Abstract
Cited2 is a transcriptional modulator involved in various biologic processes including fetal liver hematopoiesis. In the present study, the function of Cited2 in adult hematopoiesis was investigated in conditional knockout mice. Deletion of Cited2 using Mx1-Cre resulted in increased hematopoietic stem cell (HSC) apoptosis, loss of quiescence, and increased cycling, leading to a severely impaired reconstitution capacity as assessed by 5-fluorouracil treatment and long-term transplantation. Transcriptional profiling revealed that multiple HSC quiescence- and hypoxia-related genes such as Egr1, p57, and Hes1 were affected in Cited2-deficient HSCs. Because Cited2 is a negative regulator of HIF-1, which is essential for maintaining HSC quiescence, and because we demonstrated previously that decreased HIF-1α gene dosage partially rescues both cardiac and lens defects caused by Cited2 deficiency, we generated Cited2 and HIF-1α double-knockout mice. Additional deletion of HIF-1α in Cited2-knockout BM partially rescued impaired HSC quiescence and reconstitution capacity. At the transcriptional level, deletion of HIF-1α restored expression of p57 and Hes1 but not Egr1 to normal levels. Our results suggest that Cited2 regulates HSC quiescence through both HIF-1–dependent and HIF-1–independent pathways.
Introduction
Hematopoietic stem cells (HSCs) are thought to be localized in the hypoxic microenvironment of the BM and remain quiescent or differentiate into multiple blood cell lineages. Several factors have been found to regulate HSC quiescence in either a cell-intrinsic (eg, p21, p57, Bmi1, Egr1, GATA2, Gfi1, Pbx1, and others) or a cell-extrinsic (eg, Tie2/Angpt1, c-Mpl/Tpo, CXCR4/CXCL12, and others) manner.
CBP/p300-interacting transactivator with glutamic acid (E) and aspartic acid (D)–rich tail 2 (Cited2), a member of the Cited family of transcriptional modulators, is a cytokine-inducible gene1 that plays various roles during mouse development.2-6 In particular, Cited2 plays an important role in fetal liver hematopoiesis, which is supported by severely impaired fetal liver HSC function and decreased expression of genes known to be essential for hematopoiesis in Cited2-deficient fetal liver HSC/progenitors.7 Cited2 has also been implicated in tumor cell invasion and progression.8,9
Cited2 is a negative regulator of hypoxia-inducible factor 1 (HIF-1). Bhattacharya et al first demonstrated in vitro that Cited2 competes with HIF-1α for binding to p300-CH1 and inhibits HIF-1–mediated transactivation.10 Bakker et al also showed that FOXO3a inhibits HIF-1–induced apoptosis by stimulating the transcription of Cited2, which reduces the expression of the pro-apoptotic HIF-1 target genes NIX (also called “Bnip3l”) and RTP801 (also called “Ddit4”).11 In our previous studies, we showed that deletion of HIF-1α partially rescues defects in heart and lens caused by Cited2 deficiency.4,12 In addition, at the structural level, Freedman et al revealed that Cited2 and HIF-1α share a conserved “LPXL” sequence motif in their transactivation domains that interacts directly with a binding site on the CH1 region of CBP/p300.13
HIFs are recognized as master modulators of the cellular response to hypoxic stress. HIF-1α is expressed ubiquitously, whereas HIF-2α and HIF-3α exhibit a tissue-restricted mRNA expression pattern.14 In the BM, the expression of HIF-1α and HIF-3α is highest in long-term HSCs (LT-HSCs), whereas HIF-2α is mainly expressed in Lin− cells.15 HIF-3α itself is a direct transcriptional target of HIF-1α,16 which has been demonstrated to be a key regulator of the cellular hypoxia response and to inhibit proliferation of different cell types, including HSCs.17-21 Recently, Takubo et al reported that precise regulation of HIF-1α level is essential for the maintenance of the HSC quiescence.15 Stabilization of HIF-1α by deletion of the VHL gene in mouse BM impairs long-term hematopoietic reconstitution.15 Eliasson et al also found that overexpression of constitutively active HIF-1α or treatment with the HIF stabilization agents leads to reduced HSC reconstitution ability.21 These findings suggest that HIF-1 regulates HSC activity in a dose-dependent manner.
In the present study, we used a conditional knockout strategy to delete Cited2 in adult mice and demonstrated that Cited2 deficiency results in impaired HSC quiescence and hematopoietic reconstitution capacity. The defects caused by Cited2 deficiency are partially rescued by the additional deletion of HIF-1α.
Methods
Mice
The Cited2fl/+ mouse line,22 the HIF-1αfl/+ mouse line,23 and the Mx1-Cre transgenic mouse line (The Jackson Laboratory) were maintained on the C57/BL6 background (CD45.2+) and bred to generate Cited2fl/fl (referred to as wild-type), Cited2fl/fl;Mx1-Cre (referred to as Cited2Δ/Δ), and Cited2fl/flHIF-1αfl/fl;Mx1-Cre (referred to as Cited2Δ/ΔHIF-1αΔ/Δ) mice. The congenic strain of C57/BL6, B6.SJL-PtprcaPep3b/BoyJ (BoyJ; CD45.1+) was obtained from The Jackson Laboratory and used in transplantation studies. All animal studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University (Cleveland, OH).
Treatment of mice and analysis of deletion efficiency
Adult mice at the age of 5-6 weeks were treated every other day with 5 doses of 16 mg/kg body weight polyinosinic-polycytidylic acid (pI-pC; Sigma-Aldrich) via IP injection. Deletion efficiency was determined by real-time PCR using total mRNA purified from either PBMCs or sorted Lin−Sca-1+c-Kit+ (LSK) cells.
PB hematology
Peripheral blood (PB) mouse hematology was examined using HemaVet 950 (Drew Scientific).
Flow cytometry
BM cells were harvested from femurs and tibias. For LSK analysis, BM cells were stained with biotin-conjugated Abs to lineage markers (ie, Gr-1, Mac-1, Ter119, B220, and CD3; Miltenyi Biotec), and subsequently with APC-Cy7–conjugated streptavidin, APC-conjugated anti–c-Kit, PE-Cy7–conjugated anti–Sca-1, PE-conjugated anti–Flk2/Flt3, and FITC-conjugated anti-CD34. In some experiments, Alexa Fluor 700–conjugated anti-CD34 was used. For LSK analysis using signaling lymphocyte activation molecule (SLAM) markers, BM cells were stained with PE-conjugated Abs to lineage markers, APC-conjugated anti–c-Kit, PE-Cy7–conjugated anti–Sca-1, APC-Cy7–conjugated anti-CD48, and FITC-conjugated anti-CD150. All Abs were purchased from eBiosciences or BD Pharmingen. Flow cytometry was performed using an LSRII flow cytometer (BD Biosciences). Cell sorting was performed using a FACSAria cell sorter (BD Biosciences).
Cell cycle analysis and BrdU staining
BM cells were isolated from mice 1 month after pI-pC treatment. Pyronin Y (Sigma-Aldrich) and Hoechst 33342 (Sigma-Aldrich) staining was performed simultaneously within gated LSK or CD34−LSK cells as described previously.24 For bromodeoxyuridine (BrdU; Sigma-Aldrich) staining, approximately 2-3 weeks after pI-pC treatment, mice were given BrdU 160 mg/kg body weight via IP injection. In addition, 1 mg/mL of BrdU was added to the drinking water for 48 hours before analysis. A BD Pharmingen BrdU flow kit was used to evaluate BrdU incorporation within gated LSK cells.
Apoptosis
Apoptosis within gated LSK or CD34−LSK cells was determined using an annexin V–FITC kit (BD Biosciences) according to the manufacturer's instructions.
5-FU treatment
5-Fluorouracil (5-FU; APP Pharmaceuticals) was administered intravenously to mice at 250 mg/kg body weight 4 weeks after pI-pC treatment. Retro-orbital PB was collected into EDTA-coated capillary tubes to measure complete blood counts at 0, 4, 8, 12, and 16 days after 5-FU administration.
Transplantation
BM cells were harvested for transplantation from each genotype of mice approximately 2-3 weeks after pI-pC treatment. In the noncompetitive transplantation study, 5 × 105 BM cells were injected via the tail vein into lethally irradiated B6.SJL mice (11 Gy). PB analysis and lineage reconstitution studies were performed 16 weeks later. In the competitive transplantation study, 5 × 105 BM cells from each genotype of mice (CD45.2+) mixed with equal number of competitor cells (CD45.1+) were injected via the tail vein into lethally irradiated B6.SJL mice (CD45.1+). The recipient mice were analyzed for donor-derived cells at 8, 12, and 16 weeks after transplantation. Chimerism was determined by FITC-conjugated anti-CD45.2 and PE-conjugated anti-CD45.1 staining. In all of the transplantation assays, independent donor BM cells were transplanted into independent recipient mice.
Quantitative real-time PCR
Total RNA was isolated using the PicoPure RNA Isolation Kit (Applied Biosystems) and cDNA was prepared with the SuperScript III First Strand Synthesis system (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed using the iQ SYBR Green Supermix and MyiQ Real-Time PCR Detection System (Bio-Rad). Data were normalized by the abundance of an internal control 18S RNA. Sequences of primers used are available upon request.
Cell culture
EML C1 cells (obtained from Dr Schickwann Tsai, University of Utah, Salt Lake City, UT) were routinely grown in IMDM (Invitrogen) supplemented with 20% horse serum (Invitrogen) and approximately 10%-15% BHK/MKL-conditioned medium as a source of SCF.
Western blot analysis
Cells were lysed with a solution containing Tris (20mM, pH 8.0), NaCl (100mM), 1% NP-40, and protease inhibitor cocktail (Roche). Lysates were fractionated by SDS-PAGE and then transferred to PVDF membranes. Membranes were incubated with primary Abs at 4°C overnight, followed by incubation with secondary Abs conjugated to HRP. The films were developed using a chemiluminescence method (PerkinElmer).
Anti–HIF-1α Ab was purchased from Novus Biologicals. Anti-Cited2 Ab was purchased from R&D Systems. Anti-HDAC1, anti-Smad2/3, and anti–β-actin Abs were from Santa Cruz Biotechnology.
ChIP assay
Cells were treated with 1% formaldehyde, followed by the addition of glycine. Cells were then washed twice with ice-cold PBS and resuspended in radioimmunoprecipitation assay lysis buffer containing protease inhibitors. Genomic DNA was sheared to lengths of approximately 0.4- to 1-kb fragments by sonicating the cell lysate. The chromatin supernatant was incubated with the respective Abs at 4°C overnight, followed by incubation with protein G Dynabeads (Invitrogen) at 4°C for 2 hours. The pellet was then washed sequentially and eluted by elution buffer (1% SDS and 100mM NaHCO3). Reverse cross-linking was performed by adding NaCl and proteinase K and incubating at 62°C for 6 hours. Finally, DNA was purified by phenol/chloroform extraction. Primer sequences of the mouse Hes1 promoter25 and Egr1 promoter are provided in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
The significance of differences was determined by a 2-tailed Student t test. The log-rank test was used to compare survival between groups. P < .05 was considered statistically significant.
Results
Cited2 deficiency results in increased HSC apoptosis and defective HSC quiescence
We have shown previously that Cited2 is highly expressed in mouse fetal liver HSCs/progenitor cells and that Cited2 deficiency results in impaired fetal liver hematopoiesis.7 Others have also reported that Cited2 is highly expressed in mouse HSCs (CD38+CD34− LSKs) and is predicted to be associated with long-term reconstitution activity.26 To investigate the function of Cited2 in adult murine hematopoiesis, we deleted the Cited2 gene by sequential pI-pC injection, which efficiently excises Cited2 from hematopoietic tissues. Expression of Cited2 mRNA was barely detectable in LSK cells isolated from Cited2Δ/Δ mice 2 weeks after pI-pC treatment (Figure 1A). In this mouse model, the WBC count, BM cellularity, and LSK cell number were within the normal range (Figure 1B-D). In addition, in an independent mouse model in which the Cited2 gene was deleted in hematopoietic and endothelial cells using Vav-Cre,27,28 Cited2 mRNA was barely detectable in sorted LSK cells (supplemental Figure 1A), and the WBC number and BM cellularity were similar to those of wild-type controls (supplemental Figure 1B-C). These results indicate that Cited2 deficiency does not significantly affect steady-state hematopoiesis.
Conditional deletion of Cited2 results in decreased numbers of LT-HSCs. (A) pI-pC treatment induces efficient deletion of Cited2, as determined by relative expression of Cited2 mRNA normalized to 18S RNA in LSK cells (mean ± SEM, n = 8). (B-D) Approximately 2-3 weeks after pI-pC treatment, no significant difference was found between wild-type (WT) and Cited2Δ/Δ mice in WBC number (B), BM cellularity (C), and LSK cell number (D). Data shown are means ± SEM (n = 6). (E) Flow cytometric analysis shows that, compared with wild-type controls (top panel), Cited2Δ/Δ LSK cells (bottom panel) display a significantly decreased Flt3−CD34− or CD48−CD150+ fraction, which corresponds to LT-HSCs. WT and mutant mice were from the same litter and experiments were performed independently at least twice. The numbers in the Lin−-gated plots indicate the percentage of total BM mononuclear cells, whereas the numbers in the LSK-gated plots indicate the percentage within the gated population. (F) The percentage of LT-HSCs (CD48−CD150+ or Flt3−CD34−), short-term HSCs (Flt3−CD34+), and multipotent progenitors (Flt3+CD34+) in LSK cells are shown as means ± SEM (n = 4).
Conditional deletion of Cited2 results in decreased numbers of LT-HSCs. (A) pI-pC treatment induces efficient deletion of Cited2, as determined by relative expression of Cited2 mRNA normalized to 18S RNA in LSK cells (mean ± SEM, n = 8). (B-D) Approximately 2-3 weeks after pI-pC treatment, no significant difference was found between wild-type (WT) and Cited2Δ/Δ mice in WBC number (B), BM cellularity (C), and LSK cell number (D). Data shown are means ± SEM (n = 6). (E) Flow cytometric analysis shows that, compared with wild-type controls (top panel), Cited2Δ/Δ LSK cells (bottom panel) display a significantly decreased Flt3−CD34− or CD48−CD150+ fraction, which corresponds to LT-HSCs. WT and mutant mice were from the same litter and experiments were performed independently at least twice. The numbers in the Lin−-gated plots indicate the percentage of total BM mononuclear cells, whereas the numbers in the LSK-gated plots indicate the percentage within the gated population. (F) The percentage of LT-HSCs (CD48−CD150+ or Flt3−CD34−), short-term HSCs (Flt3−CD34+), and multipotent progenitors (Flt3+CD34+) in LSK cells are shown as means ± SEM (n = 4).
We further examined the numbers of the more primitive fractions within the LSK population approximately 2-3 weeks after pI-pC treatment. Flow cytometric analysis of LSK cells from Cited2Δ/Δ mice revealed a significant decrease in the pool size of LT-HSCs (defined as Flt3−CD34− or CD48−CD150+ LSKs), a moderate decrease of short-term HSCs (defined as Flt3−CD34+ LSKs), and a moderate increase of multipotent progenitors (defined as Flt3+CD34+ LSKs; Figure 1E-F).
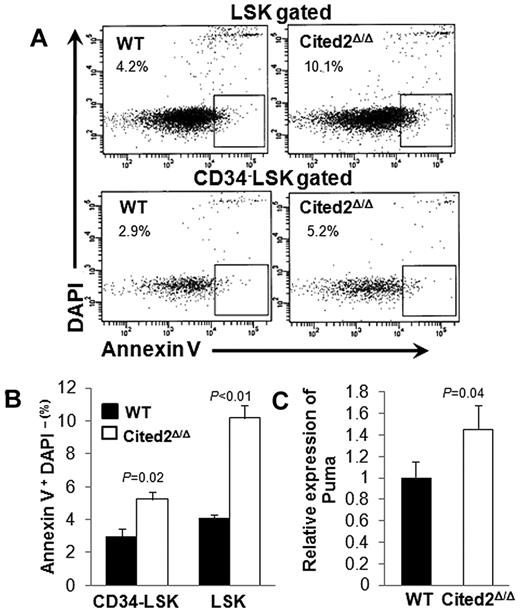
We also sought to determine whether the reduced number of LT-HSCs results from a perturbation in apoptosis or cell cycling. Apoptosis was measured by staining HSCs with annexin V and 4′,6-diamidino-2-phenylindole (DAPI) approximately 2-3 weeks after pI-pC treatment. The annexin V+DAPI− fraction within the gated LSK or CD34− LSK populations was significantly increased in Cited2Δ/Δ mice (Figure 2A-B). The expression of several pro- or anti-apoptotic proteins in LSK cells, including Puma, Bax, Bid, Bcl-2, and Bcl-xl, was examined, and only Puma, a pro-apoptotic protein, was up-regulated in Cited2Δ/Δ LSK cells (Figure 2C).
Cited2 deficiency results in elevated HSC apoptosis. (A) Representative plots of cells undergoing apoptosis (annexin V+ DAPI−) within LSK (top panel) and CD34− LSK (bottom panel) fractions. (B) Summary data of apoptotic cells shown as means ± SEM (n = 4). (C) Relative expression of Puma in LSK cells (means ± SEM, n = 4). WT indicates wild-type.
Cited2 deficiency results in elevated HSC apoptosis. (A) Representative plots of cells undergoing apoptosis (annexin V+ DAPI−) within LSK (top panel) and CD34− LSK (bottom panel) fractions. (B) Summary data of apoptotic cells shown as means ± SEM (n = 4). (C) Relative expression of Puma in LSK cells (means ± SEM, n = 4). WT indicates wild-type.
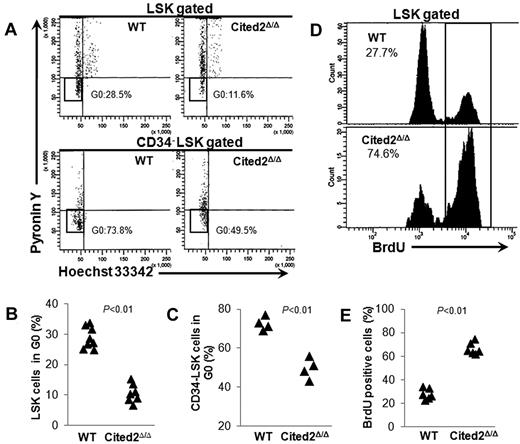
The HSC quiescent population was next determined by staining cells with the DNA dye Hoechst 33342 and the RNA dye pyronin Y. Quiescent cells at G0 phase of the cell cycle have lower levels of RNA than cells at G1. Simultaneous DNA/RNA staining allows examination of HSCs at G0.29,30 The proportion of LSK or CD34− LSK cells at G0 was significantly decreased in Cited2Δ/Δ mice compared with controls (Figure 3A-C). Consistent with a decrease in the number of quiescent HSCs, BrdU incorporation assays showed more actively cycling LSK cells in the BM of Cited2Δ/Δ mice (Figure 3D-E). These results provide supporting evidence that Cited2 is associated with the maintenance of HSC quiescence in adult hematopoiesis.
Cited2 deficiency results in loss of HSC quiescence. (A-C) Hoechst 33342 and pyronin Y staining. To analyze the proportion of cells at G0, mice were killed 4 weeks after pI-pC treatment and BM cells were stained using the DNA dye Hoechst 33342 and the RNA dye pyronin Y in conjunction with cell-surface markers. (A) Representative plots of cell cycle within gated LSKs (top panel) or CD34− LSKs (bottom panel). (B) Summary data of quiescent cells in the LSK population from WT (n = 8) or Cited2Δ/Δ (n = 7) mice. (C) Summary data of quiescent cells in the CD34− LSK population (n = 4). (D-E) BrdU staining. Wild-type and Cited2Δ/Δ mice were given BrdU via IP injection and killed 48 hours later for measurement of BrdU incorporation within LSK cells. (D) Representative plots for flow cytometric analysis. (E) Summary data of BrdU+ LSK cells (n = 6).
Cited2 deficiency results in loss of HSC quiescence. (A-C) Hoechst 33342 and pyronin Y staining. To analyze the proportion of cells at G0, mice were killed 4 weeks after pI-pC treatment and BM cells were stained using the DNA dye Hoechst 33342 and the RNA dye pyronin Y in conjunction with cell-surface markers. (A) Representative plots of cell cycle within gated LSKs (top panel) or CD34− LSKs (bottom panel). (B) Summary data of quiescent cells in the LSK population from WT (n = 8) or Cited2Δ/Δ (n = 7) mice. (C) Summary data of quiescent cells in the CD34− LSK population (n = 4). (D-E) BrdU staining. Wild-type and Cited2Δ/Δ mice were given BrdU via IP injection and killed 48 hours later for measurement of BrdU incorporation within LSK cells. (D) Representative plots for flow cytometric analysis. (E) Summary data of BrdU+ LSK cells (n = 6).
Cited2 deficiency results in impaired HSC reconstitution
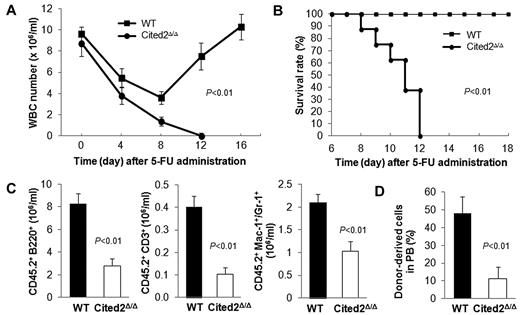
To explore the effect of Cited2 deficiency on the hematopoietic system under stress, 5-FU, a chemotherapeutic drug that kills cycling hematopoietic stem/progenitor cells, was administered to mice 4 weeks after pI-pC treatment. As expected, WBC counts dramatically decreased after 5-FU treatment in both wild-type and Cited2-knockout mice. In wild-type mice, the WBC count recovered to the normal level at approximately 16 days after 5-FU treatment. In sharp contrast, WBC counts in Cited2Δ/Δ mice continued to decrease and none of these mice survived (Figure 4A-B). The high mortality was not likely caused by Cre-mediated toxicity induced by pI-pC because no apparent difference was found in the 5-FU response between Cited2fl/fl and Mx1-Cre mice (both were treated with pI-pC before 5-FU treatment; supplemental Figure 2). Cited2fl/fl mice therefore are appropriate wild-type controls and were used throughout the study. These data indicate that Cited2 is required for proper HSC function under stress conditions.
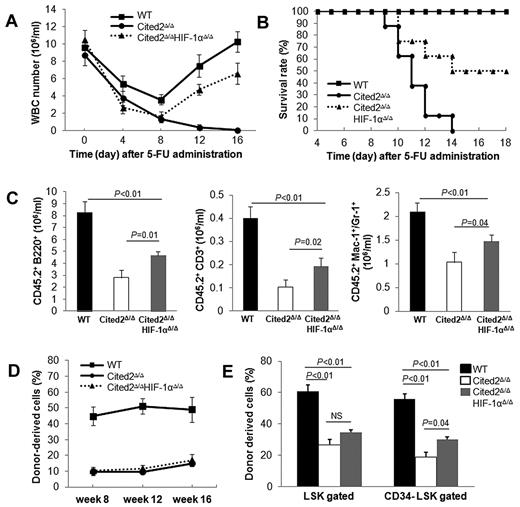
Cited2 deficiency results in decreased HSC reconstitution capacity. (A) WBC count in PB was monitored 0, 4, 8, 12, and 16 days after 5-FU treatment in WT and Cited2Δ/Δ mice (n = 8). (B) Survival time was monitored for at least 18 days. Overall survival time in Cited2Δ/Δ mice was significantly shorter than controls (n = 8). (C) Absolute numbers of donor-derived B cells (B220+), myeloid cells (Mac-1+ or Gr-1+), and T cells (CD3+) in PB 16 weeks after noncompetitive transplantation (means ± SEM, n = 6). (D) PB chimerism in recipients at 16 weeks after competitive transplantation (means ± SEM, n = 4).
Cited2 deficiency results in decreased HSC reconstitution capacity. (A) WBC count in PB was monitored 0, 4, 8, 12, and 16 days after 5-FU treatment in WT and Cited2Δ/Δ mice (n = 8). (B) Survival time was monitored for at least 18 days. Overall survival time in Cited2Δ/Δ mice was significantly shorter than controls (n = 8). (C) Absolute numbers of donor-derived B cells (B220+), myeloid cells (Mac-1+ or Gr-1+), and T cells (CD3+) in PB 16 weeks after noncompetitive transplantation (means ± SEM, n = 6). (D) PB chimerism in recipients at 16 weeks after competitive transplantation (means ± SEM, n = 4).
We next evaluated the reconstitution capacity of Cited2Δ/Δ HSCs by BM transplantation (BMT) with or without competitors. In noncompetitive BMT, we transplanted 5 × 105 BM cells from Cited2Δ/Δ or wild-type mice into lethally irradiated CD45.1 congenic recipients. Sixteen weeks later, recipient mice transplanted with Cited2Δ/Δ BM cells displayed decreased numbers of donor-derived B220+, CD3+, and myeloid (Mac-1+or Gr-1+) cells in the PB (Figure 4C). In competitive BMT, 5 × 105 BM cells from Cited2Δ/Δ or wild-type mice were injected into lethally irradiated CD45.1 congenic recipients along with equal numbers of CD45.1 competitors. Sixteen weeks after BMT, recipients of the Cited2Δ/Δ donor cells exhibited a 4-fold decrease in PB chimerism compared with that of wild-type controls (Figure 4D). Given that Cited2 deficiency results in a 2-fold decrease in LT-HSC number, we reasoned that the significantly reduced PB chimerism was caused not only by decreased LT-HSC number, but also by impaired HSC activity, suggesting that Cited2 deficiency affects both the cellular pool and the function of HSCs.
Deletion of HIF-1α partially rescues HSC quiescence and reconstitution activity
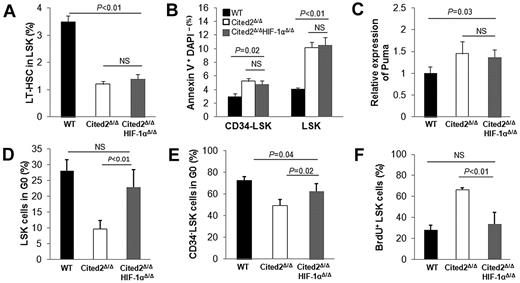
Recent studies have shown that both HIF-1α–deficient and HIF-1α–stabilized HSCs result in impaired hematopoietic reconstitution, suggesting that precise regulation of the HIF-1α level is essential for maintaining HSC quiescence and transplantation activity.15 Cited2 has been shown to be a negative regulator of HIF-1 through competitive binding to CBP/p300 with higher affinity than HIF-1α.10 These findings prompted us to hypothesize that the defects in Cited2Δ/Δ HSCs could be the result of elevated HIF-1 activity. To test this hypothesis, we generated Cited2Δ/ΔHIF-1αΔ/Δ mice. Sequential injection of pI-pC resulted in efficient deletion of Cited2 and HIF-1α in this mouse model (supplemental Figure 3A-B). Peripheral WBC count, BM cellularity, and LSK cell number from the double-knockout mice were within the normal range (data not shown). The LT-HSC (CD48−CD150+ LSK) number was similar to that of Cited2Δ/Δ mice (ie, lower than that of wild-type controls; Figure 5A). Apoptosis of HSCs was also comparable to that of Cited2Δ/Δ mice (ie, higher than that of wild-type controls; Figure 5B), and this was accompanied by up-regulation of Puma in LSK cells (Figure 5C). These results indicate that deletion of HIF-1α does not rescue aberrant apoptosis caused by Cited2 deficiency.
Deletion of HIF-1α partially restores impaired HSC quiescence caused by Cited2 deficiency. (A) Percentage of LT-HSCs (CD48−CD150+ LSKs) in LSK cells determined by FACS analysis. (B) Percentage of annexin V+DAPI− cells within LSK cells and CD34− LSK cells. (C) Relative mRNA level of Puma in LSK cells. (D) Percentage of LSK cells in G0. (E) Percentage of CD34− LSK cells in G0. (F) The percentage of BrdU+ LSK cells. All data are shown as means ± SEM (n = 4). WT indicates wild-type.
Deletion of HIF-1α partially restores impaired HSC quiescence caused by Cited2 deficiency. (A) Percentage of LT-HSCs (CD48−CD150+ LSKs) in LSK cells determined by FACS analysis. (B) Percentage of annexin V+DAPI− cells within LSK cells and CD34− LSK cells. (C) Relative mRNA level of Puma in LSK cells. (D) Percentage of LSK cells in G0. (E) Percentage of CD34− LSK cells in G0. (F) The percentage of BrdU+ LSK cells. All data are shown as means ± SEM (n = 4). WT indicates wild-type.
The status of HSC quiescence from double-knockout mice was also analyzed and compared with that of wild-type and Cited2Δ/Δ mice. Interestingly, Hoechst 33342–pyronin Y staining showed that the proportion of LSKs or CD34− LSKs at G0 was significantly improved in Cited2Δ/ΔHIF-1αΔ/Δ mice compared with the Cited2Δ/Δ mice (Figure 5D-E). This result was confirmed by a BrdU incorporation experiment, in which Cited2Δ/ΔHIF-1αΔ/Δ mice displayed fewer cycling LSK cells compared with their Cited2Δ/Δ counterparts (33.8% ± 10.9% vs 66.5% ± 1.7%; Figure 5F). Consistent with improved HSC quiescence, Cited2Δ/ΔHIF-1αΔ/Δ mice showed significantly improved survival rates after 5-FU treatment (Figure 6A-B).
Deletion of HIF-1α partially rescues impaired HSC reconstitution capacity caused by Cited2 deficiency. (A-B) 5-FU treatment. (A) WBC counts at the indicated times after 5-FU administration (n = 10). (B) Survival of mice after 5-FU administration (n = 10). The survival of double-knockout mice was significantly improved compared with Cited2-knockout mice (P < .01). (C-E) Transplantation experiments. (C) Absolute numbers of donor-derived B cells (B220+), myeloid cells (Mac-1+ or Gr-1+), and T cells (CD3+) in PB 16 weeks after noncompetitive transplantation (means ± SEM, n = 5). (D) PB chimerism in recipients 8, 12, and 16 weeks after competitive transplantation (n = 6). (E) BM chimerism in recipients 16 weeks after competitive transplantation. Data are shown as means ± SEM (n = 6). WT indicates wild-type.
Deletion of HIF-1α partially rescues impaired HSC reconstitution capacity caused by Cited2 deficiency. (A-B) 5-FU treatment. (A) WBC counts at the indicated times after 5-FU administration (n = 10). (B) Survival of mice after 5-FU administration (n = 10). The survival of double-knockout mice was significantly improved compared with Cited2-knockout mice (P < .01). (C-E) Transplantation experiments. (C) Absolute numbers of donor-derived B cells (B220+), myeloid cells (Mac-1+ or Gr-1+), and T cells (CD3+) in PB 16 weeks after noncompetitive transplantation (means ± SEM, n = 5). (D) PB chimerism in recipients 8, 12, and 16 weeks after competitive transplantation (n = 6). (E) BM chimerism in recipients 16 weeks after competitive transplantation. Data are shown as means ± SEM (n = 6). WT indicates wild-type.
We then evaluated the long-term reconstitution capacity of Cited2Δ/ΔHIF-1αΔ/Δ HSCs by BMT. In noncompetitive BMT, we transplanted a small dose (5 × 105) of BM cells from each genotype into lethally irradiated recipients. At 16 weeks after transplantation, the absolute number of donor derived B220+, CD3+, or myeloid cells in the PB was significantly decreased in recipients transplanted with Cited2Δ/Δ BM compared with wild-type controls, whereas recipients transplanted with Cited2Δ/ΔHIF-1αΔ/Δ BM had significantly enhanced PB reconstitution (Figure 6C). In competitive BMT, PB chimerism was comparable between Cited2Δ/ΔHIF-1αΔ/Δ and Cited2Δ/Δ cell recipients (Figure 6D and supplemental Figure 4) from 8-16 weeks after transplantation. In addition, we observed very low number of HIF-1αΔ/Δ–derived cells (less than 0.1%) in the PB 16 weeks after transplantation (supplemental Figure 4), suggesting that HIF-1α is crucial for HSC reconstitution capacity. After 16 weeks, the recipients were killed for BM analysis. Although we did not observe any difference in donor-derived PB chimerism (Figure 6D), CD34− LSK chimerism was significantly improved in recipients transplanted with Cited2Δ/ΔHIF-1αΔ/Δ BM compared with those transplanted with Cited2Δ/Δ BM (Figure 6E). This is consistent with the study by Takubo et al, who observed no difference in PB chimerism between VHL+/−- or wild-type transplanted recipients but improved BM chimerism from VHL+/−–transplanted recipients.15 These data indicate that deletion of HIF-1α partially rescues impaired HSC reconstitution activity caused by Cited2 deficiency.
Cited2 deficiency alters expression of HSC quiescence-related genes
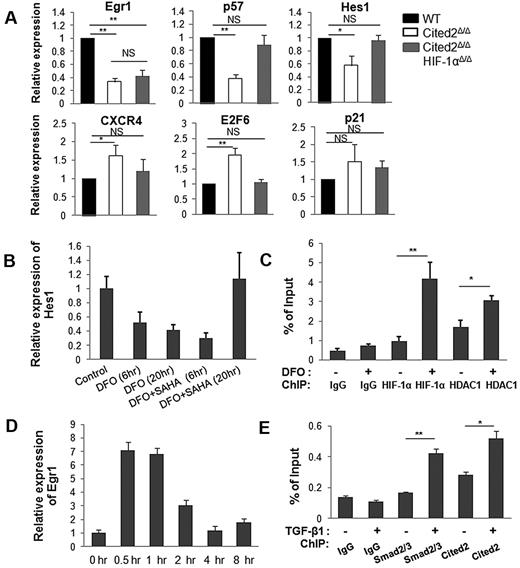
To decipher the mechanisms by which Cited2 controls HSC quiescence, expression of a set of HSC function- or quiescence-related genes was examined in sorted CD34− LSK cells from each genotype by real-time RT-PCR (summarized in Figure 7A and supplemental Figure 5). We found that Egr1, p57, and Hes1 were down-regulated 3.0-, 2.6-, and 1.7-fold, respectively, whereas E2F6 was up-regulated 1.9-fold in Cited2Δ/Δ HSCs. Interestingly, the expression of p57, Hes1, and E2F6 was restored to normal levels in Cited2Δ/ΔHIF-1αΔ/Δ HSCs. Among conventional HIF-1 target genes, the expression of CXCR4 (Figure 7A) and Enolase 1 (data not shown) was increased in Cited2Δ/Δ HSCs. Vegfa was not dysregulated in Cited2Δ/Δ HSCs (data not shown), which is consistent with the finding by Takubo et al15 that the expression of Vegfa is unaffected in HIF-1αΔ/Δ mice. These studies suggest that HIF-1 target gene expression varies depending on the cell and organ system studied, and that Vegfa is not differentially expressed in HSCs with various HIF-1α levels in vivo.
Cited2 deficiency affects the expression of HSC quiescence related genes. (A) Gene-expression analysis by real-time PCR. CD34− LSK cells were sorted for RNA isolation from each genotype. The analysis was repeated by 3 or 4 independent experiments. Two mice per genotype were used in each experiment. Data are shown as relative expression compared with WT controls (means ± SEM). (B-C) HIF-1α and HDAC1 bind to the Hes1 promoter. (B) Relative expression of Hes1 in EML C1 cells treated with DFO (100μM) with or without suberoylanilide hydroxamic acid (2μM). (C) ChIP assay in EML C1 cells was performed with anti–HIF-1α, anti-HDAC1, and IgG Abs. The precipitated DNA was subjected to quantitative real-time PCR with primers amplifying nt −235 to −1 of the Hes1 promoter. (D-E) Cited2 and Smad2/3 are recruited to the Egr1 promoter. (D) Relative expression of Egr1 in starved EML C1 cells after treatment with TGF-β1 (7.5 ng/mL) for the indicated time periods. (E) Starved EML C1 cells were treated with TGF-β1 for 1 hour and used for ChIP assay with anti-Smad2/3, anti-Cited2, and IgG Abs. The precipitated DNA was subjected to quantitative real-time PCR with primers amplifying nt −2342 to −1965 of the Egr1 promoter. These experiments were repeated 3 times. *P < .05; **P < .01. NS indicates not significant.
Cited2 deficiency affects the expression of HSC quiescence related genes. (A) Gene-expression analysis by real-time PCR. CD34− LSK cells were sorted for RNA isolation from each genotype. The analysis was repeated by 3 or 4 independent experiments. Two mice per genotype were used in each experiment. Data are shown as relative expression compared with WT controls (means ± SEM). (B-C) HIF-1α and HDAC1 bind to the Hes1 promoter. (B) Relative expression of Hes1 in EML C1 cells treated with DFO (100μM) with or without suberoylanilide hydroxamic acid (2μM). (C) ChIP assay in EML C1 cells was performed with anti–HIF-1α, anti-HDAC1, and IgG Abs. The precipitated DNA was subjected to quantitative real-time PCR with primers amplifying nt −235 to −1 of the Hes1 promoter. (D-E) Cited2 and Smad2/3 are recruited to the Egr1 promoter. (D) Relative expression of Egr1 in starved EML C1 cells after treatment with TGF-β1 (7.5 ng/mL) for the indicated time periods. (E) Starved EML C1 cells were treated with TGF-β1 for 1 hour and used for ChIP assay with anti-Smad2/3, anti-Cited2, and IgG Abs. The precipitated DNA was subjected to quantitative real-time PCR with primers amplifying nt −2342 to −1965 of the Egr1 promoter. These experiments were repeated 3 times. *P < .05; **P < .01. NS indicates not significant.
Hes1 is a well-known target of Notch signaling, but we did not observe changes in the expression of Notch molecules in Cited2Δ/Δ HSCs (data not shown). Given that deletion of HIF-1α restores decreased Hes1 to the normal level, it is reasonable to speculate that HIF-1 plays a role in this context. To further dissect HIF-1–regulated expression of the Hes1 gene, we treated EML C1 cells (a murine hematopoietic progenitor cell line) with 100μM desferrioxamine (DFO; Sigma-Aldrich) for 20 hours to stabilize HIF-1α (supplemental Figure 6). Interestingly, treatment with DFO resulted in decreased expression of Hes1 and Cited2 (Figure 7B and supplemental Figure 7), mimicking the differential gene-expression pattern in our Cited2-knockout mouse model. Several studies have demonstrated the recruitment of histone deacetylase 1 (HDAC1) by HIF-1α to chromatin through direct interaction and the modulation of several HIF-1 target genes.31-33 To determine whether HDAC1 mediates the decreased expression of Hes1, we treated EML C1 cells with a 2μM concentration of the HDAC inhibitor suberoylanilide hydroxamic acid (Sigma-Aldrich). When EML C1 cells were treated with suberoylanilide hydroxamic acid plus DFO for 20 hours, Hes1 expression was restored to the normal level compared with cells treated with DFO alone (Figure 7B). The ChIP assay was performed in parallel to analyze the binding of HIF-1α and HDAC1 to the region −235 to −1 of the Hes1 promoter, which contains hypoxia response element.25 Increased binding of both HIF-1α and HDAC1 to the Hes1 promoter was detected when EML C1 cells were treated with DFO (Figure 7C). These data suggest that in Cited2Δ/Δ HSCs, elevated HIF-1α recruits more HDAC1 to the Hes1 promoter and thus represses transcription of the Hes1 gene, whereas deletion of HIF-1α “mitigates” such repression and restores the expression of Hes1 in double-knockout HSCs.
Egr1 was profoundly down-regulated in both Cited2Δ/Δ and Cited2Δ/ΔHIF-1αΔ/Δ HSCs, indicating HIF-1–independent dysregulation of Egr1 expression in the absence of Cited2. Egr1 has previously been shown to play important roles in the maintenance of HSC quiescence and the retention of HSCs in the BM microenvironment.34 Egr1 is a Smad3 target gene35 with multiple Smad-binding elements, GTCT, or AGAC, in the proximal 3-kb region of its promoter. Our previous studies showed that Cited2 interacts with Smad3 directly and modulates the expression of its target gene, MMP9.36 Given that Egr1 expression is significantly decreased in Cited2Δ/Δ HSCs, we hypothesized that Cited2 functions as a transcriptional coactivator in modulating the expression of Egr1 mediated by Smad3. To test this possibility, EML C1 cells were starved for 18 hours in serum-free medium and then treated with 7.5 ng/mL of TGF-β1 (R&D Systems). TGF-β1 treatment strongly induced the expression of Egr1 (Figure 7D) and the phosphorylation of Smad3 (supplemental Figure 8) within 0.5 hours. The ChIP assay was then performed using Abs against Cited2 and Smad2/3. Real-time PCR using chromatin DNA showed increased occupancy of both Smad2/3 and Cited2 on the region −2342 to −1965 of the Egr1 promoter 1 hour after TGF-β1 treatment (Figure 7E), but not on the other regions (data not shown). These results suggest that Cited2 modulates the expression of Egr1 mediated by Smad3.
Discussion
The maintenance of HSCs throughout life is crucial for the constant supply of multiple lineages of blood cells under both physiologic and stressed conditions. At steady state, only a small percentage of HSCs undergo cell division, with the majority of HSCs remaining quiescent in BM niches. BM stress imposed by cytotoxic agents or radiation recruits HSCs from their quiescent niche to ensure rapid reconstitution of a damaged hematopoietic system. Therefore, stem-cell quiescence is essential to protecting the HSC pool from cell-cycle–dependent injury and from acquiring mutations during numerous rounds of replication. The molecular determinants of HSC maintenance have been widely studied, but the exact mechanisms remain unknown.
We reported previously that Cited2 deficiency is associated with defective HSC functions in the fetal liver and that Cited2 is required for fetal liver hematopoiesis.7 In the present study, we have showed that although Cited2 deficiency in adult mice has no apparent influence on steady-state hematopoiesis, as evidenced by both Mx1-Cre and Vav-Cre mouse models, Cited2Δ/Δ mice exhibit significantly reduced numbers of LT-HSCs, increased apoptosis, loss of HSC quiescence, and an impaired reconstitution capacity. Furthermore, our results show that Cited2Δ/ΔHIF-1αΔ/Δ mice display significantly improved HSC quiescence and reconstitution capacity compared with Cited2-knockout mice. These results are consistent with in vitro, in vivo, and structural studies by us and others showing that Cited2 is a negative regulator of HIF-1.4,10,12,13 Our observation that deletion of HIF-1α partially rescued the impaired HSC reconstitution capacity caused by Cited2 deficiency is also in agreement with the finding by Takubo et al that stabilization of HIF-1α by biallelic loss of VHL results in impaired reconstitution capacity.15 Although VHL may target proteins other than HIF-1α,37 these data strongly suggest that regulation of HSC function by HIF-1α is highly dose dependent.
Our finding that Cited2 deficiency results in increased apoptosis of HSCs is also in agreement with the study by Kranc et al,38 who observed increased apoptosis and elevated p53 protein level in cultured Cited2-knockout LSK cells. This suggests that Cited2 deficiency leads to perturbation of other factors in addition to HIF-1α, such as p53. Therefore, it is not surprising that HIF-1α deletion partially rescues impaired HSC quiescence (Figure 5D-F) but not the increased apoptosis (Figure 5B). Whether there is cross-talk between p53 and HIF-1 in mediating HSC functions requires further investigation. Our finding that deletion of HIF-1α does not restore increased apoptosis may partly explain the moderate but not total rescue of the impaired HSC reconstitution capacity caused by Cited2 deficiency (Figure 6C,E). In the study by Kranc et al,38 most of the mice became moribund within 6-15 days after initiation of pI-pC treatment to delete Cited2, whereas we observed normal steady-state hematopoiesis in both Mx1-Cre and Vav-Cre mouse models in which Cited2 was efficiently deleted (Figure 1 and supplemental Figure 1). Because Kranc et al started the pI-pC injections when the mice were approximately 8-12 weeks of age,38 as opposed to approximately 5-6 weeks in our study, we performed sequential pI-pC injection into 12-week-old Cited2fl/fl;Mx1-Cre mice and still observed normal hematopoiesis (data not shown). Therefore, the differences in phenotype between our Cited2Δ/Δ mice and those of Kranc et al are more likely because of different targeting vectors rather than deletion efficiency or the age of the mice.38
Expression profile analysis of CD34− LSK cells revealed that Egr1, p57, and Hes1 were significantly down-regulated and E2F6 was up-regulated in the absence of Cited2. With the exception of Egr1, the expression of these dysregulated genes was restored to the wild-type levels in Cited2Δ/ΔHIF-1αΔ/Δ mice. In addition, we observed a moderate but not significant increase of p21 expression in Cited2Δ/ΔCD34− LSK cells, which is consistent with the results of Kranc et al,38 who observed a 1.3-fold increase of p21 in Cited2-knockout LSK cells. The decrease of p57 and increase of p21 in Cited2Δ/Δ HSCs are also in agreement with the findings of Eliasson et al,21 who found that p21 was significantly up-regulated whereas p57 was down-regulated in Flt3−CD34− LSK cells under hypoxia. It is well established that E2F6 represses the transcription of E2F1 target genes such as p57 via competition with E2F1 for promoter binding to the p57 gene promoter.39-42 Therefore, the increased level of E2F6 may, at least in part, account for the decreased expression of p57 in Cited2Δ/Δ HSCs. It is worth noting that decreased expression of p57 in HSCs has been detected in many knockout mouse models in which HSC quiescence is affected, such as in CXCR4-,43 JunB-,44 Tpo-,45 and STAT546 –knockout mice. Because Cited2 is a direct target gene of STAT5,47 and because STAT5 deficiency results in decreased HSC quiescence and lower expression of p57, our findings may in part explain the loss of cell quiescence in STAT5-knockout HSCs.46
Using EML C1 cells, we have demonstrated that 1 possible mechanism for transcriptional repression of Hes1 is through the recruitment of HDAC1 by HIF-1α to the Hes1 promoter. It has been reported that HIF-1α enhances Hes1 expression by antagonizing the autoregulation of the Hes1 gene in 293 cells.25 The difference may be partly because of different cell types or distinct context-dependent mechanisms. Recently, increased HDAC1 deposition at several hematopoietic gene promoters was observed in acute myeloid leukemia and acute lymphocytic leukemia blast cells.48 Furthermore, acute lymphocytic leukemia is associated with aberrant activation of HIF-1α and decreased expression of Cited2.49 It will be of interest to further probe the network including Cited2, HIF-1α, and HDAC1 deposition and their significance in leukemogenesis.
This study demonstrates for the first time that conditional deletion of Cited2 in adult mice not only results in elevated HSC apoptosis, as reported by Kranc et al,38 but also in significant loss of HSC quiescence. Furthermore, expression profile analysis suggests that Cited2 maintains HSC quiescence by regulating the expression of Egr1, p57, Hes1, and E2F6, and that down-regulation of the latter 3 genes in the absence of Cited2 can be restored to normal levels by deletion of HIF-1α. The results of the present study provide supporting evidence that Cited2 is essential for the maintenance of HSC quiescence, at least in part by regulating HIF-1 activity. The unique structural feature of Cited2 in interacting with p300/CBP may help in the design of small compounds targeting hematopoietic disorders in which HIF plays pivotal roles.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael Sramkoski at Case Comprehensive Cancer Center for help with flow cytometric analysis; Drs Jun Yin and Yulan Qing at Case Western Reserve University for technical assistance; Dr Randall S. Johnson at the University of California, San Diego for providing the HIF-1αfl/+ mice; Dr Schickwann Tsai at the University of Utah for providing EML C1 cells; and Dr Hung-Ying Kao at Case Western Reserve University for providing suberoylanilide hydroxamic acid.
This study was supported by the National Institutes of Health (grant R01HL091896 to Y.-C.Y. and R01DK059380 to K.D.B.).
National Institutes of Health
Authorship
Contribution: Y.C., Q.L., X.H., and C.C. performed the research and analyzed the data; Z.W., S.L.D., and K.D.B. designed the research and analyzed the data; D.D. analyzed the data; and J.D. and Y.-C.Y. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.C. is Department of Pharmacology, Case Western Reserve University School of Medicine, Cleveland, OH. The current affiliation for C.C. is Brigham and Women's Hospital, Boston, MA.
Correspondence: Yu-Chung Yang, PhD, Department of Biochemistry and Cancer Center, Case Western Reserve University School of Medicine, 10900 Euclid Ave, W444, Cleveland, OH 44106; e-mail: yu-chung.yang@case.edu.