Abstract
The release of histones from dying cells is associated with microvascular thrombosis and, because histones activate platelets, this could represent a possible pathogenic mechanism. In the present study, we assessed the influence of histones on the procoagulant potential of human platelets in platelet-rich plasma (PRP) and in purified systems. Histones dose-dependently enhanced thrombin generation in PRP in the absence of any trigger, as evaluated by calibrated automated thrombinography regardless of whether the contact phase was inhibited. Activation of coagulation required the presence of fully activatable platelets and was not ascribable to platelet tissue factor, whereas targeting polyphosphate with phosphatase reduced thrombin generation even when factor XII (FXII) was blocked or absent. In the presence of histones, purified polyphosphate was able to induce thrombin generation in plasma independently of FXII. In purified systems, histones induced platelet aggregation; P-selectin, phosphatidylserine, and FV/Va expression; and prothrombinase activity. Blocking platelet TLR2 and TLR4 with mAbs reduced the percentage of activated platelets and lowered the amount of thrombin generated in PRP. These data show that histone-activated platelets possess a procoagulant phenotype that drives plasma thrombin generation and suggest that TLR2 and TLR4 mediate the activation process.
Introduction
Histones are cationic proteins that associate with DNA in nucleosomes and are involved in chromatin remodeling and regulation of gene transcription. Despite their physiologic nuclear localization, nucleosomes have been found in the circulation of both healthy subjects and patients, where they can be released from dying cells1 or actively secreted by activated inflammatory cells (neutrophils, basophils, and mast cells) in the form of “extracellular traps,” complex structures of DNA strands, histones, and cell-specific granule proteins.2,3 High blood levels of nucleosomes have been detected in several inflammatory, ischemic, autoimmune, and neoplastic diseases4 ; in some cases, a correlation with disease severity has been found.5 Whether extracellular nucleosomes are merely bystanders or active mediators in disease and which role histones play are important emerging questions. Histones are known to possess cytotoxic properties against both microorganisms6 and eukaryotic cells.7 Xu et al8 reported that extracellular histones behave as late mediators of cell damage and organ dysfunction during the hyperinflammatory reaction that characterizes sepsis, as shown by the efficacy of a neutralizing antibody against histone H4 in reducing mortality in several experimental models of murine sepsis. Moreover, direct injection of histones into mice resulted in death with pathologic lesions suggestive of a massive prothrombotic response similar to that found in sepsis, including diffuse microvascular thrombosis, fibrin and platelet deposition in the lung alveoli, and intra-alveolar hemorrhage. Fuchs et al9 recently reported that neutrophil extracellular traps (NETs) perfused with blood or platelet-rich plasma (PRP) stimulated platelets to adhere and aggregate and promote thrombus formation; NET integrity was considered to be essential in this process because treatment with DNase and heparin (which avidly binds histones) abrogated their effect. Histones H4 and H3 were found to be responsible for directly inducing the aggregation of washed human platelets.
In addition to their known role in hemostasis and thrombosis, platelets are widely recognized as mediators of inflammation and immune responses.10,11 They have been shown to express the TLRs, a major class of pattern-recognition receptors involved in the innate immune response through the recognition of microbial structures conserved among species (pathogen-associated molecular patterns) and of endogenous molecules released from damaged cells (damage-associated molecular patterns).12 TLR1, TLR2, TLR4, TLR6, TLR8, and TLR9 have been found on human platelets13,14 and were shown to be functional. For example, the TLR2 agonist PAM3-CSK4 induces platelet adhesion, degranulation, aggregation, and the formation of platelet-neutrophil aggregates,15 and lipopolysaccharide (LPS), a TLR4 agonist, induces platelet P-selectin expression and ATP secretion and primes platelets to aggregate in response to low-dose thrombin.16 Therefore, platelet TLRs might represent a bridge between inflammation and coagulation, as supported by studies demonstrating that TLRs are responsible for LPS-induced thrombocytopenia.17,18 In our laboratory, J. Xu recently found that histones specifically induce TLR2- and TLR4-mediated reporter gene expression in a cell line overexpressing different classes of TLRs.19
Given the increasing interest surrounding histones as new damage mediators and the importance of platelets in thrombosis and inflammation, we investigated the platelet-activating properties of histones, focusing mainly on the procoagulant potential of platelets in the plasma environment and in purified systems and the potential involvement of platelet TLRs in this process. In the present study, we show that histones induce a procoagulant phenotype in human platelets, thereby accelerating blood coagulation and enhancing thrombin generation, and that TLR2 and TLR4 are involved in the platelet response to histones.
Methods
Materials
The following reagents were purchased: calf thymus histones, PGE1, calf thymus DNA, Sepharose CL-2B, apyrase, BSA, LPS from Salmonella typhimurium, de-N-sulfated heparin (de-N-heparin), and phosphodiesterase type 3 inhibitor cilostazol (all from Sigma-Aldrich); recombinant histones H1, H2A, H2B, H3, and H4 (all from New England Biolabs); calf intestinal alkaline phosphatase (Psp; Promega); corn trypsin inhibitor (CTI), human prothrombin, and human activated FX (all from Haematologic Technologies); FXII or FVII-deficient plasmas (both from George King Bio-Medical); anti–tissue factor (anti-TF) mAb and the chromogenic thrombin substrate Spectrozyme TH (both from American Diagnostica); neutralizing anti–human FXII mAb (QED Bioscience); the GPIIb/IIIa antagonist tirofiban (Merck); the P2Y12 antagonist N(6)-(2-methyl-thioethyl)-2-(3,3,3-trifluoropropylthio)-beta,gammadichloromethylene-ATP (cangrelor) (The Medicines Company); blocking mAbs against human TLR2 (clone T2.5), TLR4 (HTA125), and isotype control (IgG2a, all from eBioscience); PE-labeled streptavidin and PE-labeled goat-anti–mouse IgG (both from Molecular Probes); FITC-labeled annexin V (Invitrogen); human recombinant activated protein C (APC, Xigris; Ely Lilly); unfractionated heparin (American Pharmaceutical Partners); 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS; Avanti Polar Lipids). Large unilamellar vesicles composed of 80% POPC and 20% POPS were prepared by extrusion using a 100-nm Nucleopore membrane, as described previously.20 mAb HFV-237 against human factor V/Va (FV/FVa) was prepared as described previously.21 Polyphosphate (polyP), average length 70 units, was a kind gift from Dr Thomas Staffel (BK Giulini GmbH, Ludwigshafen, Germany). mAb S12 recognizing P-selectin was kindly provided by Dr RP McEver (Oklahoma Medical Research Foundation, Oklahoma City, OK). The Tyrode buffer composition was 10 mmol/L of HEPES pH 7.5, 140 mmol/L of NaCl, 2.7 mmol/L of KCl, 0.2 mmol/L of Na2HPO4, 12 mmol/L of NaHCO3, 5.5 mmol/L of D-glucose, and 1 mmol/L of MgCl2.
Blood collection, PRP preparation, and platelet isolation
Peripheral venous blood was collected from drug-free healthy human volunteers with institutional review board approval from the Oklahoma Medical Research Foundation by puncture with a 19-gauge needle into 3.8% trisodium citrate (9:1 blood-to-citrate ratio). Alternatively, blood was collected in citrate plus CTI (50 μg/mL final concentration in whole blood) to prevent contact activation. PRP was prepared immediately by centrifugation at 180g for 10 minutes at room temperature (RT); only the top half of the PRP was collected to avoid leukocyte contamination. The platelet concentration in native PRP ranged between 300 and 500 × 103/μL. Platelet-poor plasma (PPP) with minimal platelet contamination (< 103/μL) was obtained by centrifuging PRP at 1000g for 15 minutes at RT, followed by a second centrifugation of the supernatant at 12 000g for 10 minutes. Isolated platelets were prepared by gel filtration, as described previously.22 Briefly, PRP prepared from blood drawn into acid-citrate-dextrose was applied to a Sepharose CL-2B column and platelets were eluted with Tyrode buffer, pH 7.5. Gel-filtered platelets were counted and normalized to a concentration of 2 × 105/μL in Tyrode buffer.
Thrombin generation in PRP
Plasma thrombin generation was analyzed by the calibrated automated thrombinography (CAT) method as described by Hemker et al.23 In brief, 80 μL of PRP was dispensed into round-bottom 96-well plates (Immulon 2HB; Dynex Technologies) and incubated for 10 minutes at 37°C with the indicated concentrations of histones. Analysis was performed in a final volume of 120 μL and started upon addition of 20 μL of a mixture containing 100 mmol/L of CaCl2 and 2.5 mmol/L of ZGGR-AMC (Fluca reagent; Thrombinoscope BV). Where indicated, PRP was pretreated with the following antibodies or inhibitors before treatment with histones: anti-TF antibody (30 μg/mL), anti-TLR2 or anti-TLR4 or IgG2a mAbs (50 μg/mL), PGE1 (10 μmol/L), cilostazol (50 μmol/L), tirofiban (1.5 μmol/L), Psp (10 U/mL), apyrase (10 U/mL), or cangrelor (1 μmol/L). In some experiments, histones were preincubated for 10 minutes at RT with an equal-weight concentration of DNA or with de-N-heparin. Where indicated, the experiments were performed in PPP with or without procoagulant liposomes (10 μg/mL). In some cases, clotting was triggered by 20 μg/mL of purified polyP, which was incubated in PPP for 3 minutes before starting the test. Measurements were taken every 20 seconds over time in a FluoroScan Ascent fluorometer (Thermo Scientific), and data were analyzed using Thrombinoscope software. To correct for inner-filter effects and substrate consumption, each measurement was calibrated against the fluorescence curve obtained in a sample from the same plasma added with a fixed amount of thrombin-α2-macroglobulin complex (thrombin calibrator; Thrombinoscope BV). The parameters calculated by the software were the lag time, thrombin peak, time-to-peak, and endogenous thrombin potential (ETP). All samples and calibrators were run at least in duplicate.
Platelet aggregation
Aggregation of gel-filtered platelets was optically monitored in a PAP-4 Bio/Data 4-chamber aggregometer (Bio/Data Corporation) at 37°C in the presence of 2.5 mmol/L of CaCl2 at a constant stirring rate of 1200 rpm. The percentage of aggregation was recorded 10 minutes after the addition of histones. In some experiments, histones were pretreated with APC (6 μg/mL) for 30 minutes at 37°C or with heparin (1 U/mL) for 10 minutes at RT. Where indicated, gel-filtered platelets were treated with PGE1 (4 μmol/L) before agonist addition.
Flow cytometric analysis of gel-filtered platelets
For flow cytometric analysis, gel-filtered platelets in Tyrode buffer were incubated with histones (40 μg/mL) or PBS for 15 minutes at 37°C in the presence of 2.5 mmol/L of CaCl2. Where indicated, gel-filtered platelets were preincubated with anti-TLR2, anti-TLR4, or IgG2a mAbs (50 μg/mL) for 20 minutes at RT; in some experiments, histones were pretreated with APC or heparin as detailed above. For phosphatidylserine (PS) detection, after the stimulation, platelets were diluted in 20 mmol/L of HEPES, 150 mmol/L of NaCl, pH 7.5 (HBS) with 2.5 mmol/L CaCl2 and 1% BSA, labeled with FITC–annexin V for 10 minutes at RT in the dark, and then fixed with ice-cold 1% formalin in PBS for 30 minutes on ice. For P-selectin and FV/Va evaluation, platelets were diluted in HBS and fixed with 1% formalin in PBS for 30 minutes at RT; afterward, they were washed and incubated in HBS with 1% BSA containing the mAbs S12 recognizing P-selectin or HFV-237 against FV/Va for 30 minutes and then washed again and resuspended in HBS with 1% BSA with the appropriate detection system. After labeling and a further washing step when required, platelets were diluted and analyzed. Flow cytometry was performed on a FACSCalibur (BD Biosciences) equipped with an argon-ion laser emitting at 488 nm.
Prothrombinase assay
Gel-filtered platelets were incubated with buffer or histones for 15 minutes at 37°C without stirring in the presence of 2.5 mmol/L of CaCl2. Prothrombin was then added at a final concentration of 1.4 μmol/L along with more CaCl2 to preserve the concentration of 2.5 mmol/L. Thrombin generation was started after 3 minutes by adding 2 nmol/L of FXa. Because FVa was not added, it was provided entirely by platelets. The reaction was stopped after 1.5 minutes with ice-cold buffer (HBS with 0.5% BSA) containing 10 mmol/L of EDTA, and the amount of thrombin formed was determined by the rate of hydrolysis of the chromogenic substrate Spectrozyme TH (0.1 mmol/L). The rate of change in absorbance was measured in a Thermomax microplate reader (Molecular Devices) at 405 nm, and thrombin concentrations were calculated by comparison with the rates produced by known concentrations of purified human α-thrombin.
Statistical evaluation
All data are represented as means ± SD. Statistical analyses were performed using the Student paired t test with a 2-tailed distribution. P < .05 was considered statistically significant.
Results
Histones promote thrombin generation in PRP through platelet activation
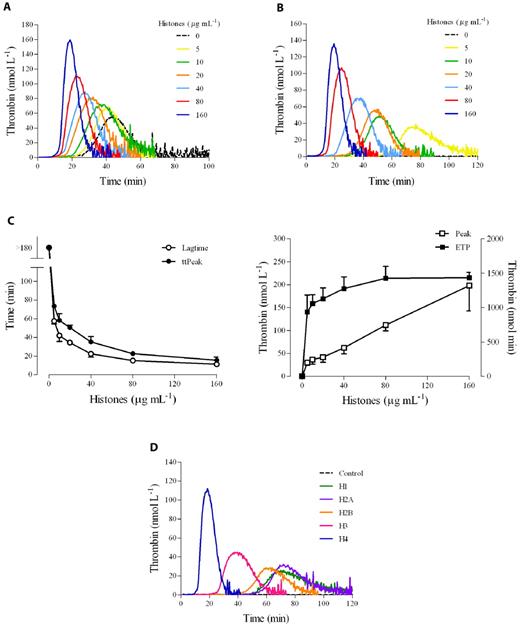
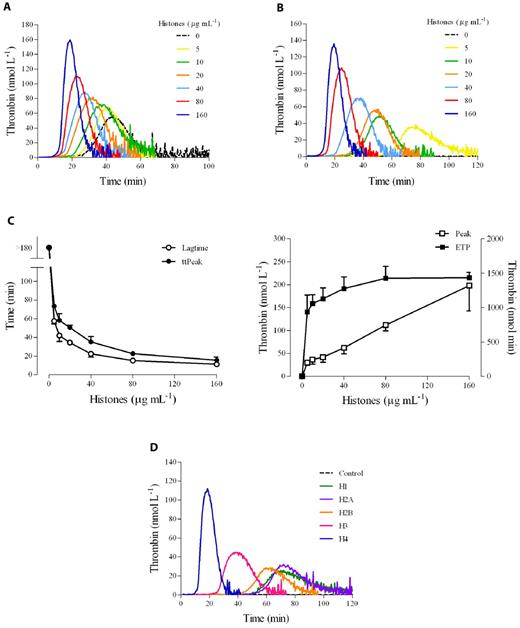
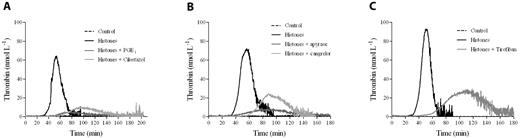
We determined the effect of a natural mixture of histones on thrombin generation in human PRP as evaluated by CAT. No trigger was added to recalcified plasma to increase the sensitivity of the test to the procoagulant potential of platelets. Histones (0-160 μg/mL) were incubated with PRP, and then calcium was added along with the fluorogenic thrombin substrate. In citrated PRP, histones dose-dependently stimulated thrombin generation (Figure 1A). In particular, they shortened both the lag time and the time-to-peak and increased the maximal thrombin generated; ETP was only slightly enhanced by the presence of histones. Under the experimental conditions used and in the absence of any platelet agonist, the clotting cascade could be initiated by the activation of the contact system.24 To avoid interference by spontaneously activated FXII on the clotting process, recalcification experiments were performed in PRP anticoagulated with citrate plus CTI, an inhibitor of FXIIa.24 The use of CTI in our assay prevents FXII activated during plasma preparation from influencing thrombin generation, but at the same time allows the intrinsic pathway to function when platelets are present because CTI is much less effective at inhibiting FXIIa generated by activated platelets than that formed in solution.25 In CTI-inhibited PRP, no thrombin was detected in the control sample during the course of the experiment, whereas a clear dose-dependent enhancement of thrombin generation was still induced by histones (Figure 1B), with an obvious shortening of the lag time and time-to-peak (about 4-fold reduction between the highest and the lowest concentrations), a striking increase of the peak (7-fold augmentation), and a more modest rise of the ETP (1.5-fold increase; Figure 1C). Similar results were obtained in whole blood based on the measurement of prothrombin fragment 1 and 2 (data not shown). To assess the relative role of the different histones present in the preparation used, experiments were performed using individual recombinant histones. As shown in Figure 1D, the capacity to induce thrombin generation in CTI-inhibited PRP could be attributed mainly to the histones H3 and, especially, H4.
Histones increase thrombin generation in human PRP. Thrombin generation was monitored in real time by CAT in the absence of any trigger. (A) Typical thrombograms in the presence of increasing concentrations of calf thymus histones (0-160 μg/mL) in recalcified citrated PRP. (B) Thrombograms in the presence of histones in recalcified CTI-inhibited PRP. Note that the control curve is flat. In both cases, a representative experiment of 4 performed with PRP from different donors is shown. The tails of the thrombograms have been cut for clarity in these and next graphs. (C) Changes in CAT parameters as a function of histone concentration in CTI-inhibited PRP. Lag time and time-to-peak (ttPeak) are shown on the left; thrombin peak and ETP on the right. Data are the means ± SD of 4 independent experiments. (D) Recalcified CTI-inhibited PRP was treated with single recombinant histones (20 μg/mL) and then thrombin generation was evaluated. A representative experiment of 3 performed with PRP from different donors is shown.
Histones increase thrombin generation in human PRP. Thrombin generation was monitored in real time by CAT in the absence of any trigger. (A) Typical thrombograms in the presence of increasing concentrations of calf thymus histones (0-160 μg/mL) in recalcified citrated PRP. (B) Thrombograms in the presence of histones in recalcified CTI-inhibited PRP. Note that the control curve is flat. In both cases, a representative experiment of 4 performed with PRP from different donors is shown. The tails of the thrombograms have been cut for clarity in these and next graphs. (C) Changes in CAT parameters as a function of histone concentration in CTI-inhibited PRP. Lag time and time-to-peak (ttPeak) are shown on the left; thrombin peak and ETP on the right. Data are the means ± SD of 4 independent experiments. (D) Recalcified CTI-inhibited PRP was treated with single recombinant histones (20 μg/mL) and then thrombin generation was evaluated. A representative experiment of 3 performed with PRP from different donors is shown.
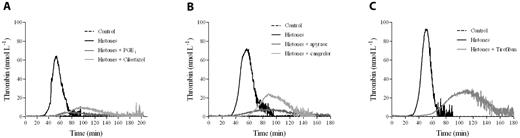
To verify the dependence of the histone effect on platelets, thrombin generation was evaluated in CTI-inhibited plasma in which platelets were omitted or substituted with POPS-containing liposomes. Histones did not induce detectable thrombin generation (data not shown), indicating that they were not able to directly drive the clotting cascade and thus induced thrombin generation in PRP by activating platelets. Therefore, we evaluated whether and to what extent platelet function inhibitors influence histone activity in CTI-inhibited PRP. Histone-induced thrombin generation was severely impaired in the presence of the common platelet inhibitors PGE1 and cilostazol (Figure 2A), which reduce platelet response to several agonists by raising the levels of intracellular cAMP. The importance of ADP and the role of its receptor P2Y12 were tested by incubating CTI-inhibited PRP with the ADP-scavenging enzyme apyrase or the specific P2Y12 antagonist cangrelor before stimulation with histones. Histone-induced thrombin generation was strongly dependent on ADP, because it was dramatically inhibited by apyrase, and the P2Y12 receptor was involved, because cangrelor significantly reduced the amount of thrombin formed in response to histones (Figure 2B). A similar reduction in the amount of thrombin formed was achieved with tirofiban, a synthetic nonpeptide inhibitor of the active glycoprotein IIb/IIIa complex (Figure 2C).
Platelet inhibitors reduce thrombin generation in histone-treated PRP. Recalcified CTI-inhibited PRP was treated with 10 μmol/L of PGE1 or 50 μmol/L of cilostazol (A), 10 U/mL of apyrase or 1 μmol/L of cangrelor (B), or 1.5 μmol/L of tirofiban (C) for 10 minutes at 37°C before stimulation with histones (40 μg/mL). Thrombin generation was then evaluated by CAT. A representative experiment of 4 performed with PRP from different donors is shown.
Platelet inhibitors reduce thrombin generation in histone-treated PRP. Recalcified CTI-inhibited PRP was treated with 10 μmol/L of PGE1 or 50 μmol/L of cilostazol (A), 10 U/mL of apyrase or 1 μmol/L of cangrelor (B), or 1.5 μmol/L of tirofiban (C) for 10 minutes at 37°C before stimulation with histones (40 μg/mL). Thrombin generation was then evaluated by CAT. A representative experiment of 4 performed with PRP from different donors is shown.
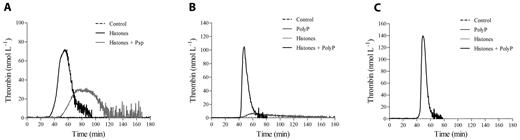
Thrombin generation in histone-treated PRP is driven by platelet-derived polyP
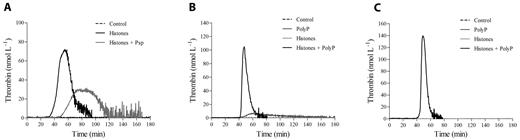
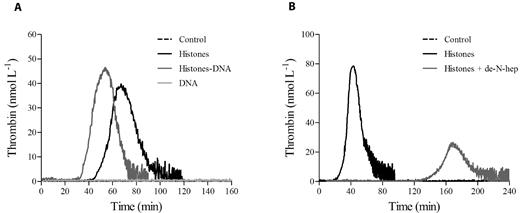
Activated platelets are thought to trigger the clotting cascade by expressing functional TF26 or by secreting polyP.27 Activation of coagulation in histone-treated CTI-inhibited PRP did not appear to be due to TF expression, because inclusion of a neutralizing anti-TF mAb in the assay failed to significantly modify the thrombin generation pattern produced by 40 μg/mL of histones (no antibody vs anti-TF mAb: lag time, 31.4 ± 6.5 vs 36 ± 5.6 minutes, nonsignificant; time-to-peak, 45.9 ± 7.7 vs 50 ± 6.6 minutes, nonsignificant; thrombin peak, 56.4 ± 12.1 vs 50.3 ± 8.5 nmol/L, nonsignificant; ETP, 1295.8 ± 34.5 vs 1213.7 ± 101 nmol/min, nonsignificant, n = 3). Moreover, histones enhanced thrombin generation when gel-filtered platelets were added to FVII-deficient plasma (data not shown). PolyP contained in platelet-dense granules activates the contact pathway through FXII27 ; the addition of Psp, which hydrolyzes polyP,28 to histone-treated CTI-inhibited PRP strongly reduced the procoagulant activity of stimulated platelets, resulting in a delayed and deeply depressed thrombin generation (Figure 3A). Surprisingly, histones still induced thrombin generation in the presence of a neutralizing anti-FXII antibody or in FXII-deficient PPP reconstituted with gel-filtered platelets (data not shown), which raises questions about the involvement of polyP as a FXII activator in this setting. We therefore hypothesized that histones might modify the ability of polyP to activate clotting. To test this, CTI-inhibited PPP was incubated with histones before the addition of purified polyP and POPS-containing liposomes, which simulated platelet activation, and thrombin generation was then measured by CAT. Whereas no thrombin was formed in the control and histone-treated samples, only traces were produced by polyP, confirming the efficacy of CTI; when histones were present along with polyP, thrombin was formed after a long latency and then increased at a high rate, reaching considerable peak values (Figure 3B). Histones thus appeared to strongly enhance the procoagulant activity of polyP in CTI-inhibited PPP, and the same effect was observed in the presence of the anti-FXII antibody (Figure 3C) and in a FXII-deficient PPP (data not shown). These results support activation of the clotting cascade at a different level and suggest that platelet-derived polyP may initiate the coagulation pathway in an FXII-independent manner when histones are present.
PolyP drives thrombin generation in histone-treated PRP. (A) Recalcified CTI-inhibited PRP was incubated with histones (40 μg/mL) in the presence of Psp (10 U/mL), and then thrombin generation was evaluated. Representative tracings of 3 experiments with PRP from different donors are shown. Recalcified CTI-inhibited PPP (B) and PPP treated with an anti-FXII antibody (150 μg/mL; C) were incubated with histones (40 μg/mL) and/or polyP (20 μg/mL), as described in “Methods,” and then thrombin generation was evaluated in the presence of liposomes. One representative experiment of 3 is shown.
PolyP drives thrombin generation in histone-treated PRP. (A) Recalcified CTI-inhibited PRP was incubated with histones (40 μg/mL) in the presence of Psp (10 U/mL), and then thrombin generation was evaluated. Representative tracings of 3 experiments with PRP from different donors are shown. Recalcified CTI-inhibited PPP (B) and PPP treated with an anti-FXII antibody (150 μg/mL; C) were incubated with histones (40 μg/mL) and/or polyP (20 μg/mL), as described in “Methods,” and then thrombin generation was evaluated in the presence of liposomes. One representative experiment of 3 is shown.
Histones induce platelet activation and expression of procoagulant activity in gel-filtered platelets
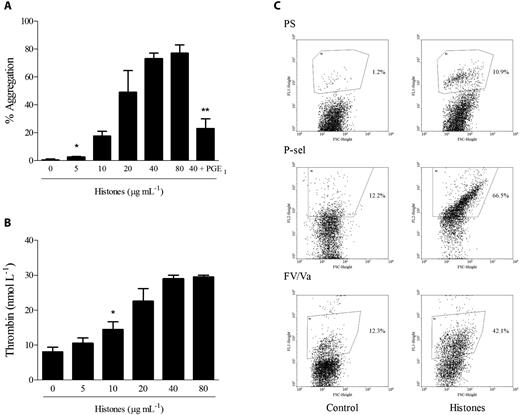
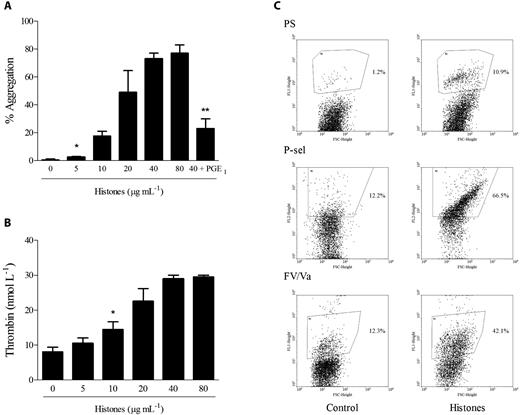
To exclude an effect of histones on plasma proteins, which could lead to the expression of a procoagulant phenotype by platelets activated by histones, we investigated the effect of histones on gel-filtered platelets with regard to the procoagulant functions of aggregation, prothrombinase activity, and secretion. The addition of histones to platelets under stirring conditions induced dose-dependent aggregation, which was inhibited by pretreatment of platelets with PGE1, thus excluding a phenomenon of passive agglutination caused by the positive charge of histones (Figure 4A). To assess the ability of histones to promote platelet prothrombinase activity, gel-filtered platelets were activated for 15 minutes with increasing concentrations of histones, and then prothrombin and FXa were added and the initial rate of thrombin formation was determined in the absence of exogenous FVa. Histone-stimulated platelets substantially increased prothrombin activation by FXa in a concentration-dependent manner (Figure 4B). Recombinant histones H3 and H4 were the most effective inducers of prothrombinase activity (data not shown). The acquisition of a procoagulant phenotype by histone-activated platelets was investigated further by assessing PS exposure and FV expression on the platelet surface by flow cytometry. The exposure of negatively charged membrane phospholipids was monitored with FITC-conjugated annexin V, and the expression of FV by an antibody recognizing both inactive and activated forms of FV. Histones (40 μg/mL) induced the appearance of a discrete, PS-positive platelet population amounting to approximately 10% of the total and an FV-positive population representing approximately 40% of the total. P-selectin translocation to the surface was also induced by histones, producing 70% P-selectin–positive platelets (Figure 4C).
Histones induce platelet aggregation, prothrombinase activity, PS exposure, and secretion. (A) Histones (0-80 μg/mL) were added to stirred gel-filtered human platelets, and aggregation was monitored for 10 minutes. Where indicated, gel-filtered platelets were pretreated with PGE1 (4 μmol/L) before the addition of histones (40 μg/mL). Data are reported as means of the maximum percentage of aggregation induced by histones ± SD of 4 independent experiments with gel-filtered platelets from different donors. *Lowest concentration producing significant aggregation (P < .05); **P < .05 compared with histones (40 μg/mL). (B) Prothrombinase activity of gel-filtered platelets stimulated with histones (0-80 μg/mL) for 15 minutes was determined after the addition of human FXa and prothrombin; the amount of thrombin generated was evaluated after 90 seconds by cleavage of the chromogenic substrate Spectrozyme TH. Data are means ± SD, n = 3. *Lowest concentration producing a significant increase (P < .05). (C) Typical flow cytometric analysis of PS exposure (top panel), P-selectin expression (middle panel), and FV/Va expression (bottom panel). Gel-filtered platelets stimulated with histones (40 μg/mL) for 15 minutes were analyzed by flow cytometry as detailed in “Methods.” Region 2 indicating a positive population for the antigen of interest was arbitrarily chosen.
Histones induce platelet aggregation, prothrombinase activity, PS exposure, and secretion. (A) Histones (0-80 μg/mL) were added to stirred gel-filtered human platelets, and aggregation was monitored for 10 minutes. Where indicated, gel-filtered platelets were pretreated with PGE1 (4 μmol/L) before the addition of histones (40 μg/mL). Data are reported as means of the maximum percentage of aggregation induced by histones ± SD of 4 independent experiments with gel-filtered platelets from different donors. *Lowest concentration producing significant aggregation (P < .05); **P < .05 compared with histones (40 μg/mL). (B) Prothrombinase activity of gel-filtered platelets stimulated with histones (0-80 μg/mL) for 15 minutes was determined after the addition of human FXa and prothrombin; the amount of thrombin generated was evaluated after 90 seconds by cleavage of the chromogenic substrate Spectrozyme TH. Data are means ± SD, n = 3. *Lowest concentration producing a significant increase (P < .05). (C) Typical flow cytometric analysis of PS exposure (top panel), P-selectin expression (middle panel), and FV/Va expression (bottom panel). Gel-filtered platelets stimulated with histones (40 μg/mL) for 15 minutes were analyzed by flow cytometry as detailed in “Methods.” Region 2 indicating a positive population for the antigen of interest was arbitrarily chosen.
APC and heparin abolish the platelet-activating potential of histones
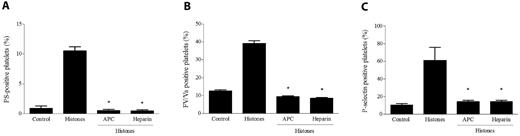
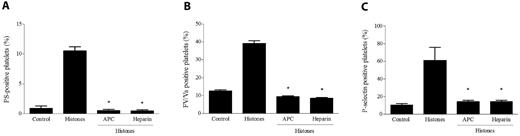
APC and heparin are of great importance in the therapy of hyperinflammatory and thrombotic conditions and possess known anti-histone properties. APC can cleave histones in vitro and in vivo, thus producing fragments that lose their ability to damage endothelial cells,8 and heparin is able to bind histones more tightly than DNA.29 We investigated the possibility that treatment of histones with these molecules could affect their ability to activate platelets. Histones preincubated with APC for 30 minutes at 37°C failed to induce platelet aggregation (data not shown) or the surface expression of P-selectin, PS, or FV, and even a short preincubation of histones with heparin similarly resulted in the complete loss of their platelet-activating potential (Figure 5). These results emphasize the effectiveness of APC and heparin in antagonizing histones as platelet activators, and indicate that platelet activation was not attributable to contaminants present in the histone preparation.
APC and heparin abolish the platelet-activating potential of histones. Histones (40 μg/mL) pretreated with APC (6 μg/mL) or heparin (1 U/mL) were added to gel-filtered platelets as described in “Methods,” and then their platelet-activating potential was evaluated by measuring PS exposure (A), FV/Va (B), and P-selectin expression (C) by flow cytometry. Data are means ± SD, n = 3. *P < .05 compared with histones.
APC and heparin abolish the platelet-activating potential of histones. Histones (40 μg/mL) pretreated with APC (6 μg/mL) or heparin (1 U/mL) were added to gel-filtered platelets as described in “Methods,” and then their platelet-activating potential was evaluated by measuring PS exposure (A), FV/Va (B), and P-selectin expression (C) by flow cytometry. Data are means ± SD, n = 3. *P < .05 compared with histones.
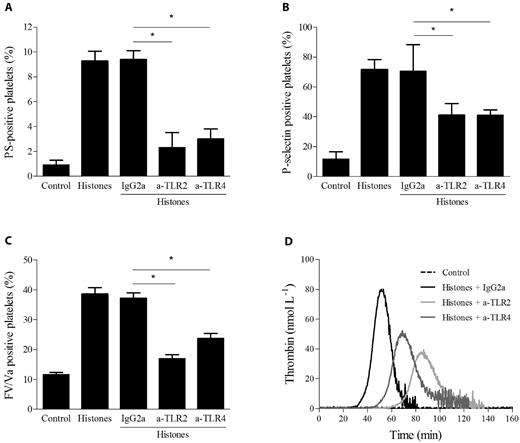
TLR2 and TLR4 are involved in platelet activation induced by histones
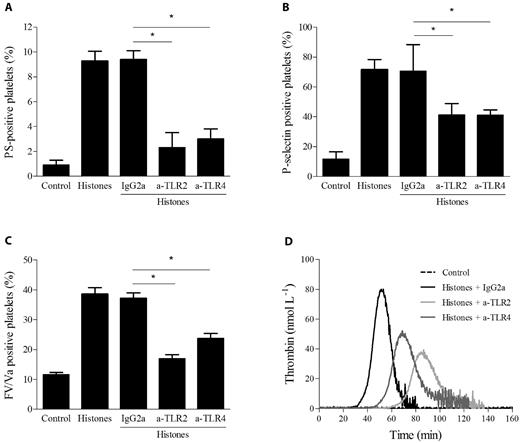
The involvement of TLR2 and TLR4 in histone-induced platelet responses was investigated by neutralizing antibodies. Gel-filtered platelets were treated for 20 minutes at RT with mAbs directed against TLR2 or TLR4 or control IgG before incubation with 40 μg/mL of histones, and the expression of PS, FV, and P-selectin was evaluated by flow cytometry. Both TLR2 and TLR4 antibodies reduced the exposure of PS (from 10% to 3%), FV (from 40% to 20%), and P-selectin (from 70% to 40%) roughly to the same extent, whereas isotype control antibody did not differ from the histones alone (Figure 6A-C). The simultaneous presence of both neutralizing antibodies did not result in any further inhibition (data not shown). These results suggest that the stimulatory effect of histones on platelet activation is, at least in part, TLR2 and TLR4 dependent. We also evaluated the possible involvement of TLR2 and TLR4 in thrombin generation induced by histones in CTI-inhibited PRP. PRP was preincubated with antibodies for 20 minutes at RT before the addition of histones. Isotype IgG2a did not modify the pattern of thrombin generation; in contrast, both anti-TLR2 and TLR4 antibodies caused an increase in the lag time and an approximately 50% reduction in the thrombin peak (Figure 6D), suggesting that histone-induced platelet activation in plasma is also partly mediated by TLR2 and TLR4. Simultaneous blockade of both receptors gave results comparable to those achieved with single antibodies (data not shown). Because treatment of CTI-inhibited PRP with up to 1 μg/mL of LPS did not induce any thrombin generation (data not shown), contamination of the histone preparation responsible for the effect can be excluded.
Histone-induced platelet activation is partially dependent on TLR2 and TLR4. Flow cytometric analysis of PS exposure (A), P-selectin expression (B), and FV/Va expression (C) in gel-filtered platelets stimulated with histones (40 μg/mL) with or without preincubation with mouse monoclonal anti–human TLR2 or TLR4 blocking Abs (50 μg/mL) or control mouse IgG2a for 20 minutes at RT. Data are means ± SD, n = 3. *P < .05. (D) Thrombin generation induced by histones (40 μg/mL) in CTI-inhibited PRP pretreated with neutralizing mAbs against TLR2, TLR4, or control Ab. Representative thrombograms of 3 experiments are shown.
Histone-induced platelet activation is partially dependent on TLR2 and TLR4. Flow cytometric analysis of PS exposure (A), P-selectin expression (B), and FV/Va expression (C) in gel-filtered platelets stimulated with histones (40 μg/mL) with or without preincubation with mouse monoclonal anti–human TLR2 or TLR4 blocking Abs (50 μg/mL) or control mouse IgG2a for 20 minutes at RT. Data are means ± SD, n = 3. *P < .05. (D) Thrombin generation induced by histones (40 μg/mL) in CTI-inhibited PRP pretreated with neutralizing mAbs against TLR2, TLR4, or control Ab. Representative thrombograms of 3 experiments are shown.
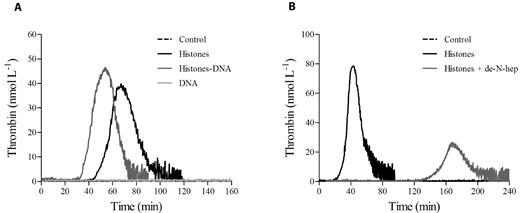
Histone-DNA complexes augment thrombin generation in PRP
Histones can be released by dying cells as free histones or in complex with DNA (nucleosomes). We hypothesized that DNA in complex with histones might modify the ability of histones to increase thrombin generation. We therefore evaluated thrombin generation in CTI-inhibited PRP incubated with histone-DNA complexes in the absence of any trigger and compared it with the complex formed between histones and de-N-heparin, a modified heparin devoid of anticoagulant activity.30 As shown in Figure 7, unlike de-N-heparin, which antagonized histones, the presence of DNA enhanced histone-dependent thrombin generation.
Histone-DNA complexes increase thrombin generation in PRP. Thrombin generation tracings in recalcified CTI-inhibited PRP stimulated with DNA-histone complexes (1:1 weight ratio; A) or de-N-heparin histone complexes (0.5:1 weight ratio; B), as described in “Methods.” One representative tracing of 3 experiments is shown.
Histone-DNA complexes increase thrombin generation in PRP. Thrombin generation tracings in recalcified CTI-inhibited PRP stimulated with DNA-histone complexes (1:1 weight ratio; A) or de-N-heparin histone complexes (0.5:1 weight ratio; B), as described in “Methods.” One representative tracing of 3 experiments is shown.
Discussion
A pathogenic relationship between extracellular histones and thrombosis has been recently proposed based on the observation that injection of histones in mice induces thrombotic lesions reminiscent of those found in severe sepsis.8 Another recent study9 demonstrated that NETs induced platelet adhesion and aggregation and fibrin deposition occurred on their surface, suggesting that these structures can promote thrombus formation. The histone component of NETs was deemed of primary importance because recombinant H3 and H4 were found to induce aggregation of washed platelets. However, whether and how histone-platelet interaction affects blood coagulation remains unclear. In the present study, we provide new evidence that histones can promote coagulation and thrombin formation directly through platelet-dependent mechanisms. The effects of histones were first evaluated by CAT in PRP. In recalcified citrated PRP, in which coagulation is thought to be triggered by contact system activation,24 histones induced a dose-dependent enhancement of thrombin generation, as evinced mainly from the changes in the kinetic parameters of the thrombogram (shortening of the lag time and time-to-peak and increased thrombin peak). This suggests earlier platelet activation with consequent impact on the coagulation dynamics. In CTI-inhibited PRP, in which contact system activation is almost abolished, histones still induced thrombin generation in a concentration-dependent manner, whereas no thrombin could be detected in control samples. The observation that ETP was modestly influenced by increasing concentrations of histones might be explained by its lower sensitivity to platelet activation with respect to the other parameters.31,32 The effect of histones was entirely platelet dependent, as shown by the following observations: (1) histones failed to induce detectable thrombin formation in CTI-inhibited PPP even in the presence of an exogenous procoagulant surface, indicating that they are not able to trigger thrombin generation by themselves; and (2) histone-induced thrombin generation in CTI-inhibited PRP was strongly reduced by several agents that interfere with platelet function at different levels, implying that the histone effect requires cellular signaling. These findings raise the question of how histone-activated platelets can trigger blood coagulation in CTI-inhibited PRP.
Activated platelets have long been known to amplify clotting reactions, and recent evidence indicates that they can also trigger the clotting cascade by different pathways. TF expression in platelets is still a matter of debate.26,33 Under our experimental conditions, neither a TF-inhibiting antibody nor the absence of FVII reduced the histone-induced enhancement of thrombin generation in PRP, thus ruling out an involvement of endogenous TF. Secretion of the inorganic polymer polyP by activated platelets is an emerging mechanism of clotting activation that sheds new light on the pathophysiological importance of the contact system.28 Severe deficiency in FXII does not cause hemorrhage34 but protects from thrombosis, which implies a role in pathologic rather than in physiologic hemostasis.35 PolyP, along with RNA,36 might well represent “foreign” surfaces that bring about thrombin generation in disease by promoting activation of FXII. Treatment of CTI-inhibited PRP with Psp delayed the lag time and reduced the thrombin formed in the presence of histones, which argues for a direct participation of polyP released from platelets in thrombin generation dynamics. Because platelet polyP is reportedly a rather weak activator of FXII,37 and because the effect of histones was not appreciably modified by blocking FXII or by the absence of FXII, we hypothesize that other mechanisms of clotting activation might be operative when polyP and histones are simultaneously present. The experiments performed in CTI-inhibited and FXII-deficient PPP showed that the requirement for FXII by purified polyP was bypassed in the presence of histones, which may be able to modulate polyP properties. Studies are ongoing to clarify these observations.
Experiments performed with isolated platelets showed that histones induce a wide spectrum of platelet responses. They induced aggregation of platelets in a concentration-dependent manner, which is in agreement with recently published data.9 At variance with the latter study performed with recombinant histones, the mixture of histones we used was from a natural source and most probably had undergone posttranslational modifications that did not impair the pro-aggregating properties. More relevant in the context of our study is the observation that platelets, upon stimulation with histones, acquired a procoagulant phenotype as evidenced by increased surface expression of FV/Va and exposure of PS at levels that efficiently promoted thrombin formation in an assay using purified FXa and prothrombin. Histones also up-regulated the expression of platelet P-selectin, an adhesion molecule involved in platelet adhesion to the endothelium, platelet-leukocyte aggregation, induction of TF expression by monocytes, and uptake of TF-bearing microparticles, all implicated in thrombus formation.38 Recombinant APC, the only drug approved by the US Food and Drug Administration for the treatment of sepsis, and heparin, a widely used anticoagulant agent, almost completely inhibited the whole spectrum of platelet responses to histones, thus extending previous findings on the anti-histone properties of these compounds.
We also evaluated the possible involvement of TLRs in histone-induced platelet activation. Their role in the recognition of foreign pathogens and the initiation of the inflammatory response is indispensable for host defense. There is also considerable emerging evidence that self-recognition through TLRs may play an important role in inflammation and autoimmunity. Known endogenous ligands released from damaged tissues such as heat-shock proteins,39,40 high-mobility group box-1,41-43 and extracellular matrix fragments interact with TLR2 and TLR4,44,45 fibrinogen and β-defensin interact with TLR4,46,47 mRNA interacts with TLR3,48 and DNA interacts with TLR9,49,50 and all of these ligands may be present at elevated levels at sites of tissue injury and inflammation. Histones are newly recognized TLR agonists, because they have been found to activate TLR2 and TLR4 in established cell lines.19 We found that these receptors also mediate, at least in part, histone-induced platelet activation. In experiments with gel-filtered platelets, blockade of TLR2 and TLR4 significantly reduced the population positive for P-selectin, PS, and FV/Va. Moreover, thrombin generation in histone-treated CTI-inhibited PRP was impaired in the presence of TLR2- and TLR4-neutralizing antibodies. The observation that when using both gel-filtered platelets and PRP, the platelet response to histones was not completely abolished by TLR2 and TLR4 blockade suggests that additional mechanisms of activation might be operative, among which are an engagement of platelet FcRγII by the Fc fragment of the bound antibodies, leading to the activation of surrounding platelets or to a nonspecific detergent effect of histones on the platelet membrane.6,7
Not all histones possess the same potency. H4 and, to a lesser degree, H3 are the most powerful, which is in agreement with the findings of Fuchs et al9 with respect to platelet aggregation. Although further studies are needed to specifically assess the relationship between structural differences of histones and platelet-stimulating activity, the prominent importance of histones H4 and H3 compared with other histones can be inferred from in vivo experimental studies.8 Finally, extracellular histones may come from dying cells as free histones or associated with DNA in the form of nucleosomes, or may come from inflammatory cells as part of NETs. It is difficult to establish which form is predominant, especially in the setting of diffuse tissue damage and inflammation. The addition of DNA to histones serves as a mimic of NETs/nucleosomes, and the finding that it did not inhibit the ability of histones to increase thrombin generation in PRP reinforces the pathophysiologic importance of the observation.
Our study shows for the first time that, both in a physiologic environment and in purified systems, histones have the capacity to promote blood coagulation and thrombin formation mainly by inducing platelet activation and subsequent expression of a procoagulant phenotype, and that the effects of histone are partly mediated by TLR2 and TLR4. This hitherto undescribed mechanism might have pathophysiological implications. For example, it might contribute to the formation of platelet-rich thrombi in the microcirculation of histone-treated mice or LPS-treated or septic animals. It is remarkable in this context that the effects of histones were observed with concentrations that can be attained in vivo in Escherichia coli–treated baboons,8 in which the plasma level of total histones was estimated to be approximately 70 μg/mL, and in human patients, where similar levels were found (Xu J, unpublished observations, August 2010). Moreover, plasma concentrations may underestimate the amount of histones locally associated with cells51 at sites of cellular release and/or altered blood flow, where they could be much higher. Nuclear proteins released from dying cells, including histones, have been implicated as mediators of inflammation and cell injury in a variety of severe human diseases such as sepsis, trauma, ischemia, autoimmune diseases, and cancer, all of which are known to be associated with thrombosis of the macro- and/or microcirculation. Our findings might help aid our understanding of how blood coagulation is promoted in these pathologic conditions. For example, in sepsis, histone activation may make a contribution to platelet consumption. More importantly, however, the ability of histones to interact with the activated platelets, probably through polyP, provides a mechanism to generate a “super” platelet procoagulant activity. Because the histone levels appear to increase most at the onset of organ failure, this potent procoagulant function may contribute to the disseminated intravascular coagulation seen in late-stage sepsis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the American Heart Association (to G.L.D.) and the National Institutes of Health (R01 HL047014 to J.H.M.). C.T.E. is an investigator of the Howard Hughes Medical Institute. F.S. is supported by a fellowship from the University of Bari, Bari, Italy.
National Institutes of Health
Authorship
Contribution: F.S. and C.T.A. designed and executed the experiments, analyzed the data, and wrote the manuscript; J.H.M. provided the polyP and contributed useful comments; G.L.D. and P.F. were involved in platelet isolation and flow cytometry experiments and contributed useful comments; N.L.E. contributed helpful comments and assisted in manuscript preparation; and C.T.E. oversaw the overall execution of the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles T. Esmon, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: esmonc@omrf.org.