Abstract
The lower gastrointestinal tract (LGI) and liver are the GVHD target organs most associated with treatment failure and nonrelapse mortality. We recently identified regenerating islet-derived 3-α (REG3α) as a plasma biomarker of LGI GVHD. We compared REG3α with 2 previously reported GI and liver GVHD diagnostic biomarkers, hepatocyte growth factor (HGF) and cytokeratin fragment 18, in 954 hematopoietic cell transplantation patients. All 3 biomarkers were significantly elevated in LGI GVHD compared with non-GVHD diarrhea; REG3α discerned LGI GVHD from non-GVHD diarrhea better than HGF and cytokeratin fragment 18. Although all 3 biomarkers predicted nonresponse to therapy at day 28 in LGI GVHD patients, only REG3α and HGF concentrations predicted 1-year nonrelapse mortality (P = .01 and P = .02, respectively). Liver GVHD without GI involvement at GVHD onset and non-GVHD liver complications were uncommon; all 3 biomarkers were elevated in liver GVHD, but did not distinguish GVHD from other causes of hyperbilirubinemia.
Introduction
Acute GVHD, a leading cause of nonrelapse mortality (NRM) after allogeneic hematopoietic cell transplantation (HCT), is measured by dysfunction in the skin, liver, and gastrointestinal (GI) tract.1-3 We recently identified regenerating islet-derived 3-α (REG3α), an antimicrobial protein expressed in Paneth cells, as a biomarker of GVHD of the lower GI (LGI) tract that can differentiate LGI GVHD from non-GVHD diarrhea. Concentrations of this biomarker at onset of GVHD can predict response to treatment and NRM.4 Hepatocyte growth factor (HGF) has been reported as a biomarker that is elevated in GVHD of the GI tract and liver as part of a panel of 4 GVHD biomarkers.5 Similarly, cytokeratin fragment 18 (KRT18), an apoptotic protein, was described previously as a biomarker of visceral GVHD in a cohort of 55 patients,6 and has recently been correlated with response to GVHD therapy.7 Liver involvement has been historically observed in up to 36% of GVHD patients,8,9 although a recent cohort demonstrated a declining incidence.10 Liver involvement at GVHD onset occurs in 6%-20% of patients8,9,11 and has been associated with poor response to GVHD therapy and increased NRM.8,9,12 There are no validated biomarkers specific to liver GVHD. We compared the diagnostic and prognostic utility of REG3α, HGF, and KRT18 for LGI and liver GVHD.
Methods
Patients and samples
All plasma/serum samples and patient information were collected after obtaining patient consent in accordance with the Declaration of Helsinki for institutional review board–approved studies at the University of Michigan (n = 826), Regensburg, Germany (n = 88), and Kyushu, Japan (n = 40) for patients receiving HCT between January 2000 and November 2010, as described previously (and see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).4 Patients were divided into 6 groups: (1) patients with newly diagnosed GVHD diarrhea with or without skin involvement but without liver involvement (LGI GVHD), (2) patients with diarrhea inconsistent with GVHD either by clinical or histologic criteria (non-GVHD diarrhea), (3) patients with liver GVHD with or without skin involvement but without GI involvement (liver GVHD), (4) patients with liver complications attributable to non-GVHD causes (non-GVHD liver), (5) patients who presented with isolated skin GVHD (skin GVHD), and (6) patients at similar time points who never developed GVHD symptoms (no GVHD). Patient numbers and characteristics are shown in Table 1 and in supplemental Methods. Non-GVHD liver complications were included if patients never developed acute GVHD and experienced hyperbilirubinemia within 120 days of HCT in the absence of chronic GVHD. Causes of non-GVHD diarrhea and liver complications are described in supplemental Table 1. All samples were obtained at GVHD onset within 48 hours of initiation of systemic GVHD therapy and 1 sample was analyzed per patient. Patients who had non-GVHD complications before GVHD onset had samples evaluated only at GVHD onset. The rare incidence of liver involvement at GVHD onset did not permit analysis of 2 independent validation sets as was performed for REG3α in LGI GVHD.4
ELISAs
KRT18 ELISA kits were purchased from Peviva AB (M30 Apoptosense ELISA) and performed according to manufacturer protocol. Samples (diluted 1:2) and standards were run in duplicate. Absorbance was measured with a SpectraMax M2 (Molecular Devices), and results were calculated with SoftMax Pro Version 5.4 software (Molecular Devices). Other biomarker ELISAs were performed as described previously (and see supplemental Methods).4,5
Statistical analysis
Biomarker concentrations from individual patient samples were compared using 2-sample t tests applied to log-transformed concentrations. Differences in characteristics between patient groups were assessed with a Kruskal-Wallis test for continuous values and χ2 tests of association for categorical values. Receiver operating characteristic (ROC) areas under the curve (AUCs) were estimated nonparametrically. NRM was modeled with cumulative incidence regression methods as described by Fine and Gray.13
Results and discussion
Biomarker concentrations were measured in samples from N = 954 allogeneic HCT recipients from the University of Michigan (n = 826), University Medical Center Regensburg (n = 88), and Kyushu University (n = 40; Table 1). The incidence of liver involvement at onset of GVHD requiring systemic corticosteroids (35 of 285; 12%) in our patient dataset was comparable to previous reports.8,9,11,14 There was no statistical difference in biomarker concentrations based on GVHD prophylaxis whether a calcineurin inhibitor was combined with either methotrexate or mycophenolate mofetil (supplemental Table 2). All 3 biomarkers were significantly elevated in LGI GVHD compared with non-GVHD diarrhea and asymptomatic patients (Figure 1A-C). REG3α and HGF concentrations were also elevated in LGI GVHD compared with isolated skin GVHD, whereas KRT18 concentrations were not. This is consistent with the KRT18 tissue expression profile,15 but differs from findings reported by Luft et al that KRT18 concentrations are not elevated in skin GVHD.6
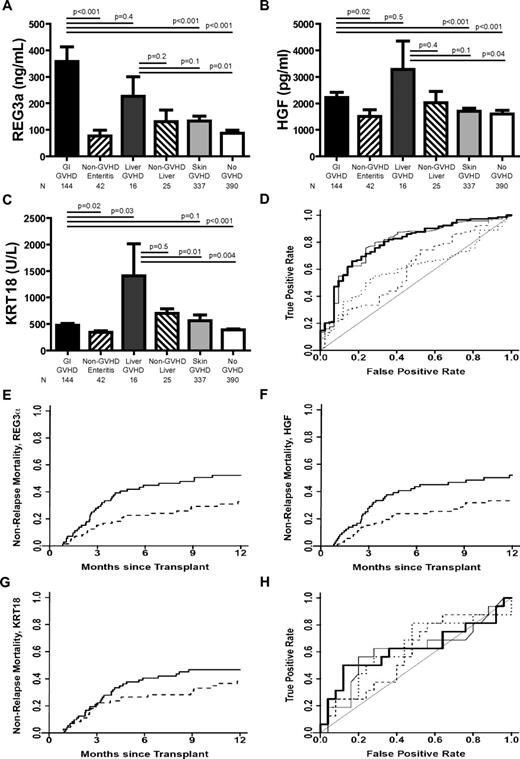
Biomarkers at the onset of GVHD symptoms. (A-C) REG3α, HGF, and KRT18 concentrations, respectively, at the onset of symptoms consistent with GVHD in 954 HCT patients. (D) ROC curves comparing biomarker concentrations at the onset of LGI GVHD without liver GVHD (n = 144) and non-GVHD diarrhea (n = 42). REG3α (thin solid line): AUC = 0.79; HGF (dotted line): AUC = 0.60; KRT18 (dashed line): AUC = 0.60; composite of all 3 biomarkers (thick solid line): AUC = 0.80. (E-G) NRM in patients with LGI GVHD at onset with onset concentrations above the median (solid line; n = 89) versus patients with onset concentrations below the median (dotted line; n = 89) for REG3a, HGF, and KRT18, respectively. (E) REG3a: > 135 ng/mL versus ≤ 135 ng/mL; 52% versus 33%, P = .01. (F) HGF: > 1398 pg/mL versus ≤ 1398 pg/mL; 52% versus 33%, P = .02. (G) KRT18 > 373 U/L versus ≤ 373 U/L; 47% versus 38%, P = .3. (H) ROC curves comparing biomarker concentrations at the onset of liver GVHD without GI GVHD (n = 16) and non-GVHD liver complications (n = 25); REG3α (thin solid line): AUC = 0.61; HGF (dotted line): AUC = 0.59; KRT18 (dashed line): AUC = 0.63; composite of all 3 biomarkers (thick solid line): AUC = 0.62.
Biomarkers at the onset of GVHD symptoms. (A-C) REG3α, HGF, and KRT18 concentrations, respectively, at the onset of symptoms consistent with GVHD in 954 HCT patients. (D) ROC curves comparing biomarker concentrations at the onset of LGI GVHD without liver GVHD (n = 144) and non-GVHD diarrhea (n = 42). REG3α (thin solid line): AUC = 0.79; HGF (dotted line): AUC = 0.60; KRT18 (dashed line): AUC = 0.60; composite of all 3 biomarkers (thick solid line): AUC = 0.80. (E-G) NRM in patients with LGI GVHD at onset with onset concentrations above the median (solid line; n = 89) versus patients with onset concentrations below the median (dotted line; n = 89) for REG3a, HGF, and KRT18, respectively. (E) REG3a: > 135 ng/mL versus ≤ 135 ng/mL; 52% versus 33%, P = .01. (F) HGF: > 1398 pg/mL versus ≤ 1398 pg/mL; 52% versus 33%, P = .02. (G) KRT18 > 373 U/L versus ≤ 373 U/L; 47% versus 38%, P = .3. (H) ROC curves comparing biomarker concentrations at the onset of liver GVHD without GI GVHD (n = 16) and non-GVHD liver complications (n = 25); REG3α (thin solid line): AUC = 0.61; HGF (dotted line): AUC = 0.59; KRT18 (dashed line): AUC = 0.63; composite of all 3 biomarkers (thick solid line): AUC = 0.62.
We compared the diagnostic ability of REG3α, HGF, and KRT18 as biomarkers for LGI GVHD using ROC curves. REG3α performed better than KRT18 and HGF as a diagnostic biomarker distinguishing LGI GVHD from non-GVHD diarrhea (Figure 1D; AUC = 0.79, 0.60, and 0.60, respectively). The combination of all 3 biomarkers into a composite panel provided minimal additional diagnostic utility to that of REG3α alone (AUC = 0.80). Positive and negative predictive values for diagnostic utility of all 3 biomarkers are listed in supplemental Table 3. REG3α concentrations maintained diagnostic utility in patients experiencing diarrhea within 14 days of HCT, whereas HGF and KRT18 did not (supplemental Table 4). All 3 biomarkers, when measured at the onset of LGI GVHD, predicted nonresponse to therapy at 28 days16-18 (supplemental Figure 1 and supplemental Table 5).
We divided patients into groups of equal size according to whether biomarker concentrations were above (high) or below (low) the median concentration at the onset of LGI GVHD. High REG3α and HGF concentrations correlated with significantly higher 1-year NRM, whereas there was no significant correlation between NRM and high KRT18 concentrations. (Figure 1E-G; P = .01, P = .02, and P = .3, respectively). Biomarker concentrations were comparable at onset between patients receiving systemic corticosteroids alone (n = 102) and those receiving multiagent therapy (n = 40) as initial GVHD treatment (supplemental Table 6).
In the present study and as reported recently,10 liver GVHD without GI involvement at the onset of disease was uncommon (n = 16; 3% of GVHD patients and 2% of all patients), as were liver complications early after HCT (n = 25; 3% of all patients). REG3α and HGF concentrations were elevated in liver GVHD compared with asymptomatic patients, but were comparable to concentrations in patients with LGI GVHD, non-GVHD hyperbilirubinemia, and isolated skin GVHD (Figure 1A-B). KRT18 concentrations were significantly higher in patients with liver GVHD than in all other patients except those with non-GVHD liver complications (Figure 1C). None of the 3 biomarkers effectively distinguished liver GVHD from non-GVHD liver complications (Figure 1H; AUC = 0.61, 0.59, and 0.63, respectively; composite panel AUC = 0.62). Most patients with non-GVHD liver complications had sinusoidal obstruction syndrome (n = 20), which can often be distinguished clinically from GVHD. Cases in which biopsies were required to determine the etiology of hyperbilirubinemia during the period in which patients typically develop acute GVHD were very uncommon in our patient cohort (n = 7).
When including all patients with concomitant GI and liver involvement at GVHD onset regardless of other organ involvement (n = 35), REG3α concentrations at the onset of liver GVHD were significantly higher than concentrations at onset of non-GVHD hyperbilirubinemia (supplemental Figure 2). Comparing concentrations from all 35 liver GVHD patients with those from the 25 patients with non-GVHD liver complications, the AUC of the REG3α ROC curve improved to 0.69, whereas curves for KRT18 and HGF performed more poorly (0.54 and 0.57, respectively), reinforcing the strength of REG3α as an LGI GVHD biomarker and lack of specificity of KRT18 and HGF as visceral GVHD biomarkers.
In conclusion, REG3α performs better than HGF and KRT18 as a diagnostic biomarker of LGI GVHD. All 3 biomarkers predicted day 28 nonresponse to therapy, and both REG3α and HGF are good prognostic markers for 1-year NRM in patients with LGI GVHD. These findings should be validated in a prospective, multicenter study. Hyperbilirubinemia was an uncommon occurrence in our patient cohort, and the preliminary findings from this study warrant further investigation. In addition, a dedicated proteomics search should be performed to identify potential biomarkers specific to liver GVHD pathophysiology. This search should then be validated in a multicenter trial because of the rarity of this post-HCT complication and to minimize any potential center effect.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the National Institutes of Health (RC1-HL-101102, P01-CA039542, and T32-HL007622), the Hartwell Foundation, and the Doris Duke Charitable Foundation. J.L.M.F. is a clinical research professor of the American Cancer Society and a visiting fellow of the Oxford All Souls College. S.P. is an investigator of the Eric Hartwell fund and the Amy Strelzer Manasevit Research Program.
National Institutes of Health
Authorship
Contribution: A.C.H. designed and planned the experiments, performed the research, performed the data collection and quality assurance, analyzed the data, and wrote the manuscript; J.L.M.F. planned the study, interpreted the data, and wrote the manuscript; T.M.B. was the study statistician and wrote the manuscript; E.H., T.T., J.E.L., S.W.C, K.L., K.A., D.R.C., and P.R. contributed to patient accrual, clinical data collection, and quality assurance, to discussions of the research, and to writing of the manuscript; M.V.L. performed the experiments and wrote the manuscript; and S.P. conceived and planned the study design, performed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Sophie Paczesny, Blood and Marrow Transplant Program, University of Michigan Comprehensive Cancer Center, Room 6410, 1500 E Medical Center Dr, Ann Arbor, MI, 48109-5942; e-mail: sophiep@med.umich.edu.