Abstract
Dendritic cells (DCs) are essential in inducing adaptive immune responses against bacteria by expressing cytokines that skew T-cell responses toward protective Th17 cells. Although it is widely recognized that induction of these cytokines by DCs involves activation of multiple receptors, it is still incompletely characterized which combination of receptors specifically skews Th17-cell responses. Here we have identified a novel role for FcγRIIa in promoting human Th17 cells. Activation of DCs by bacteria opsonized by serum IgG strongly promoted Th17 responses, which was FcγRIIa-dependent and coincided with enhanced production of selected cytokines by DCs, including Th17-promoting IL-1β and IL-23. Notably, FcγRIIa stimulation on DCs did not induce cytokine production when stimulated individually, but selectively amplified cytokine responses through synergy with TLR2, 4, or 5. Importantly, this synergy is mediated at 2 different levels. First, TLR-FcγRIIa costimulation strongly increased transcription of pro-IL-1β and IL-23p19. Second, FcγRIIa triggering induced activation of caspase-1, which cleaves pro-IL-1β into its bioactive form and thereby enhanced IL-1β secretion. Taken together, these data identified cross-talk between TLRs and FcγRIIa as a novel mechanism by which DCs promote protective effector Th17-cell responses against bacteria.
Introduction
Protection against different classes of pathogens requires the activation of Ag-presenting dendritic cells (DCs) to express factors that promote the development of distinct effector Th-cell subsets, which are specialized to combat the class of pathogen involved.1 Effective T cell–mediated immunity against extracellular bacteria requires DCs to produce IL-1β, IL-6, and IL-23 that contribute to the development of Th17 cells.2,3 The pathogen-induced production of cytokines by DCs is induced on sensing of pathogens by pattern-recognition receptors (PRRs), including TLRs, C-type lectins, Nod-like receptors, and RIG-I–like receptors.4-7 Although triggering of individual PRRs is known to induce cytokine production, it is becoming increasingly clear that the ultimate amount and the profile of cytokine production by DCs crucially depends on cross-talk between multiple PRRs.8-10 However, our knowledge on these cross-talk mechanisms is likely to be still largely incomplete.10
In this respect, the role of FcγRs, the family of high- and low-affinity receptors for IgG, in the induction of cytokine production did not receive much attention. IgG is the most prevalent immunoglobulin in the blood and body tissues.11,12 Because of the high levels of IgG directed against numerous polyreactive bacterial Ags, invading bacteria are efficiently opsonized as soon as they penetrate the body's barriers, even during primary infection.13-17 IgG opsonization can directly lead to pathogen inactivation via complement activation, but can also result in a variety of responses by different effector immune cells such as cell degranulation, production of reactive oxygen species (ROS), or Ab-dependent cellular cytotoxicity (ADCC).18,19 In addition, binding of opsonized pathogens to low-affinity FcγRs on DCs mediates phagocytosis, degradation and subsequent presentation of pathogen-derived Ags to T cells.20 FcγR stimulation also induces DC maturation.21-23 However, the triggering of FcγRs on DCs results in no or only low production of cytokines and has not been demonstrated to play a major role in polarization of human T-cell responses in healthy donors.21,22
In the present study, we have taken into account that in most conditions DCs will engage bacteria that are IgG opsonized and that such DCs will be simultaneous triggered via FcγRs and bacterial sensors. We here report that the engagement of DCs with opsonized bacteria resulted in strongly up-regulated production of selected cytokines, including IL-1β and IL-23, which favored the development of Th17 cells. This effect was fully dependent on stimulation of the low-affinity IgG receptor FcγRIIa (also known as CD32a), which synergized with TLRs for the amplification of Th17-promoting cytokines by both enhancing cytokine transcription and by activating caspase-1. Taken together, these data identified cross-talk between TLRs and FcγRIIa as a novel mechanism by which DCs promote the development of protective effector T cells in response to bacteria.
Methods
Binding ELISA with bacteria
Sheep blood agarose plates (Biomerieux) were inoculated with Klebsiella pneumoniae (clinical isolate), Escherichia coli (ATCC 8738), Salmonella typhimurium (clinical isolate), Staphylococcus aureus (RN4220), or Staphylococcus epidermidis (RP62a) and incubated at 37°C overnight. Bacteria were removed from the plate, washed and coated overnight in PBS in 96-well Maxisorp plates (Nunc). Wells were washed 3 times, blocked with PBS containing 1% bovine serum albumin (Sigma-Aldrich) and subsequently incubated with irrelevant human IgG (Humira; Sanquin Blood Supply), human serum (Lonza), or 5 μg/mL purified IgG (Nanogam; Sanquin Blood Supply), washed 3 times, and incubated with anti–human IgG-HRP (MH16-1; Sanquin Blood Supply).
Cells and stimulation
Monocyte-derived DCs and monocytes were isolated and cultured as described previously.24 Memory CD4+ T cells were obtained by isolating human PBMCs from heparinized human peripheral blood by density gradient centrifugation on Lymphoprep (Nycomed), Percoll (Pharmacia), and finally purification using a MACS isolation kit (Miltenyi Biotec) using CD45RO+-PE (Dakopatts) and anti-PE beads (Miltenyi Biotec). Bacteria were opsonized by preincubation for 2 hours in human serum or 500 μg/mL purified IgG and were washed 3 times afterward in PBS.
Stimulation with S aureus and K pneumoniae was performed in X-VIVO 15 culture medium (Lonza) using 10 bacteria/DC. Stimulation of DCs with 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) or 10 μg/mL peptidoglycan (PGN; Invivogen) was done in Iscove Modified Dulbecco Median (IMDM; Gibco) with 2.5% FCS (Lonza). DCs were stimulated with plate-bound IgG by overnight coating of 96-well Maxisorp plates at room temperature with 1 μg/mL Nanogam, human IgG1 (Sigma-Aldrich), IgG2 (Sigma-Aldrich), or IgG3 purified from Nanogam (Sanquin Blood Supply) diluted in PBS. Both IgG-coated and control wells were blocked by 2-hour incubation with PBS with 10% FCS.
FcγRIIa was blocked by preincubating the cells with 20 μg/mL IV.3 (StemCell Technologies) for 30 minutes at 37°C, after which stimuli and culture medium was added resulting in a final concentration of 5 μg/mL. Uptake of bacteria was inhibited by preincubating DCs for 2 hours at 37°C in X-VIVO medium containing 10μM Cytochalasin D (Sigma-Aldrich).
In vitro differentiation of CD4+ T cells
For Th-cell polarization, bacteria were opsonized and cocultured with 2000 DCs and 20 000 allogeneic CD4+ T cells per well in Maxisorp plates in X-VIVO 15 culture medium in the presence of 10 pg/mL S aureus enterotoxin B (SEB; Sigma-Aldrich). After 4 days, cells were transferred to 96-well round-bottom plates (Greiner Bio-One) and every 2 days half of the medium was replaced by IMDM with 2.5% FCS and 10 U/mL IL-2 (Chiron) and wells were splitted if necessary. Resting cells were restimulated at day 12 with 0.1 μg/mL phorbal myristate acetate (PMA), 1 μg/mL ionomycin, and 10 μg/mL brefeldin A (Sigma-Aldrich) for 5 hours. Cells were washed, fixed with 4% paraformaldehyde (Merck) for 15 minutes, washed again, permeabilized with 0.5% saponin (Calbiochem) in PBS containing 1% bovine serum albumin and incubated with anti–IL-17–biotin (eBioscience) followed by streptavidin-PE (BD Pharmingen) and anti–IFNγ-FITC (BD Biosciences) for 30 minutes at room temperature and analyzed by flow cytometry. For measuring cytokines in supernatant, cells were restimulated with 1 μg/mL anti-CD3 (clone 1XE; Sanquin Blood Supply) and anti-CD28 (15E8; Sanquin Blood Supply) for 24 hours. IL-17 was measured using coated anti–IL-17A (eBioscience) and biotinylated anti–IL-17A (eBioscience), and recombinant IL-17A from R&D Systems as a standard.
ELISA and quantitative PCR
DCs (50 000/well) or monocytes (100 000/well) were stimulated in 96-well Maxisorp plates and supernatants were harvested after 24 hours and stored at −20°C until the levels of IL-1β (Endogen), IL-6 (Biosource), IL-12 (UCytech), IL-23 (eBioscience), and TNFα (eBioscience) were measured by sandwich ELISA, as described previously.25 mRNA production was determined using quantitative RT-PCR (iCycler iQ Multi-Color Real Time PCR Detection System; Bio-Rad). DCs were lysed at the indicated time points, after which mRNA extraction was done using the NucleoSpin RNA Isolation Kit (Macherey-Magel) and cDNA synthesis using the MBI Fermentas Kit. For each sample, the normalized amount of target mRNA was calculated from the obtained threshold cycle (Ct) values (defined as the number of PCR cycles where the fluorescence signal exceeds the detection threshold value) for both target and GAPDH mRNA with normalized amount of target (Nt) = 2Ct(GAPDH) − Ct(target). Data were expressed as relative mRNA expression, which represents the mRNA induction compared with unstimulated cells (which was set at 1). For an overview of used primers, see supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
FcγR expression and FLICA
FcγR expression was determined by staining cells with anti-CD64 (Sanquin Blood Supply), anti-CD32A (IV.3), or anti-CD16 (Sanquin Blood Supply) followed by PE-conjugated goat anti–mouse (Jackson ImmunoResearch Laboratories), or directly labeled anti-CD32B-AF488 (2B6; Sanquin Blood Supply), and analysis by flow cytometry. Caspase-1 activation was determined using the FLICA Apoptosis Detection Kit for Caspase-1 (Immunochemistry Technologies) according to the manufacturer's guidelines. Fluorescence of the cells was assessed using flow cytometry.
Statistics
Data were analyzed for statistical significance using the paired t test with GraphPad Prism Version 5.0 software (GraphPad Software).
Results
Opsonization of bacteria promotes Th17 responses
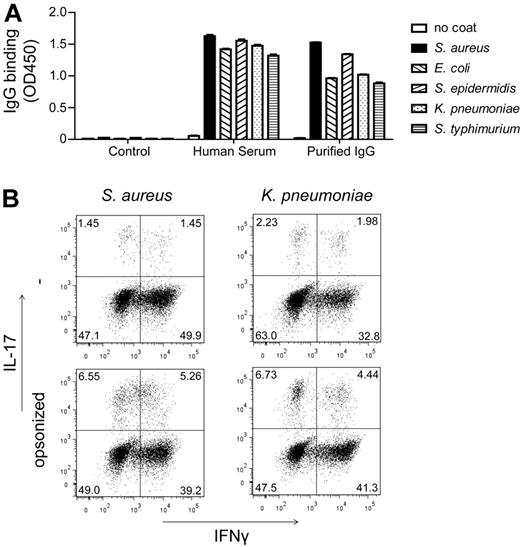
On penetrating the body's barriers, bacteria are exposed to high concentrations of IgG, which is expressed in both blood and tissue fluids. Here, we set out to determine whether IgG opsonization of bacteria affects DC-mediated Th-cell polarization. First, we determined to what extent human serum indeed contains bacteria-specific IgGs by testing the binding of IgG in pooled human serum to a variety of bacterial species by ELISA. All different bacterial strains we tested were recognized by serum IgG (Figure 1A), indicating that these bacteria are opsonized by IgG on exposure. The different bacterial strains were recognized by purified IgGs in a similar fashion as by serum IgGs (Figure 1A), thereby confirming IgG specificity.
Opsonization of bacteria promotes Th17 responses. (A) Bacteria were coated on a 96-well plate, which were blocked and incubated with a control Ab, human serum, or purified IgG, followed by incubation with anti–human IgG-HRP and were subsequently developed. Data shown are mean ± SEM of triplicate measurement from 1 representative experiment of 3. (B) DCs and CD4+ T cells were incubated with bacteria that were preincubated or not in human serum. Intracellular levels of IFNγ and IL-17 were measured at day 12, after 5 hours of restimulation with PMA, ionomycin, and BFA. FACS plots shown are from 1 representative of 3 different donors.
Opsonization of bacteria promotes Th17 responses. (A) Bacteria were coated on a 96-well plate, which were blocked and incubated with a control Ab, human serum, or purified IgG, followed by incubation with anti–human IgG-HRP and were subsequently developed. Data shown are mean ± SEM of triplicate measurement from 1 representative experiment of 3. (B) DCs and CD4+ T cells were incubated with bacteria that were preincubated or not in human serum. Intracellular levels of IFNγ and IL-17 were measured at day 12, after 5 hours of restimulation with PMA, ionomycin, and BFA. FACS plots shown are from 1 representative of 3 different donors.
To investigate whether opsonization of bacteria affects Th-cell polarization, we incubated opsonized and nonopsonized bacteria with human monocyte-derived DCs and CD4+ T cells in serum-free culture medium. To assess Th1 and Th17 skewing, we determined the production of IFNγ and IL-17, respectively, by intracellular FACS staining on restimulation of the T cells after 12 days. Strikingly, opsonization by human serum IgG strongly enhanced Th17 induction for both Gram-positive S aureus and Gram-negative K pneumonia (Figure 1B). Notably, no consistent differences were observed for Th1 responses (Figure 1B). Taken together, these data indicate that IgG opsonization of bacteria skews adaptive immune responses toward Th17.
Opsonization of bacteria modulates cytokine production by human DCs
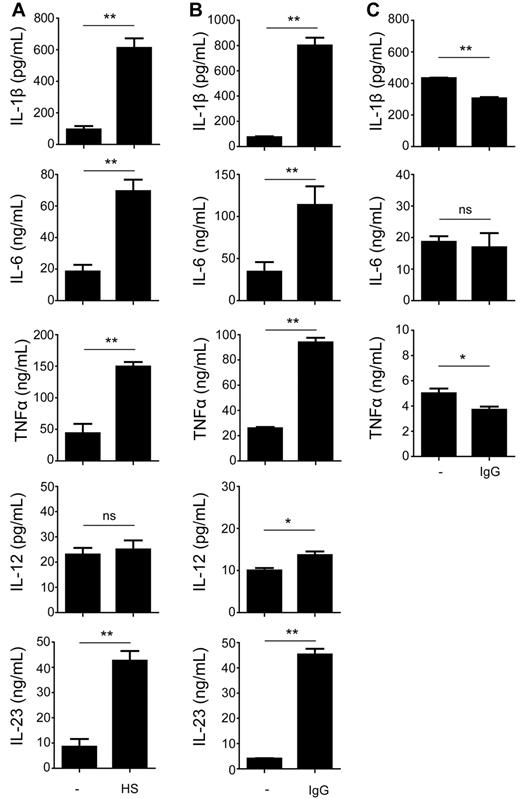
Next, we set out to determine whether the modulated T-cell polarization in response to opsonization of bacteria was mediated by an altered production of polarizing cytokines by DCs. DCs were cocultured with opsonized or nonopsonized S aureus and, after 24 hours, supernatants were harvested and subsequently analyzed for presence of cytokines. Compared with nonopsonized bacteria, activation of DCs with bacteria that were preincubated in human serum resulted in strongly enhanced levels of TNFα and Th17-promoting cytokines IL-1β, IL-6, and IL-23 (Figure 2A). Notably, production of the classic Th1-associated cytokine IL-12 was not affected (Figure 2A). Opsonization of bacteria with purified IgG led to an up-regulation of cytokine production in a similar manner as for whole human serum, with increased IL-1β, IL-6, IL-23, and TNFα production, but only very little increase in IL-12 (Figure 2B). Interestingly, incubation with opsonized bacteria did not lead to enhanced cytokine production by monocytes (Figure 2C). Instead, IL-1β and TNFα production was moderately decreased on activation with opsonized bacteria, while IL-12 and IL-23 production by monocytes was undetectable, both after incubation with opsonized or nonopsonized bacteria. Thus, these results demonstrate that opsonization of bacteria with IgG from serum strongly and selectively modulates cytokine responses by DCs, but not by monocytes.
Opsonization of bacteria modulates cytokine production by human DCs. (A-B) DCs were stimulated for 24 hours with S aureus that was preincubated or not in (A) human serum (HS) or (B) purified IgG. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 6 experiments with different donors. (C) Monocytes were stimulated as described under panel B, after which cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
Opsonization of bacteria modulates cytokine production by human DCs. (A-B) DCs were stimulated for 24 hours with S aureus that was preincubated or not in (A) human serum (HS) or (B) purified IgG. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 6 experiments with different donors. (C) Monocytes were stimulated as described under panel B, after which cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
Up-regulation of cytokine production is mediated by FcγRIIa
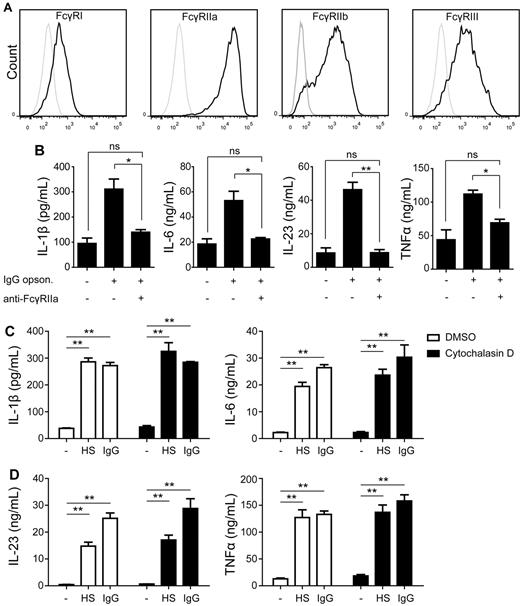
One of the main receptors on DCs for recognition of IgG-opsonized bacteria is the family of FcγRs. To determine which FcγRs are expressed on DCs, we assessed expression by flow cytometry. DCs displayed a low to moderate expression of FcγRI (CD64), FcγRIIb (CD32b), and FcγRIII (CD16), but highly expressed FcγRIIa (CD32a; Figure 3A). To determine whether FcγRs are responsible for the enhanced cytokine production by DCs induced by opsonized bacteria, we blocked different FcγRs with specific Abs during DC activation and assessed cytokine production. Blocking of FcγRI and FcγRIII did not have any effect on cytokine production induced by opsonized bacteria (data not shown). In contrast, blocking of FcγRIIa inhibited the up-regulation of IL-1β, IL-6, IL-23, and TNFα production induced by opsonized bacteria and reduced it to the level induced by nonopsonized bacteria (Figure 3B). Thus, FcγRIIa is responsible for the up-regulation of cytokine production by DCs activated by IgG-opsonized bacteria.
Up-regulation of cytokine production is mediated by FcγRIIa. (A) FcγR expression on immature DCs was measured by FACS. Black line indicates expression; and gray line, control. Data shown are from one representative experiment of 3 experiments with different donors. (B) DC were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa, after which cells were stimulated with IgG-opsonized or nonopsonized S aureus. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. (C) DCs were preincubated for 2 hours with DMSO or 10μM Cytochalasin D, after which cells were stimulated with S aureus that was preincubated or not in human serum (HS) or purified IgG. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
Up-regulation of cytokine production is mediated by FcγRIIa. (A) FcγR expression on immature DCs was measured by FACS. Black line indicates expression; and gray line, control. Data shown are from one representative experiment of 3 experiments with different donors. (B) DC were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa, after which cells were stimulated with IgG-opsonized or nonopsonized S aureus. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. (C) DCs were preincubated for 2 hours with DMSO or 10μM Cytochalasin D, after which cells were stimulated with S aureus that was preincubated or not in human serum (HS) or purified IgG. Cytokine levels were determined in the supernatant by ELISA. Data shown are from 1 representative experiment of 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
To determine whether internalization of opsonized bacteria is required for the amplification of cytokines, we blocked internalization using actin-polymerization inhibitor cytochalasin.26 Importantly, blocking internalization did not prevent the enhanced cytokine production induced by IgG opsonization (Figure 3C) indicating that the induction of cytokines by FcγRIIa does not depend on internalization.
FcγRIIa ligation up-regulates cytokine production via synergy with TLR stimulation
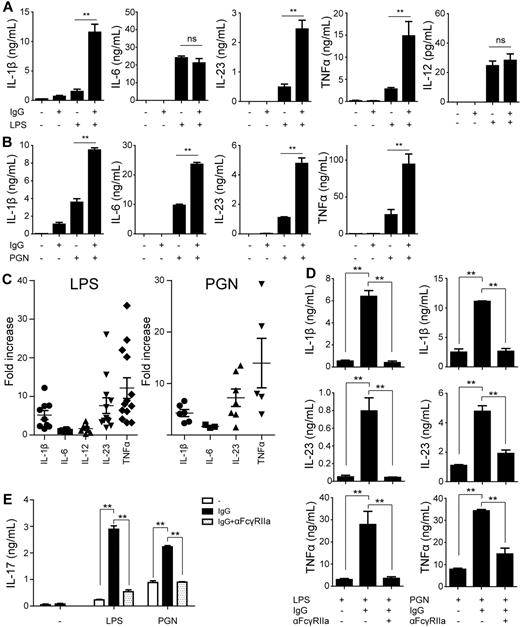
Our data showed that FcγRIIa ligation by opsonized bacteria strongly up-regulated the production of particular cytokines. However, in previous studies, FcγRIIa has been described as a poor inducer of cytokine responses by DCs. To more specifically investigate the role of FcγRIIa in cytokine induction, we stimulated DCs overnight with plate-bound IgG, which is recognized by low-affinity FcγRs such as FcγRIIa.18 Consistent with previous findings, stimulation with complexed IgG alone did not induce any detectable amount of cytokines, with the exception of little amounts of IL-1β and IL-8 (Figure 4A-B, supplemental Figure 1). However, besides FcγRs, opsonized bacteria are recognized through various PRRs, which sense microbial structures that induce cytokine production. The best-studied PRRs belong to the family of TLRs, of which TLR2, 4, and 5 are known to play a major role in recognition of bacteria.27,28 As expected, TLR4 stimulation by LPS and TLR2 stimulation by PGN alone induced production of all measured cytokines (Figure 4A-B, supplemental Figure 1), while stimulation of TLR5 by flagellin induced very little amounts (supplemental Figure 2A). To mimic the condition of stimulation with IgG-opsonized bacteria, we stimulated DCs simultaneously with complexed IgG and TLR ligands. Strikingly, costimulation of LPS with IgG strongly amplified TLR4-induced production of IL-1β, IL-23, and TNFα, but did not affect—or only moderately affected—production of IL-6, IL-8, IL-10, and IL-12 (Figure 4A, supplemental Figure 1). Similarly, stimulation with complexed IgG up-regulated PGN-induced IL-1β, IL-23, and TNFα production (Figure 4B). In contrast to LPS stimulation, coligation with PGN moderately increased production of IL-6, while IL-12 remained undetectable both with and without IgG coligation (Figure 4B). Cytokine production induced by TLR5-ligand flagellin was modulated in a similar manner as PGN (supplemental Figure 2A). To compensate for potential donor differences, we calculated the fold-increase of the different cytokines on costimulation for multiple donors. In line with the previous results, stimulation with complexed IgG strongly up-regulated LPS- and PGN-induced IL-1β, IL-23, and TNFα, while IL-6 and IL-12 production remained generally unaffected (Figure 4C). Next, to determine whether the enhanced cytokine production induced by complexed IgG is dependent on FcγRIIa, we blocked the receptor by preincubating DCs with an FcγRIIa-specific Ab. IgG-induced up-regulation of IL-1β, IL-23, and TNFα was completely blocked by anti-FcγRIIa (Figure 4D, supplemental Figure 2B). To ensure that the observed block did not result from unrecognized effects from using whole anti-FcγRIIa IgGs, we generated Fab fragments of the anti-FcγRIIa Ab. Anti-FcγRIIa Fabs displayed an almost identical block of IgG-induced cytokine production compared with whole Ab (supplemental Figure 3A). In addition, the specific block of FcγRIIb, which is closely related to FcγRIIa, did not inhibit IgG-induced cytokine up-regulation (supplemental Figure 3B), demonstrating that the effect fully depends on FcγRIIa.
FcγRIIa ligation up-regulates Th17-promoting cytokines via synergy with TLR stimulation. (A-B) DCs were stimulated with plate-bound IgG, (A) LPS, (B) PGN, or a combination. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 5 experiments with different donors. (C) Fold-increase in cytokine production on LPS or PGN stimulation combined with plate-bound IgG stimulation compared with LPS or PGN stimulation without plate-bound IgG. For every cytokine, every dot represents a different donor tested in an individual experiment. (D) DCs were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa, after which cells were stimulated with LPS or PGN in combination with plate-bound IgG. Data shown are from 1 representative experiment of 3 independent experiments with different donors. (E) DCs were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa. Subsequently, the cells were stimulated with LPS or PGN in combination with plate-bound IgG and cocultured with CD4+ T cells. IL-17 levels were measured by ELISA at day 12, after restimulation with a CD3-specific and a CD28-specific Ab. Data shown are from 1 representative of 3 different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
FcγRIIa ligation up-regulates Th17-promoting cytokines via synergy with TLR stimulation. (A-B) DCs were stimulated with plate-bound IgG, (A) LPS, (B) PGN, or a combination. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 5 experiments with different donors. (C) Fold-increase in cytokine production on LPS or PGN stimulation combined with plate-bound IgG stimulation compared with LPS or PGN stimulation without plate-bound IgG. For every cytokine, every dot represents a different donor tested in an individual experiment. (D) DCs were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa, after which cells were stimulated with LPS or PGN in combination with plate-bound IgG. Data shown are from 1 representative experiment of 3 independent experiments with different donors. (E) DCs were preincubated for 30 minutes with or without a blocking Ab against FcγRIIa. Subsequently, the cells were stimulated with LPS or PGN in combination with plate-bound IgG and cocultured with CD4+ T cells. IL-17 levels were measured by ELISA at day 12, after restimulation with a CD3-specific and a CD28-specific Ab. Data shown are from 1 representative of 3 different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
Thus, consistent with previous findings, FcγRIIa is a poor inducer of cytokines when stimulated alone. However, FcγRIIa synergizes with TLR2, 4, and 5 for the selective up-regulation of IL-1β, IL-23 and TNFα, thereby correlating with the up-regulated cytokine profile observed after opsonization of bacteria.
Costimulation of TLRs and FcγRIIa promotes Th17 responses
Cytokines IL-1β and IL-23, which are up-regulated by TLR-FcγRIIa costimulation, are pivotal for human Th17 development. To determine whether TLR-FcγRIIa costimulation, similar to what occurs on stimulation with opsonized bacteria, indeed induces Th17 responses, we cocultured stimulated DCs with CD4+ T cells and quantitatively determined IL-17 secretion in the supernatant on restimulation after 12 days. As expected from the little amount of cytokine production, stimulation with complexed IgG alone did not induce any IL-17 secretion (Figure 4E). However, stimulation of DCs with complexed IgG strongly enhanced both LPS- and PGN-induced IL-17 production by T cells (Figure 4E). The enhanced IL-17 production was mediated by FcγRIIa because addition of a blocking Ab attenuated IL-17 production to the level of TLR stimulation alone (Figure 4E). Thus, FcγRIIa cooperates with TLRs on DCs for the promotion of Th17 responses.
FcγRIIa ligation strongly increases sensitivity to TLR ligands and relies on IgG1 and IgG2
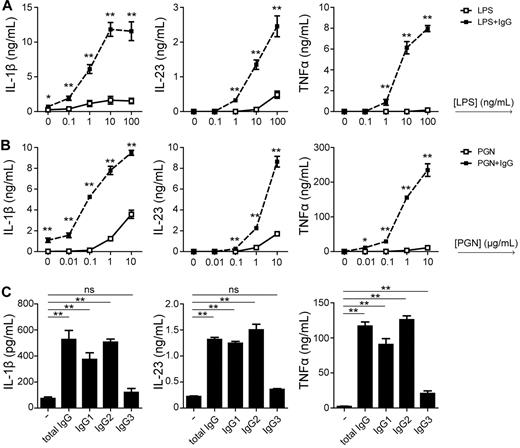
To investigate the effect of IgG stimulation on TLR responses in more detail, we stimulated DCs at serial-step dilutions of LPS and PGN and measured cytokine production after costimulation with plate-bound IgG. Strikingly, IgG costimulation promoted TLR-induced IL-1β, IL-23, and TNFα even at TLR agonist concentrations that were 100- to 1000-fold below optimum, which are concentrations that did not induce any detectable cytokines in the absence of FcγR costimulation (Figure 5A). This demonstrates that FcγRIIa stimulation drastically increases the sensitivity of DCs for TLR ligands.
FcγRIIa ligation increases sensitivity to TLR ligands and relies on IgG1 and IgG2. (A) DCs were stimulated with plate-bound IgG and different concentrations of LPS or PGN. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 5 experiments with different donors. (B) DCs were stimulated with LPS in combination with either plate-bound total IgG, IgG1, IgG2, or IgG3. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
FcγRIIa ligation increases sensitivity to TLR ligands and relies on IgG1 and IgG2. (A) DCs were stimulated with plate-bound IgG and different concentrations of LPS or PGN. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 5 experiments with different donors. (B) DCs were stimulated with LPS in combination with either plate-bound total IgG, IgG1, IgG2, or IgG3. After 24 hours, cytokine levels in the supernatant were determined by ELISA. Data shown are from 1 representative experiment of at least 3 experiments with different donors. Error bars indicate SEM; *P < .05, **P < .01; ns indicates not significant; paired t test.
In addition, we assessed the role of different IgG subclasses. Human serum and tissue fluids contain a mixture of different IgG subclasses, which may differently stimulate FcγRIIa and consequently give rise to different responses. Therefore, we selectively costimulated DCs with either IgG1, IgG2, or IgG3. IgG4 was omitted considering its extremely low affinity to FcγRs.29 While costimulation with IgG1 and IgG2 up-regulated IL-1β, IL-23, and TNFα in a similar manner as total IgG, costimulation with IgG3 hardly induced any up-regulation of cytokines (Figure 5B). These data indicate that the subclass of IgG plays an important role in the orchestration of Th17 responses against bacteria.
Taken together, FcγRIIa stimulation strongly enhances the sensitivity of DCs for TLR agonists, and FcγRIIa-induced up-regulation of cytokines is mainly induced by IgG1 and IgG2.
FcγRIIa ligation modulates cytokine production through both enhanced transcription and caspase-1 activation
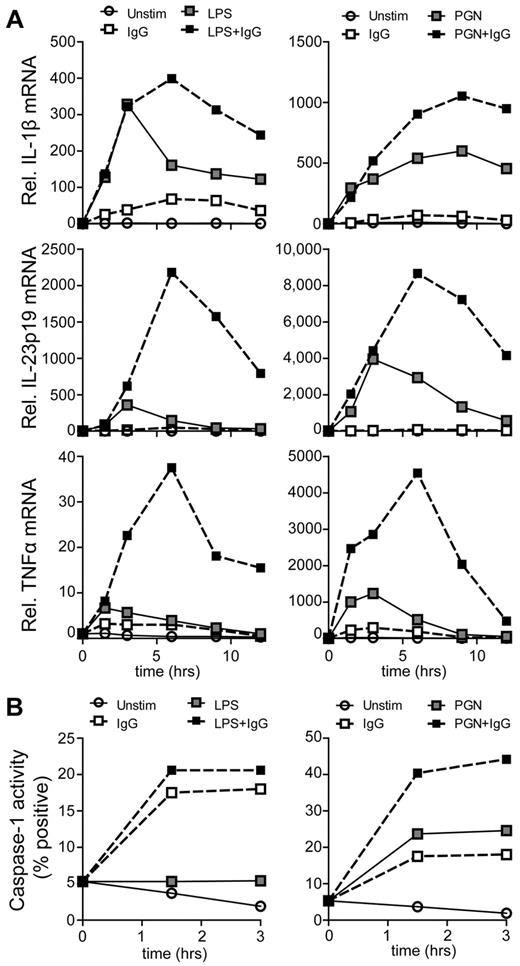
Regulation of cytokine production can be organized at several levels. To determine whether the up-regulation of cytokines by TLR and FcγRIIa coligation is orchestrated at the level of transcription, we determined mRNA expression over time by real-time PCR after costimulation with TLR ligands and plate-bound IgG. Similar to protein production of the cytokines, stimulation of DCs with LPS or PGN alone induced transcription of IL-1β, IL-12p35, IL-12p40, IL-23p19, and TNFα, while stimulation with complexed IgG merely induced little amounts of IL-1β mRNA, but not other cytokine genes (Figure 6A, supplemental Figure 4). However, costimulation of TLRs with FcγRIIa strongly enhanced IL-23p19 and TNFα transcription, correlating with the enhanced production at the protein level. Remarkably, IL-12p40 mRNA production was strongly reduced after costimulation with IgG (supplemental Figure 4), indicating that for particular genes FcγRIIa costimulation also induces transcriptional down-regulation. Taken together, these data suggest that the synergy between TLRs and FcγRIIa for induction of IL-23 and TNFα is mediated at the level of transcription.
FcγRIIa ligation enhances cytokine transcription and activates caspase-1. (A) Relative mRNA expression was determined in DCs that were not stimulated, stimulated with plate-bound IgG, LPS, PGN, or a combination of LPS or PGN with plate-bound IgG. Lysates were made at the indicated time points, after which mRNA expression of the indicated genes was determined by real-time PCR. Expression is normalized to GAPDH and set at 1 for unstimulated cells at 0 hours. Data shown are from 1 representative experiment of 5 experiments using different donors. (B) Caspase-1 activation was determined in DCs that were unstimulated, stimulated with plate-bound-IgG, LPS, PGN, or a combination of LPS or PGN with plate-bound IgG. After stimulation at indicated time points, cells were washed and incubated with a fluorescent caspase-1 inhibitor for 1 hour, after which cells were washed and fluorescence was measured using flow cytometry. Data shown are from 1 representative experiment of 3 experiments using different donors.
FcγRIIa ligation enhances cytokine transcription and activates caspase-1. (A) Relative mRNA expression was determined in DCs that were not stimulated, stimulated with plate-bound IgG, LPS, PGN, or a combination of LPS or PGN with plate-bound IgG. Lysates were made at the indicated time points, after which mRNA expression of the indicated genes was determined by real-time PCR. Expression is normalized to GAPDH and set at 1 for unstimulated cells at 0 hours. Data shown are from 1 representative experiment of 5 experiments using different donors. (B) Caspase-1 activation was determined in DCs that were unstimulated, stimulated with plate-bound-IgG, LPS, PGN, or a combination of LPS or PGN with plate-bound IgG. After stimulation at indicated time points, cells were washed and incubated with a fluorescent caspase-1 inhibitor for 1 hour, after which cells were washed and fluorescence was measured using flow cytometry. Data shown are from 1 representative experiment of 3 experiments using different donors.
Remarkably, IL-1β production seemed to be substantially less up-regulated on the mRNA level than on the protein level after TLR-FcγRIIa costimulation (Figures 6A and 4C, respectively), suggesting that other mechanisms of synergy between these 2 receptors are involved. The release of biologically active IL-1β is known to be a 2-step process: besides transcription and translation, caspase-1 needs to be activated, which processes pro-IL-1β to functional IL-1β.30,31 To investigate whether caspase-1 is involved, we determined its activation after stimulation using fluorescent caspase-1–binding compound FAM-YVAD-FMK and analyzed results by flow cytometry. Strikingly, stimulation with complexed IgG rapidly activated caspase-1 (Figure 6B), indicating that FcγR stimulation alone is sufficient for inflammasome activation. In addition, costimulation with IgG further increased LPS- and PGN-induced caspase-1 activation in an additive manner (Figure 6B). Overall, these data indicate that Th17 induction after IgG opsonization of bacteria is mediated by synergy between TLRs and FcγRIIa on DCs, which enhances the production of Th17-polarizing cytokines via both enhanced transcription and caspase-1 activation.
Discussion
Polarization of Th-cell responses is crucial for pathogen clearance because it tailors immune responses to the class of pathogen involved.1 Here we have identified opsonization of bacteria by IgG as a new mechanism by which the immune system promotes Th17 responses, which is essential to efficiently combat extracellular pathogens. IgG opsonization of bacteria selectively up-regulates the production of TNFα and Th17-polarizing cytokines IL-1β, IL-6, and IL-23 by human DCs, which fully depends on low-affinity IgG receptor FcγRIIa. Notably, FcγRIIa stimulation does not directly lead to cytokine production by DCs, but cooperates with TLR stimulation for the amplification of cytokine production. This synergy is regulated at 2 levels. First, coligation increases the transcription of pro-IL-1β, IL-23p19, and TNFα. Second, FcγRIIa activates caspase-1 independent of TLR stimulation, thereby further enhancing the production of IL-1β. Importantly, Th17 induction via this mechanism is likely to occur particularly in tissues that are located just below the epithelial barriers that are most frequently the target of bacterial infections and contain abundant amounts of DCs and interstitial IgGs. Thus, TLR-FcγR cross-talk on DCs may be a universally relevant natural mechanism to counteract bacterial infections in numerous sites in the human body.
On infection, pathogens are recognized by multiple microbial sensors on Ag-presenting cells. The ultimate immune response against a given pathogen critically depends on the interaction between these different receptors.8-10 The main current concept is that this cross-talk is predominantly induced between receptors belonging to the families of PRRs, with an underexposed role for other receptors. Here we show that FcγRIIa also plays an important role in pathogen recognition via cross-talk with TLRs. In contrast to PRRs, FcγRIIa does not recognize pathogen-associated molecular patterns (PAMPs) expressed by microbes, but instead recognizes pathogens on opsonization with IgG. Most likely as a result of previous pathogen exposure, IgG cross-reactivity and expression of common microbial structures recognized by IgGs, invasion of the body by pathogens such as bacteria rapidly leads to opsonization.13-17 Recognition of IgG-opsonized microbes by FcγRs has previously been described to mediate various effects on DCs, such as pathogen uptake, degradation of pathogen-derived structures, Ag presentation of pathogen-derived peptides, and DC maturation.18 However, the capacity of FcγRs to induce production of polarizing cytokines by human DCs is considered to be limited. Here we show a new function of FcγRs in production of Th17-promoting cytokines by DCs via cross-talk with PRRs. As such, this synergy adds another layer of complexity to recognition of pathogens.
Stimulation of FcγRIIa affected TLR-induced cytokine production in a selective manner. Some cytokines were strongly amplified (IL-1β, IL-23, and TNFα), while others were not, or were only moderately, affected (IL-8, IL-10, and IL-12). Notably, the effect on IL-12 production could only be assessed on TLR4 stimulation, because TLR2-ligand PGN is known to be a poor inducer of IL-12.32 The observation that IL-12 is not enhanced by coligation of TLRs and FcγRIIa was confirmed when DCs were stimulated with opsonized bacteria, indicating that this effect also holds true for whole pathogens. Modulation of IL-6 production appears to be dependent on the type of TLR involved: IgG costimulation does not affect TLR4-induced IL-6 production, but does up-regulate TLR2- and TLR5-induced IL-6 production. IgG opsonization of S aureus enhanced IL-6 production by DCs, which may suggest that the modulation of cytokine production for bacteria mainly results from cross-talk with TLR2. Combined, these data strongly suggest that the observed Th17 induction by opsonization of bacteria is mediated via specific up-regulation of particular polarizing cytokines by DCs: besides up-regulating Th17-promoting IL-1β, IL-6, and IL-23, TLR-FcγRIIa cross-talk does not enhance IL-12 production, which is pivotal for Th1 development and in turn can inhibit Th17 responses.33,34 In addition, FcγRIIa costimulation enhances TNFα production. Although TNFα produced by plasmacytoid DCs has recently been suggested to play a role in Th22 development, the role of TNFα in T-cell polarization is still incompletely characterized.35 Whether or how IgG opsonization modulates the polarization of immune responses to other classes of pathogens is not yet clear. For example, viral clearance requires Th1 and cytotoxic lymphocyte responses instead of Th17 and therefore FcγRIIa-mediated Th17 promotion would be counterproductive. However, Th17 induction against viruses by this mechanism may be less likely to occur. For example, IgG opsonization of pathogens requires specific antimicrobial IgGs to be present already at the time of infection. While IgGs directed against numerous bacteria are pre-existent at the time of infection, Abs against viruses are generally highly specific for viral proteins of a particular (sub)type of virus and are therefore not (yet) available during primary infection, suggesting that viral opsonization and subsequent TLR-FcγRIIa cross-talk is less likely to occur.14,15,36-38 In addition, viruses are recognized through different PRRs than bacteria, and cytokine induction by these viral-sensing PRRs may be differently modulated on costimulation with FcγRIIa. Further research on FcγRIIa cross-talk with different families of PRRs will shed light on how this mechanism is involved in conferring pathogen-class specific immunity to other microorganisms. Taken together, these data indicate that TLR-FcγRIIa cross-talk on DCs during stimulation with bacteria enhances the production of particular polarizing cytokines that promote Th17 responses.
The main FcγR responsible for the observed effects is FcγRIIa, which is expressed on most hematopoietic cells besides lymphocytes.39 Notably, FcγRIIa is restrictively expressed in primates, suggesting that the observed effects may be specific for these species.39 Alternatively, in other species, FcγR-TLR cross-talk could be mediated by other receptors. For example, in mice, FcγRIIIa is closely related to human FcγRIIa, even though there are differences in intracellular domains and cellular expression between the human and murine orthologue, and FcγRIII in mice has been associated previously with induction of Th2 responses.40,41 On most human cell types including DCs, FcγRIIa is coexpressed with the closely related, but inhibitory, isoform FcγRIIb.22 While complexed IgG stimulates both FcγRIIa and FcγRIIb, the specific up-regulation of cytokines by IgG stimulation was fully dependent on FcγRIIa. Importantly, FcγRIIa is a low-affinity receptor that selectively interacts with IgG in the form of immune complexes, such as expressed on opsonized pathogens, and not with monomeric Abs.39 This feature may be essential for shaping Th-cell responses in an appropriate manner. Monomeric IgGs are present in high concentrations in blood and tissues, but do not induce or amplify cytokine production via triggering of FcγRIIa or other (high- or low-affinity) FcγRs. In contrast, only on pathogen entry and opsonization will the complexed IgG on the microbe stimulate FcγRIIa leading to enhanced cytokine production. Nevertheless, recognition of immune complexes alone, that is, in absence of TLR stimulation, is also not sufficient for induction of immune responses by DCs. Although FcγRIIa stimulation induces DC maturation, we and others have shown that FcγRIIa stimulation alone induces little production of proinflammatory cytokines and hence are unlikely to mediate T-cell polarization.21-23 Therefore, FcγRIIa activation can only act in concert with a second “danger” signal such as TLR agonists to potentiate immune responses.
Our data show that the mechanism of cross-talk between FcγRIIa and TLRs for the induction of cytokines is orchestrated on at least 2 different levels. First, FcγRIIa-TLR cross-talk acts at the transcriptional level by selectively amplifying TLR-induced transcription of IL-1β, IL-23p19, and TNFα, while IL-6 transcription is up-regulated depending on the TLR involved (supplemental Figure 5). In addition, IL-12p40, the subunit required for both functional IL-12 and IL-23, was down-regulated after TLR-FcγRIIa costimulation. However, because IL-12p40 is usually produced in abundance, IL-23 production most likely mainly depends on enhanced transcription of IL-23p19. As a second mechanism, FcγRIIa up-regulates cytokine production via activation of caspase-1. Production of bioactive IL-1β depends both on gene transcription as well as cleavage of transcribed pro-IL-1β into its functional form by caspase-1.30,31 While TLRs are potent inducers of IL-1β transcription, caspase-1 activation by TLR stimulation is limited, especially by LPS.42,43 Importantly, here we show that FcγRIIa stimulation, independent of TLR stimulation, activates caspase-1, thereby identifying FcγRs as a new class of receptors that can activate caspase-1. Consequently, TLRs and FcγRIIa collaborate for the induction of IL-1β: TLR stimulation induces IL-1β transcription, while FcγRIIa stimulation enhances IL-1β transcription and activates (LPS) or enhances (PGN) activation of caspase-1, leading to functional IL-1β. Taken together, these 2 mechanisms enhance the production of particular cytokines by TLRs, while not inducing cytokine production on its own.
Previously, FcγRIIa has been shown to internalize and deliver DNA-containing immune complexes to lysosomes that contain TLR9 in plasmacytoid DCs, and in this manner facilitate cytokine production.44 However, blocking internalization of bacteria in DCs did not prevent the enhanced cytokine production induced by IgG opsonization. This indicates that, in contrast to several other FcγR-dependent effects or effects on other cell-types, TLR-FcγRIIa synergy in DCs for induction of Th17 cytokines is not dependent on internalization. Most likely, FcγRIIa-mediated effects are induced by direct induction of a downstream signaling cascade that modulates TLR responses. It is tempting to speculate on a vital role for spleen tyrosine kinase (Syk), which has previously been described to be involved in several FcγRIIa-mediated effects and is also known to modulate gene transcription and inflammasome activation.39,45,46 In addition, induction of different signaling pathways may explain the distinct effects of different IgG subclasses. Although stimulation of FcγRIIa with IgG1 or IgG2 strongly amplified TLR-induced cytokine production, stimulation with IgG3 had little effect. Paradoxically, FcγRIIa has a higher affinity for IgG3 than for IgG1 or IgG2.47 However, recently it has been shown that for both FcαRI and FcγRIII binding of immunoglobulins with different affinity induces the recruitment of different signaling molecules.48,49 Hence, the lower affinity of IgG1 and IgG2 compared with IgG3 for FcγRIIa may induce a different signaling cascade, resulting in distinct effects on TLR-induced cytokine production. Importantly, only minute concentrations of TLR agonists were required for synergy with FcγRIIa. This underlines that FcγRIIa stimulation strongly increases the sensitivity of DCs for TLR ligands, resulting in the promotion of Th17 responses even at very low bacterial presence. Furthermore, this increased sensitivity may be highly relevant for therapeutic purposes such as vaccine development. Remarkably, cross-talk between FcγRIIa and TLRs seems to be specific for DCs. Opsonization of bacteria did not lead to any increase in cytokine production by monocytes, the cell type from which the in vitro–generated DCs are derived. This difference between cell types does not seem to be dependent on receptor expression because both TLRs and FcγRIIa are highly expressed on monocytes.22 Hence, these findings suggest that cell-intrinsic signaling properties are required for TLR-FcγRIIa cross-talk. What these properties are and whether they are present in other cell types besides DCs is currently under investigation.
Taken together, our data indicate that IgG opsonization of bacteria, besides enhancing uptake and Ag presentation, is a natural mechanism to tailor immune responses to effectively combat extracellular pathogens. From a therapeutic point of view, induction of this TLR-FcγRIIa synergy mechanism on DCs may be a useful tool to skew immune responses to clear pathogenic infections more efficiently.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Rispens and N. Derksen for generation of Fab fragments, K. Szegedi for experimental help, and M. A. W. P. de Jong for critically reading the manuscript.
This work was supported by grants from the Netherlands Organization for Scientific Research (NWO; Rubicon, project no. 825.09.028; and VENI, project no. 91611012) and the Academic Medical Center (AMC Postdoc grant 2010).
Authorship
Contribution: J.d.D., L.T.C.V., T.W., F.J.M.M., and L.d.B. performed experiments; T.W.K. contributed vital reagents; J.d.D., S.A.J.Z., M.L.K., and E.C.d.J. designed experiments; and J.d.D., M.L.K., and E.C.d.J. devised the concept, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeroen den Dunnen, PhD, Department of Cell Biology and Histology, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: j.dendunnen@amc.nl.
References
Author notes
M.L.K. and E.C.d.J. contributed equally to this study, sharing last authorship.