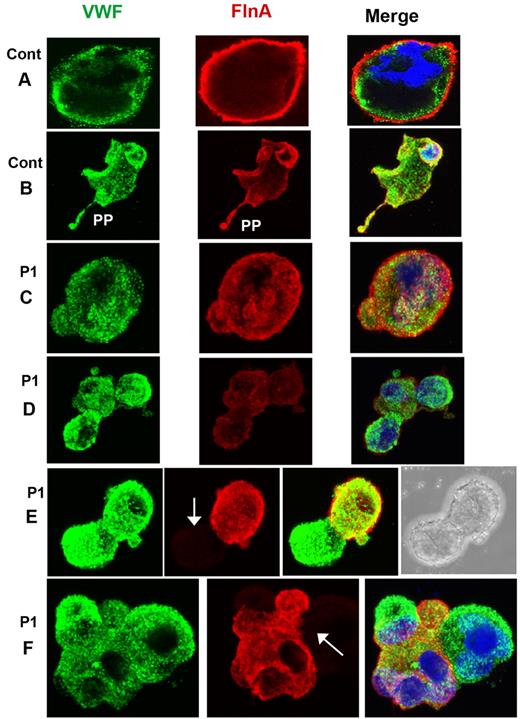
FLNa was visualized in smears of acetone-permeabilized platelets for the patients and compared with α-granule VWF by 2-color confocal microscopy (Figure 2). For control platelets, FLNa was mostly found as compact staining in a peripheral layer that was distinct from the punctate more central α-granule staining of VWF. The distribution of FLNa for the patients was abnormal, with a population of the largest platelets showing labeling for VWF but not for FLNa (illustrated for P1 and P2 in Figure 2); also the distribution of FLNa seemed more punctiform, a situation most exaggerated for P3. The percentage of the platelet population lacking FLNa is 20% ± 1% for P1, 21% ± 1% for P2 and 11% ± 7% for P3. Mostly, platelets lacking FLNa were the largest platelets. The presence of a positive and negative population for FLNa content was confirmed by flow cytometry performed on permeabilized platelets using the same antibodies; again the negative platelets tended to be larger than the positive population (illustrated for P2 in supplemental Figure 4).
Abnormal presence of FLNa in platelets of 3 patients. Two-color confocal microscopy comparing the distribution of antibodies detecting FLNa (green fluorescence) and VWF (red fluorescence) in fixed and permeabilized platelets. Note that for P1 and P2, the platelets lacking FLNa are the largest, whereas for P3 there is a loss of the more peripheral FLNa organization typical for control platelets. (Right and bottom) Projections of the images on the z-axis showing a cottony appearance. Western blotting with anti-FLNa antibody. Note the signs of FLNa degradation that were more extensive for P3.
Abnormal presence of FLNa in platelets of 3 patients. Two-color confocal microscopy comparing the distribution of antibodies detecting FLNa (green fluorescence) and VWF (red fluorescence) in fixed and permeabilized platelets. Note that for P1 and P2, the platelets lacking FLNa are the largest, whereas for P3 there is a loss of the more peripheral FLNa organization typical for control platelets. (Right and bottom) Projections of the images on the z-axis showing a cottony appearance. Western blotting with anti-FLNa antibody. Note the signs of FLNa degradation that were more extensive for P3.
Western blotting showed an abnormal presence of FLNa in platelets of all 3 patients with low-molecular-mass forms. For P1, FLNa of normal migration was reduced to ∼ 50% of usual platelet levels. For P2, the migration is close to normal and band intensity represents ∼ 80% of the control. The most abnormal pattern was observed for P3 where a series of bands of faster migration predominate (Figure 2). These were consistently present in platelets taken over a 2-year period, although their proportion was variable. One possibility is that they result from proteolytic degradation.
In vitro cultures of MKs
MK cultures also were performed for P1 and P3. MKs developed normally for the patients and were able to form proplatelets, but they did so less efficiently than the control. Indeed, at days 11 to 12 of culture, the percentage of proplatelet-forming MKs was 17% ± 1.9% for the control, 11% ± 0.9% for P1, and 3% ± 1.1% for P3 (for whom the results were statistically significant).
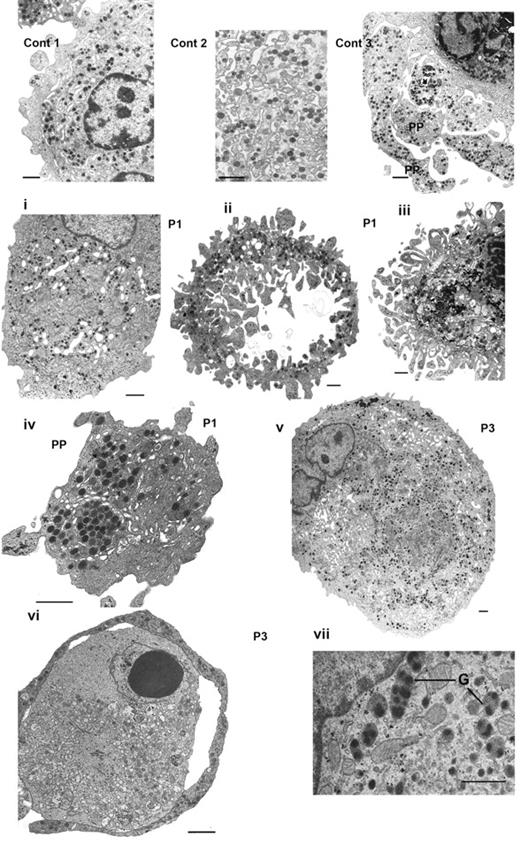
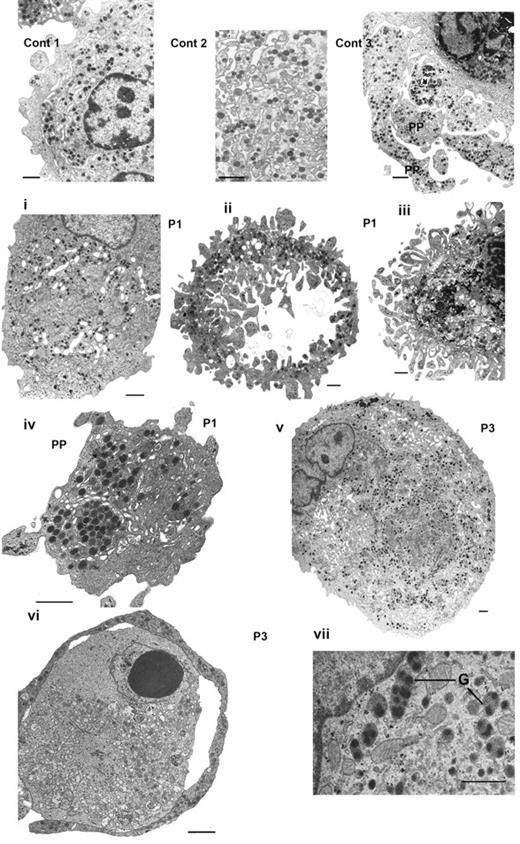
The morphology of MKs at day 14 in culture was examined by EM (Figure 3). First shown are images for control MKs: (1) a normal mature MK with a well-developed demarcation membrane system (DMS) and numerous α-granules, (2) a field at higher magnification illustrating the DMS and granules, and (3) the characteristic presence of proplatelets. Abnormalities observed for the MKs from P1 are illustrated in Figure 3i-iv and those from P3 in Figure 3v-vii. In Figure 3i, the MK of P1 contains a large number of granules with a heterogeneous distribution and a well-developed DMS concentrated in discrete domains. In Figure 3ii, the MK has lost much of its cytoplasm as well as the nucleus, giving a disparate structure but one that still contains α-granules inside the cytoplasmic external ring. In Figure 3iii, the nucleus is still present inside the cell, but the cytoplasm seems disorganized and frayed; this probably represents an intermediate stage to that seen in Figure 3ii). Figure 3iv shows the section of an abnormal large proplatelet with granules abnormally concentrated in discrete areas. For P3, a very large MK shows alternating areas with granules and others with DMS (Figure 3v). Figure 3vi illustrates a proplatelet-like outer ring containing α-granules but also shows signs of an abnormal cytoplasmic structure, and shows abnormal granules with inside each structure multiple nucleoids. Globally, these abnormalities concern approximately half of the MKs examined, with the most characteristic feature being the apparent fragility of the structures examined.
Abnormal MKs observed by EM. Control MKs (Cont) are illustrated in thetop panel: (1) a normal mature MK, (2) DMS and granules shown at higher magnification, and (3) the formation of proplatelets (PP). Next is shown a panel of MKs obtained in vitro culture from CD34+ blood cells from P1 (i-iv) and from P3 (v-vii). Panel i shows heterogeneity in granule and DMS distribution in a large mature MK. Panel ii shows granules are present only in the peripheral ring, whereas the cytoplasm has a disrupted frayed structure with segments of cytoplasm devoid of granules, note also the presence of ghost structures in the center. In panel iii, the nucleus is still present; at the periphery fragments seem to be detaching from the main body of the MK and are often without granules. In panel iv, an abnormal proplatelet has granules concentrated in discrete zones. In panel v, a large MK from P3 has a heterogeneous distribution of granules and abnormal membrane complexes. In panel vi, an abnormal MK with at the periphery a ring-like proplatelet structure containing α-granules; there are signs of apoptosis in part of the cytoplasm and inside the nucleus. Panel vii illustrates immature α-granules (G) with several nucleoids inside of 1 structure. Bars indicate 1 μm.
Abnormal MKs observed by EM. Control MKs (Cont) are illustrated in thetop panel: (1) a normal mature MK, (2) DMS and granules shown at higher magnification, and (3) the formation of proplatelets (PP). Next is shown a panel of MKs obtained in vitro culture from CD34+ blood cells from P1 (i-iv) and from P3 (v-vii). Panel i shows heterogeneity in granule and DMS distribution in a large mature MK. Panel ii shows granules are present only in the peripheral ring, whereas the cytoplasm has a disrupted frayed structure with segments of cytoplasm devoid of granules, note also the presence of ghost structures in the center. In panel iii, the nucleus is still present; at the periphery fragments seem to be detaching from the main body of the MK and are often without granules. In panel iv, an abnormal proplatelet has granules concentrated in discrete zones. In panel v, a large MK from P3 has a heterogeneous distribution of granules and abnormal membrane complexes. In panel vi, an abnormal MK with at the periphery a ring-like proplatelet structure containing α-granules; there are signs of apoptosis in part of the cytoplasm and inside the nucleus. Panel vii illustrates immature α-granules (G) with several nucleoids inside of 1 structure. Bars indicate 1 μm.
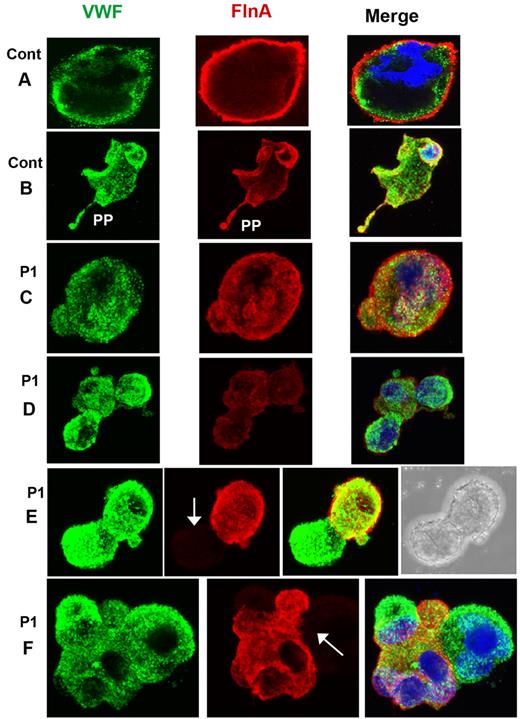
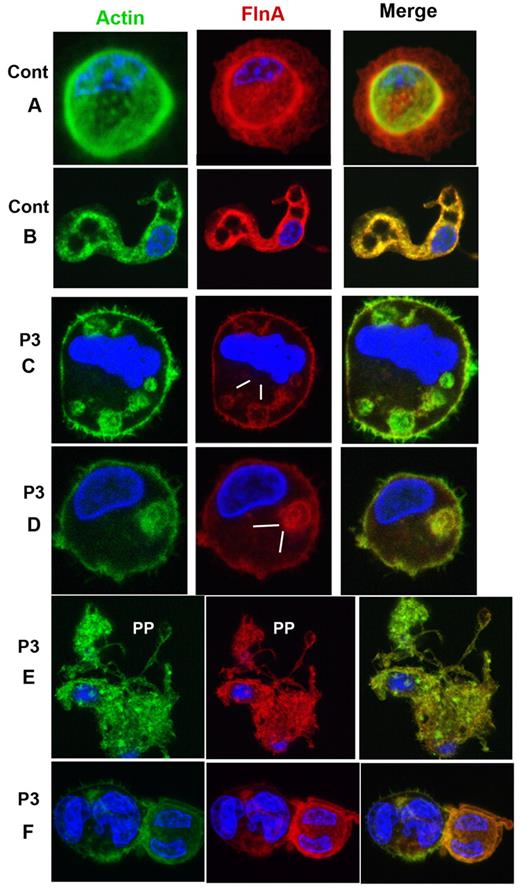
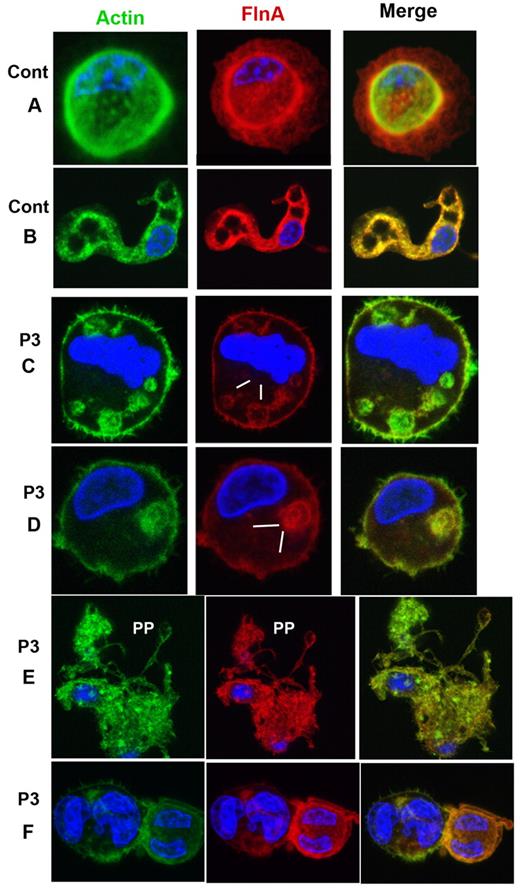
MKs grown from CD34+ cells also were studied for P1 by confocal microscopy after spreading on polylysine-coated glass slides and dual labeling for FLNa and VWF. Representative images for control MKs are shown in Figure 4A-B. For P1, a population of mature MKs normally expressed both proteins (Figure 4C-D); however, a population of MKs with only VWF labeling was also apparent (Figure 4E-F). Figure 5 illustrates mature MKs from P3 double stained for actin filaments and FLNa. For the control cells, the staining was homogeneous, with colocalization of both proteins (Figure 4A-B). For P3, all MKs were positive for both markers, but staining was heterogeneous within the cytoplasm; some areas were positive and other areas had no labeling, suggesting that the same MK can produce platelets with different amounts of FLNa (Figure 5C-D). Nevertheless, peripheral rings were positive for FLNa (Figure 5C-D). It is interesting to note that actin filaments showed the same distribution. Formation of proplatelets can be seen (Figure 5E), and these were labeled for both actin and FLNa, but with an overall morphology suggestive of a disintegration of the MK. Smaller MKs with prominent nuclei had a more normal structure (Figure 5F).
Distribution of FLNa and VWF in mature MKs cultured from CD34+ blood cells of P1. (A-B) Mature MKs from a control donor with the concomitant presence of FLNa and VWF also in the proplatelets. For P1 (C-F), MKs are shown normally containing VWF and FLNa (C), whereas others contain decreased amounts of FLNa (D) or are devoid in FLNa (E-F). Arrows indicate the presence of MKs devoid in FLNa. In panel E, the right side shows the presence of a nucleus, confirming the identification of a cell as an MK by refractory microscopy, in other cells, nuclei are labeled by 4,6-diamidino-2-phenylindole (blue).
Distribution of FLNa and VWF in mature MKs cultured from CD34+ blood cells of P1. (A-B) Mature MKs from a control donor with the concomitant presence of FLNa and VWF also in the proplatelets. For P1 (C-F), MKs are shown normally containing VWF and FLNa (C), whereas others contain decreased amounts of FLNa (D) or are devoid in FLNa (E-F). Arrows indicate the presence of MKs devoid in FLNa. In panel E, the right side shows the presence of a nucleus, confirming the identification of a cell as an MK by refractory microscopy, in other cells, nuclei are labeled by 4,6-diamidino-2-phenylindole (blue).
Distribution of FLNa and actin in mature MKs cultured from CD34+ blood cells of P3, with isolated thrombocytopenia. (A-B) Mature MKs of a control donor illustrating the concomitant presence of FLNa and actin also seen in extending proplatelets (B). For P3 (C-F), MKs contain FLNa heterogeneously distributed in patches; interestingly, the same distribution is observed for actin filaments (C-D, zones devoid in FLNa). Panel E shows an MK forming PP; this MK has an irregular distribution of FLNa, the MK seems to be partially disrupted. (F) Small MKs with large nuclei show a colocalization of actin and FLNa.
Distribution of FLNa and actin in mature MKs cultured from CD34+ blood cells of P3, with isolated thrombocytopenia. (A-B) Mature MKs of a control donor illustrating the concomitant presence of FLNa and actin also seen in extending proplatelets (B). For P3 (C-F), MKs contain FLNa heterogeneously distributed in patches; interestingly, the same distribution is observed for actin filaments (C-D, zones devoid in FLNa). Panel E shows an MK forming PP; this MK has an irregular distribution of FLNa, the MK seems to be partially disrupted. (F) Small MKs with large nuclei show a colocalization of actin and FLNa.
Discussion
Our results reinforce the concept that familial thrombocytopenias and platelet anisotropy because of defects in MK-expressed genes often involve the link between membrane adhesion receptors and the cytoskeleton. They imply that thrombocytopenia with enlarged round platelets can occur if any 1 of the 3 partners in the VWF-GPIb-FLNa axis is defective and confirm a key role for GPIbα-mediated signaling in megakaryocytopoiesis. Classically, in the Bernard–Soulier syndrome, giant platelets are formed in the absence or with nonfunctional GPIb-IX-V complexes.21 GPIbα is the main adhesion receptor of platelets for VWF and its cytoplasmic domain binds to the platelet cytoskeleton via FLNa.3,4,7 For our patients, GPIb was normally expressed or increased as determined either by flow cytometry (supplemental Table 1) or by Western blotting (data not shown); results contrasting with the model of FlnA-deficient mice where GPIb was decreased.15 A link to GPIb-mediated signaling and enlarged platelets comes from von Willebrand disease type 2B (VWD2B) where gain-of-function mutations in VWF result in an enhanced interaction between GPIbα and VWF.19,20 Other examples of defects leading to giant platelet formation include MHY9-related disease. Here, autosomal dominant mutations of the MYH9 gene encoding the nonmuscle myosin heavy chain type IIA lead to macrothrombocytopenia and a multifactorial phenotype with manifestations including different combinations of leukocyte inclusions, kidney disease, deafness, and cataracts.21,22 Both in VWD2B and MYH9-related disease, the proportions of abnormal large platelets differ widely between patients and also in patients with the same mutation belonging or not to the same family, suggesting a role for other genetic factors.22,23 As observed for patients with FLNA mutations, the presence of a population of normal-sized platelets often contributes to the anisotropy observed. An initial report concerning patients with the tubulin (TUBB1) p.Q43P polymorphism indicates that this polymorphism also is associated with the presence of round large platelets and that some of these patients can develop a thrombocytopenia.24 But additional characteristics include an abnormal distribution of a ring of microtubules and a peripheral zone devoid in granules, thus differing from patients with FLNA mutations. More recently, it was shown that the mutation TUBB1 p.R1318W25 was responsible for macrothrombocytopenia.
In view of the increasing number of macrothrombocytopenias now described, immunofluorescence analysis will be useful for clinical practice: the presence of myosin precipitates is in favor of a MYH9 syndrome,26 the presence of platelet aggregates and VWF at the periphery of the platelets are arguments in favor of VWD2B,20 and a severe reduction in α-granules that can be evaluated by labeling of intragranular proteins confirms the gray platelet syndrome.27 Here, we show that abnormal FLNa with a population of negative platelets could be added to the panel of immunofluorescence tests for patients with macrothrombocytopenia.
The phenotype of FlnA-deficient platelets has recently been described in a mouse model.15 Platelets from FlnA+/− mice possessed macrothrombocytopenia and long tail–bleeding times. In this model, platelets specifically lacking FlnA were generated using Cre-lox technology; FlnAloxp/y GATA1-Cre males were more thrombocytopenic and their platelets were larger. Differences between our results for human patients and mouse FlnA deficiency include (1) the enlarged human platelets tended to be round, (2) the decrease in GPIb expression and its degradation were not seen in patients, and (3) leukocyte numbers were increased in mice but normal in patients. Comparison between human disease and the mouse model must take into account the fact that FLNA and Flna are located on the X chromosome and thus concerned by X chromosome inactivation.28 Furthermore, the defects in our patients are variable; they correspond in the first patient to a stop codon present on repeat 15, resulting in an abnormal protein; in the second case to a deletion of several exons from exon 31-32 to exon 46-48; and in the third to a previously undescribed missense mutation in a very conserved region of repeat 16 and absent from the 600 control chromosome examined.
Western blotting revealed some additional FLNa bands; in addition to that corresponding to native FLNa, there is a band of 100 kDa for P1, whereas P3 showed more heterogeneity. These bands are highly suggestive of a proteolytic process. It should be noted that results remained consistent for P3 during the 2 years of the study. Proteolysis has already been reported for FLNa that can be cleaved by calpain, to give bands of 200 and 100 kDa.29 We cannot exclude that FLNa degradation modifies the scaffolding function of FLNa for signaling molecules, leads to proteosomal degradation,30,31 or both. For P2, 80% of FLNa was normal in Western blotting, suggesting either that any truncated protein is too unstable to be maintained associated with the platelets or that the mRNA is unstable.
Confocal microscopy investigating the localization of platelet FLNa revealed an absence of FLNa for ∼ 20% of the platelets for the patients with the stop codon (P1) and for the patient with a deletion (P2), a number that is close to that observed in FlnA+/− female mice.15 This population of negative platelets recognized by their VWF content included the largest forms. For P3 with the novel missense mutation, the number of FLNa-negative platelets was lower, but the distribution of FLNa inside each platelet was particularly heterogeneous. Furthermore, the FLNa had a punctiform texture compared with the compact structure found for the controls, a finding more exaggerated for P3 and perhaps linked to the FLNa degradation. All of these abnormalities prompted us to examine megakaryocytopoiesis for the patients. MKs grown in vitro from peripheral CD34+ blood cells of the patients also showed cells that were negative for FLNa. Because for P1 and P2 a negative population of platelets for FLNa also was detected, this suggests not only that these platelets have been produced from FLNa-negative MKs but also that FLNa is not essential for platelet production. For P3, only a few platelets were devoid in FLNa, but within the MK cytoplasm the distribution of FLNa was very heterogeneous. Interestingly, in mature MKs instead of a normal proplatelet development, sometimes frayed structures and signs of degradation were present, suggesting that the FLNa defect favors an abnormal disruption into fragments, often not containing granules, suggesting that these MKs were only partially effective in platelet production. This phenomenon can result from peripheral cytoskeletal instability.7 A role for FLNa in α-granule maturation, trafficking, or both also might be suggested by our results.
Very recently, Jurak Begonja et al16 studying the megakaryocytopoiesis of FlnAloxp PF4-Cre mice detected MKs totally devoid of FlnA that produced enlarged fragile platelets with decreased surface expression of GPIbα. In the patients studied by us with X-linked inheritance and heterozygous mutations, the defects are more subtle except for the thrombocytopenia (P1 and P3) and the platelet anisotropy that was found for all 3 patients. Such findings had been noted previously in isolated reports, but these emphasized the other, more obvious phenotypes, including FLNA-PVNH, of the filaminopathies A. The X random inactivation measured in lymphocytes for the 3 patients showed that ∼ 50% of cells are normal and half affected; however, this can be different for the MKs because each tissue can have different X inactivation. Individual MKs can therefore either show defects in FLNa or have normal protein. The fact that no more than 20% of platelets were lacking FLNa may suggest a rapid clearance of negative platelets as found in the mouse model.16 Thrombocytopenia was more severe for P3 with FLNa present in almost all of the platelets, suggesting that the missense mutation has specific consequences on platelet production, clearance, or both. For P2, the normal platelet count with the presence of platelets lacking FLNa suggests that the equilibrium between platelet production and destruction can be maintained.
The pathologic mechanisms of filaminopathies A are probably attributed to loss of partner binding or aberrant interactions caused by mutations.3,31 However, the effect of most of these mutations on partner interactions remains to be elucidated at the molecular level. Interestingly, in the mouse model, adhesion of platelets and thrombus formation to collagen was decreased.15 A previously undescribed FLNa interaction with the tyrosine kinase Syk was shown to be important for dense granule secretion. In a parallel study, we have also examined the interaction of our patients' platelets with collagen under flow and the platelet signaling pathways involved in thrombus buildup. Our results in which we describe how loss of platelet function despite an often normal aggregation response in vitro also contributes to the bleeding manifestations of the patients will be the subject of a second manuscript (M.B. et al, manuscript in preparation). Although we cannot exclude that endothelial cell or vascular defects contribute to the bleeding, we are accumulating evidence for shear dependent defects on thrombus formation and or stability under flow. These relate to altered platelet reactivities on collagen- and VWF-coated surfaces.
In conclusion, our study identifies a new subtype of filaminopathy A in which inherited platelet macrothrombocytopenia is the primary phenotype. In view of the fact that ITP is diagnosed by exclusion and that the molecular causes of many patients with congenital thrombocytopenias are unknown, we believe that FLNA mutations should be considered as a new contributory cause. Our results also extend the concept that inherited thrombocytopenias because of defects in megakaryocyte-expressed genes often involve an abnormal link between membrane adhesion receptors and the cytoskeleton.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Frédéric Martins, Elodie Guérineau, Annie Paponneau, Robert Combrié, Audrey Bidet, Julie Bouron, Xavier Pillois, and Sophie Salome-Desnoulez for technical assistance.
This study was supported in part by grants from Gis-Maladies Rares, Inserm (ANR-08-GENO-028-03) and DIATROC Program Hospitalier de Recherche Clinique.
Authorship
Contribution: P.N., A.N., and C.G. were responsible for study design and coordination; N.D., M.B., and I.C. designed data and performed experiments; I.Y.-M., P.R., A.-C.P., F.A., A.K., J.-M.D.L., C.R., and E.B. participated in the research; G.S., P.F., D.C., B.A., and D.L. commented on the study design; P.N., A.N., C.G., I.C., N.D., W.V., and M.B. wrote the manuscript; and all authors were responsible for revisions of draft manuscripts.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paquita Nurden, Centre de Référence des Pathologies Plaquettaires, Laboratoire d'Hématologie, Hôpital Cardiologique, 33604 Pessac, France; e-mail: paquita.nurden@cnrshl.u-bordeaux2.fr.
References
Author notes
P.N. and N.D. contributed equally to this work.