Abstract
The mammalian target of rapamycin (mTOR) signaling pathway plays a critical role in growth and survival of BCR-ABL transformed cells. AMPK kinase is a metabolic sensor that exhibits suppressive effects on the mTOR pathway and negatively regulates mTOR activity. We report that AMPK activators, such as metformin and 5-aminoimidazole-4-carboxamide ribonucleotide, suppress activation of the mTOR pathway in BCR-ABL–expressing cells. Treatment with these inhibitors results in potent suppression of chronic myeloid leukemia leukemic precursors and Ph+ acute lymphoblastic leukemia cells, including cells expressing the T315I-BCR-ABL mutation. Altogether, our data suggest that AMPK is an attractive target for the treatment of BCR-ABL–expressing malignancies and raise the potential for use of AMPK activators in the treatment of refractory chronic myeloid leukemia and Ph+ acute lymphoblastic leukemia.
Introduction
Chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL) are characterized by the presence of the abnormal reciprocal translocation between chromosomes 9 and 22, which results in expression of the abnormal BCR-ABL kinase fusion protein.1,2 The constitutive tyrosine kinase activity of BCR-ABL leads to engagement of a plethora of antiapoptotic and pro-proliferative effector cascades, including PI3K/mammalian target of rapamycin (mTOR)3-7 and MAPK pathways.8,9 Inhibition of mTOR and its effectors plays an important role in the generation of the antileukemic effects of BCR-ABL inhibitors,3-7,10 whereas there is evidence that combinations of rapamycin with imatinib mesylate or nilotinib result in enhanced antileukemic responses.3-7,10 The better understanding of the structure of distinct complexes formed by mTOR (mTORC1 and mTORC2) and the development of catalytic inhibitors of mTOR have led to efforts to overcome the resistance that BCR-ABL–expressing cells develop in many cases. Two different catalytic inhibitors (PP242 and OSI-027) have been recently shown to block growth of BCR-ABL–expressing cells, including cells with BCR-ABL mutations that are resistant to nilotinib, imatinib, or dasatinib.11,12
In the present study, we determined whether targeting CML cells using a different approach, involving activation of the 5′ AMP-activated protein kinase (AMPK) pathway, results in antileukemic effects. For these studies, we used the pharmacologic activators of AMPK, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and metformin. AICAR is transported in the cell by the adenosine transporter and is metabolized to an AMP analog, ZMP, which in turn binds to the γ-subunit of AMPK, thus enabling the activating phosphorylation of AMPK by LKB1 on Thr172 to occur.13 On the other hand, metformin is transported in the cells via the Oct-1 transporter, resulting in increased cytoplasmic AMP, thereby activating AMPK.14,15 Our data demonstrate that AICAR and metformin inhibit the mTOR signaling cascade in BCR-ABL cells and suppress the growth of different CML-derived cell lines, as well as primitive progenitors from CML patients. Importantly, both AICAR and metformin are effective in suppressing the growth of different types of cells expressing the T315I-BCR-ABL mutation, which renders cells resistant to all pharmacologic inhibitors currently approved for the treatment of CML.
Methods
Cells and reagents
K562, KT1, and BV173 cells were grown in RPMI 1640 medium supplemented with 10% FBS and gentamicin. Antibodies against the phosphorylated forms of acetyl-CoA carboxylase (ACC), S6 kinase (S6K), rpS6, 4E-BP1, AKT, and TSC2 and against ACC, rpS6, 4E-BP1, AKT, and PARP were purchased from Cell Signaling Technology. Antibodies against S6K, TSC2, and tubulin were purchased from Sigma-Aldrich. The antibody against GAPDH was purchased from Millipore. AICAR and metformin were purchased from Sigma-Aldrich. Rapamycin was purchased from Calbiochem/EMD. Imatinib mesylate was purchased from ChemieTek. PP242 was purchased from Chemdea. The Ba/F3-BCR-ABL or Ba/F3-T315I-BCR-ABL transfectants were kindly provided by Dr Brian J. Druker (Howard Hughes Medical Institute and Oregon Health & Science University, Portland, OR).
Cell lysis and immunoblotting
Cell lysis, immunoprecipitation, and immunoblotting were performed as in previous studies.12,16,17 Cells were treated with AICAR (1mM), metformin (10mM), imatinib mesylate (5μM), PP242 (0.5μM), or rapamycin (20nM) for the indicated times, unless otherwise indicated. In some experiments, equal amounts of protein from the same experiment were resolved separately by SDS-PAGE and immunoblotted with antibodies against either the phosphorylated form or against the total protein.
Cell proliferation and apoptosis assays
Human leukemic hematopoietic progenitor cell assays
Bone marrow or peripheral blood from CML patients was collected, after obtaining informed consent in accordance with the Declaration of Helsinki, approved by the Institutional Review Board of Northwestern University. Clonogenic hematopoietic leukemic progenitor assays in methylcellulose were performed as in previous studies.12
Results and discussion
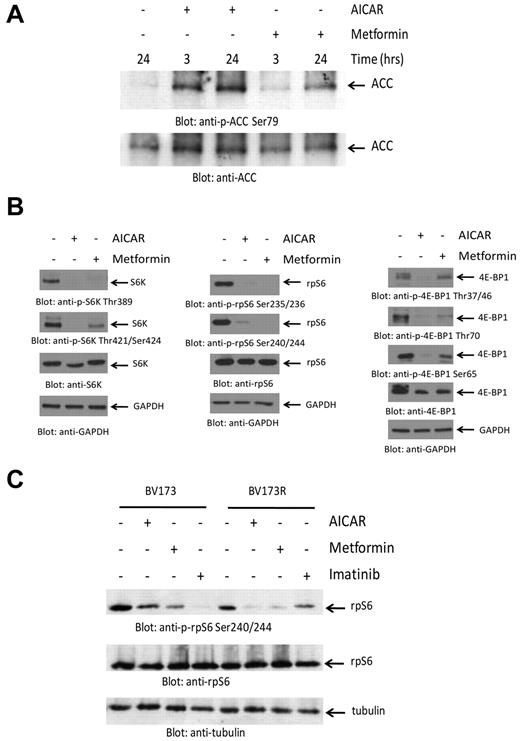
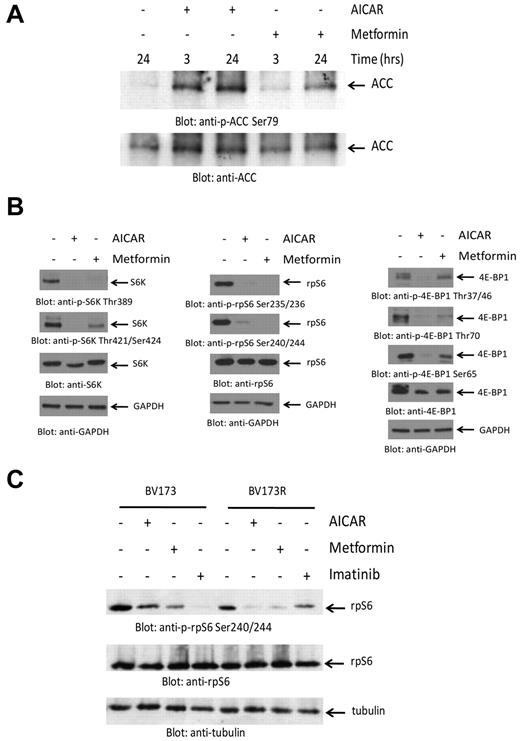
In initial experiments, we determined whether treatment of BCR-ABL–expressing cells with metformin or AICAR results in induction of the kinase activity of AMPK. Both AICAR and metformin induced phosphorylation of ACC at Ser79 and of TSC2 at Ser1387, reflecting induction of AMPK kinase activity18,19 (Figure 1A; supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To determine whether such engagement of AMPK results in inhibition of mTOR activity, we examined their effects on the engagement of elements of the mTOR pathway. As shown in Figure 1B, both AICAR and metformin were potent suppressors of phosphorylation of the S6K on Thr389 and Thr421/Ser424 (Figure 1B left panels), as well as phosphorylation of the downstream effector of S6K and rpS6 on Ser235/236 and Ser240/244 (Figure 1B middle panels). Similarly, both metformin and AICAR suppressed phosphorylation of the other major target for mTOR kinase activity, 4E-BP1, at all different sites examined, including Thr37/46, Ser65, and Thr70 (Figure 1B right panels). Similar results were obtained when the CML-derived KT1 cell line was studied (supplemental Figure 1C). When time course experiments were performed, we found that the reduction in mTOR signaling occurred in a time-dependent manner with a maximal effect at 24 hours (supplemental Figure 2). Notably, AICAR or metformin did not block phosphorylation of AKT on Ser473, indicating that AMPK activation does not inhibit mTORC2 activity (supplemental Figure 3). We also determined whether AMPK activation results in mTOR suppression in cells expressing the T315I-BCR-ABL mutation. We determined the effects of metformin or AICAR in imatinib mesylate-sensitive (BV173) and imatinib mesylate-resistant (BV173R) Ph+ ALL cells.20 Consistent with our observations in other BCR-ABL–expressing cell lines, we found that phosphorylation of rpS6 was inhibited by both AICAR and metformin in both WT BCR-ABL–expressing BV173 cells, as well BV173R cells, which express T315I-BCR-ABL (Figure 1C).
Suppression of mTOR activity by AMPK activators in BCR-ABL–expressing cells. (A) K562 cells were treated with either AICAR or metformin, as indicated. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with an antibody against the Ser79 phosphorylated form of ACC. The same blot was stripped and reprobed with an anti-ACC antibody, as indicated. (B) K562 cells were treated with either AICAR or metformin for 24 hours. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (C) BV173 or BV173R cells were treated with AICAR, metformin, or imatinib mesylate for 24 hours as indicated. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
Suppression of mTOR activity by AMPK activators in BCR-ABL–expressing cells. (A) K562 cells were treated with either AICAR or metformin, as indicated. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with an antibody against the Ser79 phosphorylated form of ACC. The same blot was stripped and reprobed with an anti-ACC antibody, as indicated. (B) K562 cells were treated with either AICAR or metformin for 24 hours. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (C) BV173 or BV173R cells were treated with AICAR, metformin, or imatinib mesylate for 24 hours as indicated. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
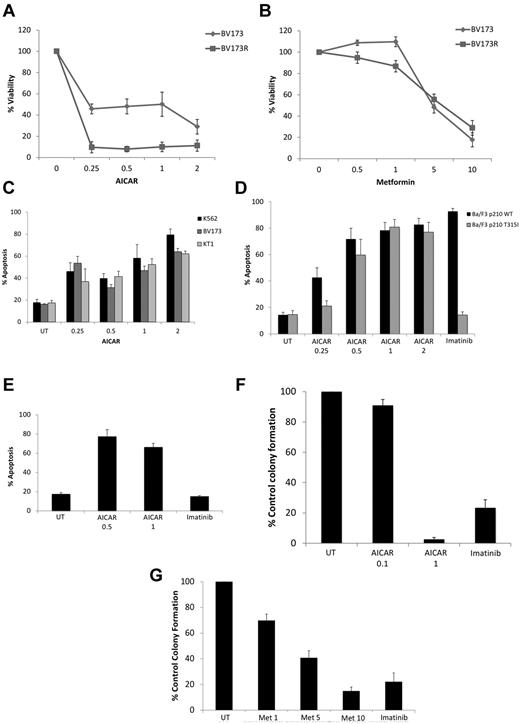
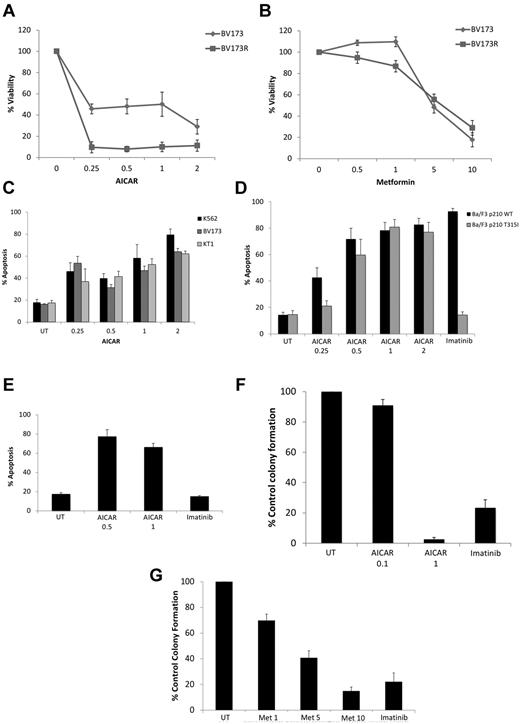
To examine the functional consequences of engagement of AMPK in the context of BCR-ABL transformation, we determined the effects of AICAR or metformin on growth and/or survival of BCR-ABL–expressing cells. Treatment of cells with AICAR suppressed proliferation of various BCR-ABL cell lines, including K562 and KT1 (supplemental Figure 4A-B), BV173 cells, as well as BV173 cells expressing T315I–BCR-ABL (Figure 2A). Similar results were seen when metformin was used (Figure 2B; supplemental Figure 4C-D). Treatment with AICAR also resulted in induction of apoptosis in K562, KT1, and BV173 (Figure 2C) and Ba/F3 cells stably transfected with WT–BCR-ABL (Figure 2D), as well as Ba/F3 stably transfected with T315I–BCR-ABL (Figure 2D) or BV173R-expressing T315I–BCR-ABL (Figure 2E). In addition, when the effects of AMPK activators on primitive leukemic progenitors were examined in clonogenic assays in methylcellulose, we found potent, dose-dependent, suppression of leukemic CFU-GM colony formation from different CML patients (Figure 2F-G).
Antileukemic effects of AMPK activators. (A) BV173 or BV173R cells were treated for 5 days with the indicated concentrations of AICAR (mM), and cell viability was determined by methyl-thiazolyl-tetrazolium assays. Data are expressed as percentage control cells and represent mean ± SE of 4 experiments. (B) BV173 and BV173R cells were treated for 5 days with the indicated concentrations of metformin (mM), and cell viability was determined by methyl-thiazolyl-tetrazolium assays. Data are expressed as percentage control cells and represent mean ± SE of 4 experiments. (C) K562, KT1, and BV173 cells were treated with increasing concentrations of AICAR (mM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data represent mean ± SE of 4 experiments. (D) Ba/F3 cells stably expressing WT-BCR-ABL or T315I-BCR-ABL were treated with either AICAR (mM) or imatinib mesylate (1μM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data represent mean ± SE of 4 experiments. (E) BV173R cells expressing the T315I mutation were treated with the indicated concentrations of AICAR (mM) or imatinib (5μM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data are mean ± SE of 4 experiments. (F-G) Effects of different concentrations of AICAR (mM; F), metformin (mM; G), and imatinib mesylate (1μM) on primary leukemic progenitor colony formation (CFU-GM) from CML patients in clonogenic assays in methylcellulose. Data are expressed as percentage control leukemic CFU-GM colony formation of untreated samples and represent mean ± SE of 7 experiments for panel F and 9 experiments for panel G.
Antileukemic effects of AMPK activators. (A) BV173 or BV173R cells were treated for 5 days with the indicated concentrations of AICAR (mM), and cell viability was determined by methyl-thiazolyl-tetrazolium assays. Data are expressed as percentage control cells and represent mean ± SE of 4 experiments. (B) BV173 and BV173R cells were treated for 5 days with the indicated concentrations of metformin (mM), and cell viability was determined by methyl-thiazolyl-tetrazolium assays. Data are expressed as percentage control cells and represent mean ± SE of 4 experiments. (C) K562, KT1, and BV173 cells were treated with increasing concentrations of AICAR (mM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data represent mean ± SE of 4 experiments. (D) Ba/F3 cells stably expressing WT-BCR-ABL or T315I-BCR-ABL were treated with either AICAR (mM) or imatinib mesylate (1μM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data represent mean ± SE of 4 experiments. (E) BV173R cells expressing the T315I mutation were treated with the indicated concentrations of AICAR (mM) or imatinib (5μM) for 72 hours, as indicated. Apoptosis was assessed by annexin V/PI staining. Data are mean ± SE of 4 experiments. (F-G) Effects of different concentrations of AICAR (mM; F), metformin (mM; G), and imatinib mesylate (1μM) on primary leukemic progenitor colony formation (CFU-GM) from CML patients in clonogenic assays in methylcellulose. Data are expressed as percentage control leukemic CFU-GM colony formation of untreated samples and represent mean ± SE of 7 experiments for panel F and 9 experiments for panel G.
AMPK is a serine/threonine kinase, which functions as energy sensor of AMP/ATP ratio changes by AMP binding on its γ-subunit, resulting in a conformational change that allows for LKB1-mediated activating phosphorylation of AMPK at Thr172 position in the α-subunit.21,22 Because of its regulatory effects on mTOR, there has been recently an emerging interest on the potential use of AMPK activators as antitumor agents. AICAR and metformin are AMPK activators that exhibit antineoplastic activities and exhibit AMPK-dependent effects,23,24 although they also have AMPK-independent properties.25,26 Recent evidence has suggested that AICAR exhibits potent inhibitory effects against different types of leukemia and solid tumor cells in vitro,25,27-29 including BCR-ABL–transformed cells.25 Our studies demonstrate that AICAR exhibits potent effects on primitive leukemic progenitors from different patients with CML and, for the first time, demonstrate that it suppresses the growth and induces apoptosis of Ph+ ALL cells (BV173), including Ph+ ALL cells carrying the T315I mutation. As AICAR only inhibits TORC1 and not TORC2 signals, it is probable that a different mechanism, unrelated to effects on mTOR, is involved in the proapoptotic response. Importantly, our data show, for the first time, that another AMPK activator, metformin, suppresses growth of different BCR-ABL–expressing lines and primary leukemic progenitors from CML patients, including cells expressing the T315I-BCR-ABL mutation. These findings are particularly interesting, in view of the emerging high interest of clinically developing metformin as an antineoplastic agent.30 The abilities of metformin and AICAR to generate antileukemic responses also raise the possibility that these agents may be useful to target leukemic stem cells in CML. This is particularly important, as there is clear evidence now that, even in imatinib-sensitive cases, the leukemic stem cells are resistant31 and future efforts to investigate this possibility are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Brian Druker (Knight Cancer Institute, Oregon Health & Science University, Portland, OR) for providing the Ba/F3 transfectants.
This work was supported by a Veterans Administration Merit Review grant, the Leukemia & Lymphoma Society (grant LLS-6166-09), and the National Institutes of Health (grants R01CA121192 and R01CA77816).
National Institutes of Health
Authorship
Contribution: E.V. performed research, analyzed data, and contributed to manuscript writing; J.K.A. and H.G. performed research; N.J.D. contributed vital reagents and edited the manuscript; and L.C.P. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonidas C. Platanias, Robert H. Lurie Comprehensive Cancer Center and Division of Hematology/Oncology, Northwestern University Medical School, Lurie 3-125, 303 E Superior Ave, Chicago, IL 60611; e-mail: l-platanias@northwestern.edu.