Abstract
Proplatelet production represents a terminal stage of megakaryocyte development during which long, branching processes composed of platelet-sized swellings are extended and released into the surrounding culture. Whereas the cytoskeletal mechanics driving these transformations have been the focus of many studies, significant limitations in our ability to quantify the rate and extent of proplatelet production have restricted the field to qualitative analyses of a limited number of cells over short intervals. A novel high-content, quantitative, live-cell imaging assay using the IncuCyte system (Essen BioScience) was therefore developed to measure the rate and extent of megakaryocyte maturation and proplatelet production under live culture conditions for extended periods of time. As proof of concept, we used this system in the present study to establish a mechanism by which trastuzumab emtansine (T-DM1), an Ab-drug conjugate currently in clinical development for cancer, affects platelet production. High-content analysis of primary cell cultures revealed that T-DM1 is taken up by mouse megakaryocytes, inhibits megakaryocyte differentiation, and disrupts proplatelet formation by inducing abnormal tubulin organization and suppressing microtubule dynamic instability. Defining the pathways by which therapeutics such as T-DM1 affect megakaryocyte differentiation and proplatelet production may yield strategies to manage drug-induced thrombocytopenias.
Introduction
Platelets are formed and released into the bloodstream by specialized precursor cells called megakaryocytes. During the final stages of development, megakaryocytes remodel their cytoplasm into long, microtubule-based proplatelet extensions from which platelets are produced. Each day, an adult human produces approximately 100 billion platelets1 to maintain a circulating concentration of 1.5-4 × 108 platelets/mL. Platelets are essential for blood clotting, and the rate of platelet production can increase by a factor of 10 or more when the demand for platelets suddenly increases. Conversely, when platelet numbers are very low, a patient is at serious risk of death from hemorrhage. Recent efforts to generate useful numbers of clinically viable platelets for infusion by reproducing elements of the BM vascular niche have had limited success.2,3 Significant improvements in platelet quality and yield will require the ability to assay multiple individual components of the BM microenvironment empirically in high throughput for quantitative improvements in megakaryocyte maturation and proplatelet production before they are incorporated in scaled biomimetic systems.
Drug-induced thrombocytopenia can be triggered by a wide range of medications through the inhibition of megakaryocyte development and platelet biogenesis and/or the clearance of platelets in the blood.4,5 Approximately 10 persons per million in the United States and Europe are affected by drug-induced thrombocytopenia per year, which can range from mild to life-threatening.6 The slow recovery of platelet levels in such patients is a serious medical problem and has resulted in a steady increase in the demand for platelet transfusions. Whereas platelet survival and clearance rates during drug development are typically measured through infusion studies using flow cytometry,7 quantification of the rate and extent of proplatelet production is not amenable to this approach because it requires direct visualization to establish at what stage thrombocytopoiesis is affected. Mouse primary fetal liver megakaryocyte cultures currently represent the most desirable culture method to study proplatelet production ex vivo and can be used to test thrombocytopoiesis-related drug effects apart from platelet clearance.8
Quantification of megakaryocyte differentiation and proplatelet production is complicated by significant limitations in our ability to culture these cells. These limitations include the asynchronous differentiation of hematopoietic stem cells in culture, which is necessary to visualize cultures over extended periods of time (4 days for mice and 14 days for humans), the relatively low number of megakaryocytes generated (< 10% of fetal liver cell culture population), asynchronous proplatelet production, and variable resultant cellular morphologies.9 Practical limitations of imaging megakaryocyte differentiation and proplatelet production have made thresholding individual cells and high-throughput quantification of key stages across multiple experimental parameters in culture even more challenging. Furthermore, light refraction through the meniscus results in uneven background illumination and affects image contrast, high-energy incident light can kill cells during repeated exposure, and the necessity to maintain cells under standard culture conditions makes it impractical to visualize multiple cell-culture replicates simultaneously over extended periods of time. As a result, most studies to date have relied on static snapshots of megakaryocyte differentiation from a limited number of time points. Live-imaging approaches have traditionally yielded mostly qualitative data from a small numbers of cells and are therefore subject to selection bias.
In the present study, we describe a novel high-content, quantitative, live-cell imaging assay to measure the rate and extent of megakaryocyte differentiation and proplatelet production in vitro. To demonstrate proof of concept, we then apply this assay to a clinically relevant case of drug-induced thrombocytopenia caused by the human epidermal growth factor receptor 2–positive (HER2+) breast cancer drug T-DM1 and elucidate the mechanism by which platelet production is inhibited.
Methods
Reagents
T-DM1, 5B6-DM1, and trastuzumab were provided by Genentech.
Drug treatment
Cultured mouse megakaryocytes and mouse/human washed platelets were incubated separately with 100 μg/mL of T-DM1, 5B6-DM1 (trastuzumab specificity control), and trastuzumab (Ab control) for up to 72 hours.
Mouse/human whole blood platelets
Murine blood was obtained by retroorbital bleed into 0.1 volume of Aster-Jandl anticoagulant from anesthetized mice as described previously.7 Human blood was obtained by venipuncture from healthy laboratory volunteers as described previously.10 Collections were performed in accordance with ethics regulation with Brigham and Women's Hospital institutional review board approval and informed consent was obtained according to the Declaration of Helsinki.
Megakaryocyte suspension cultures
Mouse fetal liver cells were collected from CD1 mice (Charles River Laboratories) on embryonic day 13.5 and cultured at 37°C and 5% CO2 in the presence of 0.5% tissue culture supernatant from a fibroblast cell line engineered to secrete recombinant human thrombopoietin for 5 days. Round megakaryocytes were isolated by BSA gradient sedimentation on day 4 and cultured for an additional 24 hours to visualize proplatelet production, as described previously.9 All studies complied with institutional guidelines approved by the Boston Children's Hospital Animal Care and Use Committee and the Boston Children's Hospital Institutional Animal Care and Use Committee.
IncuCyte system
Mouse fetal liver cells (day 0) or isolated megakaryocytes (day 4) were transferred to a 24-well plate and imaged using the IncuCyte HD system ((Essen BioScience) Frames were captured at 1-hour intervals from 4 separate 950 × 760–μm2 regions per well using a 20× objective. Cultures were maintained at 37°C in an XL-3 incubation chamber (Carl Zeiss) throughout and run in quadruplicate. Rates and extent of megakaryocyte differentiation and proplatelet production were measured in ImageJ Version 1.45r software using investigator-coded software (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Values from all 4 regions of each well were pooled and averaged across all 4 replicates.
Expression of EGFP–β1-tubulin construct
β1-tubulin was subcloned into XhoI and EcoRI sites of the modified enhanced green fluorescent protein–murine stem cell virus (EGFP-MSCV) retroviral vector (a gift from R. A. Shivdasani, Dana-Farber Cancer Institute, Boston, MA). Retroviruses were produced by cotransfecting viral and pCL helper plasmids into 50% confluent HEK293T cells using Fugene-6 (Roche). Medium was changed 24 hours later, and viral supernatants were harvested at 72 hours. Fetal liver cells were infected at day 2.5 of culture with retroviral supernatants in the presence of 8 μg/mL of polybrene by centrifugation at 800g at 25°C for 1.5 hours and incubation at 37°C for 1.5 hours. Cells were cultured in fresh medium with thrombopoietin, and EGFP–β1-tubulin was visualized in isolated megakaryocytes on the IncuCyte system 48 hours after infection (day 4).
Immunofluorescence microscopy
Cultured mouse megakaryocytes and mouse/human platelets were probed as described previously.11 Briefly, samples were centrifuged onto poly-L-lysine (1 μg/mL)–coated cover slides in either 4% formaldehyde (for megakaryocytes and resting platelets) or in platelet resuspension buffer (10mM HEPES, 140mM NaCl, 3mM KCl, 0.5mM MgCl2, 5mM NaHCO3, and 10mM glucose, pH 7.4) and allowed to spread on a glass slide for 15 minutes at room temperature before fixation (for spread platelets). For immunofluorescence microscopy, samples were permeabilized with 0.5% Triton X-100 and blocked in immunofluorescence blocking buffer (1% BSA, 0.05% sodium azide, and 10% FCS in 1× PBS) overnight before Ab labeling.12 To demarcate permeabilized cells, samples were incubated with a rabbit polyclonal primary Ab for mouse or human β1-tubulin generated against the C-terminal peptide sequence LEDSEEDAEEAEVEAEDKDH and CKAVLEEDEEVTEEAEMEPEDKGH, respectively (Genemed Synthesis). Samples were treated with a secondary goat anti–rabbit Ab conjugated to an Alexa Fluor 568 (Invitrogen). T-DM1 was probed with a mouse mAb against the complementary determining region of trastuzumab, clone 6C6, conjugated to an Alexa Fluor 488 (Genentech). As background controls, slides were incubated with the appropriate secondary Ab alone, and all images were adjusted to account for nonspecific binding of Abs. Samples were examined with an Axiovert 200 microscope (Carl Zeiss) equipped with a 63× (numeric aperture, 1.4) Plan-ApoChromat oil-mmersion objective, and images were obtained using a charged coupled device camera (Hamamatsu). Images were analyzed using Metamorph Version 7.7.2.0 image analysis software (Molecular Devices) and ImageJ Version 1.45r software.
Flow cytometry
Mouse/human platelets were collected from whole blood, washed, and examined under resting conditions or after activation for 5 minutes at 37°C with 1 U/mL of thrombin (Roche Diagnostics). Samples were probed with PE-conjugated mouse anti–P-selectin Abs (BD Biosciences) and run on a FACSCalibur flow cytometer (BD Biosciences). Platelets were gated by their characteristic forward- and side-scattering as they passed through the detector, and the percentage of fluorescently conjugated platelet-sized events was calculated after subtraction of a PE-conjugated IgG Ab specificity control (BD Biosciences). Analysis of platelet activation marker surface expression was performed for at least 3 different samples. Statistical significance was established using a 1-tailed Student t test for paired samples.
Preparation of photomicrographs
Digital images were analyzed using ImageJ Version 1.45r software. Dividing lines separate different images or separate regions of the same image. No specific features within an image were enhanced, obscured, moved, removed, or introduced, and adjustments made to the brightness, contrast, and color balance were applied linearly to the whole image.
Results
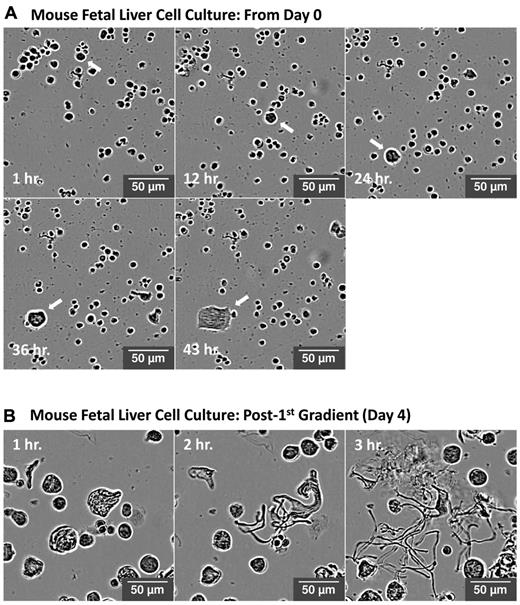
To overcome the major practical limitations of high-throughput imaging of live cells and to enable quantification of the rate and extent of megakaryocyte differentiation and proplatelet production from primary mouse cultures, we used the IncuCyte HD system with a 20× objective. By placing the microscope inside the incubator, the IncuCyte system allowed us to collect live-cell images of the megakaryocyte cultures at 1-hour intervals over 72 hours. The high-definition imaging mode provided by IncuCyte uses a combination of changes to the optical path of light and software analysis to eliminate the visual effect caused by refraction of light through the meniscus. This results in a reduced image halo, improved contrast, and more even background illumination relative to standard phase-contrast imaging. Because the incident light is of low energy and illumination time is minimal because of software-driven focus, this instrument is well suited for live-cell imaging over long periods of time. Figure 1A represents a time course of a mouse fetal liver hematopoietic stem cell culture grown in the presence of thrombopoietin for 4 days. The arrow highlights a single progenitor cell that undergoes cytoplasmic differentiation over time to become a megakaryocyte. Figure 1B represents a time course from a round megakaryocyte culture after gradient sedimentation on day 4. In this figure, a single mature megakaryocyte is observed undergoing proplatelet production over the course of 3 hours. Quantitative analysis of mouse fetal liver cell and round megakaryocyte cultures in Figure 2 represents a major technological advance in our ability to study previously inaccessible kinetics of megakaryocyte cell cultures, and was performed in ImageJ Version 1.45r software using investigator-coded software (please see the supplemental Methods). Analysis enabled the real-time detection of both the rate and extent of megakaryocyte differentiation and proplatelet production for at least 100 cells over multiple cell-culture replicates. Quantification was confirmed by manual counts of megakaryocyte number and percent of proplatelet production and was consistent with previously published data.13
Visualization of megakaryocyte differentiation and proplatelet production in vitro. Representative high-definition differential interference contrast images of a fetal liver cell culture over time using the IncuCyte system. (A) Arrow highlights a single progenitor cell undergoing cytoplasmic differentiation over 43 hours to become a megakaryocyte. (B) A single mature megakaryocyte undergoing proplatelet production over 3 hours.
Visualization of megakaryocyte differentiation and proplatelet production in vitro. Representative high-definition differential interference contrast images of a fetal liver cell culture over time using the IncuCyte system. (A) Arrow highlights a single progenitor cell undergoing cytoplasmic differentiation over 43 hours to become a megakaryocyte. (B) A single mature megakaryocyte undergoing proplatelet production over 3 hours.
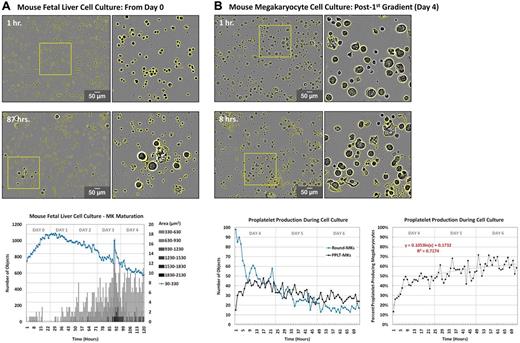
Quantification of the rate/extent of megakaryocyte differentiation and proplatelet production in vitro. Quantitative analysis of fetal liver cell and round megakaryocyte cultures was performed in ImageJ Version 1.45r software. Images were corrected for background illumination and thresholded based contrast. (A) Cells were categorized as hematopoietic stem cells (area < 330 μm2, blue line) or round megakaryocytes of increasing size (circularity ≥ 0.4, area ≥ 330 μm2, gray bars). Objects were normalized to initial (day 0) object counts and data from each time point was averaged across 4 separate regions. (B) Cells ≥ 330 μm2 in area were categorized as round megakaryocytes (circularity ≥ 0.4, blue line) or proplatelet-megakaryocytes (circularity < .0.4, black line). Objects were normalized to initial (day 4) object counts and are expressed as total number of objects (top right) and percentage of proplatelet-producing megakaryocytes (bottom right). Data from each time point were averaged across 4 separate regions.
Quantification of the rate/extent of megakaryocyte differentiation and proplatelet production in vitro. Quantitative analysis of fetal liver cell and round megakaryocyte cultures was performed in ImageJ Version 1.45r software. Images were corrected for background illumination and thresholded based contrast. (A) Cells were categorized as hematopoietic stem cells (area < 330 μm2, blue line) or round megakaryocytes of increasing size (circularity ≥ 0.4, area ≥ 330 μm2, gray bars). Objects were normalized to initial (day 0) object counts and data from each time point was averaged across 4 separate regions. (B) Cells ≥ 330 μm2 in area were categorized as round megakaryocytes (circularity ≥ 0.4, blue line) or proplatelet-megakaryocytes (circularity < .0.4, black line). Objects were normalized to initial (day 4) object counts and are expressed as total number of objects (top right) and percentage of proplatelet-producing megakaryocytes (bottom right). Data from each time point were averaged across 4 separate regions.
T-DM1 inhibits megakaryocyte differentiation and proplatelet production
As proof of concept, we examined the mechanism of trastuzumab entasinsine–induced thrombocytopenia. T-DM1 is an Ab-drug conjugate currently in clinical development for HER-2+ cancer that incorporates the intracellular delivery of the potent antimicrotubule agent DM1 with the antitumor properties of the humanized Ab trastuzumab. HER2 is overexpressed in 20% of breast cancers.14 Human studies of T-DM1 in patients with advanced HER2+ breast cancer have demonstrated transient thrombocytopenia (< 150 000 platelets/μL) as a major side effect in approximately 54% of cases beginning as soon as 1 day after T-DM1 administration and reaching a nadir by approximately day 815 ; of these, 12.5% were of grades 3 and 4 (< 50 000 platelets/μL). Whereas these patients received 4.8 mg/kg of T-DM1, declines in platelet levels occurred in almost all patients receiving T-DM1 at doses > 1.2 mg/kg.15 Patients in these studies were treated with T-DM1 on a 3-week schedule, and platelet numbers were recovered by day 15, suggesting that T-DM1 may affect platelet production.
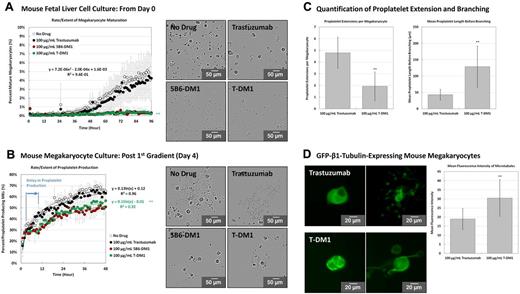
To determine whether T-DM1 affectsg megakaryocyte differentiation and proplatelet production, mouse fetal liver cell cultures and mature megakaryocytes were incubated with 100 μg/mL of T-DM1, 5B6-DM1 (nonspecific humanized Ab-drug conjugate control), trastuzumab (Ab alone), or a vehicle control for up to 4 days and analyzed on the IncuCyte system using investigator-coded software (please see the supplemental Methods). This represents a physiologically relevant (circulating) concentration of the Ab-drug complex in humans undergoing T-DM1 treatment, and was not toxic to cells in culture. T-DM1 and 5B6-DM1 incubations resulted in complete inhibition of megakaryocyte differentiation (Figure 3A and supplemental Video 1). Likewise, mature megakaryocytes treated with either T-DM1 or 5B6-DM1 experienced a 6-hour delay in proplatelet extension. Delay in proplatelet production has not been reported previously, and would not have been possible to detect by conventional analysis. Proplatelet production was also reduced significantly in T-DM1– and 5B6-DM1–treated samples and consisted of fewer unbranched proplatelets per megakaryocyte relative to vehicle control (Figure 3B-C and supplemental Video 2). Trastuzumab alone had no effect on megakaryocyte differentiation or proplatelet production, suggesting that the microtubule-disrupting agent DM1 causes inhibition. Because proplatelet extension is powered by microtubules, we examined microtubule organization during proplatelet production. Cultured mouse megakaryocytes were retrovirally transfected to express EGFP–β1-tubulin and treated with either trastuzumab or T-DM1 (Figure 3D and supplemental Video 3). Megakaryocytes incubated with T-DM1 showed thicker microtubules relative to trastuzumab control, which was confirmed by quantification of mean fluorescence intensity across the proplatelet shaft. Abnormal tubulin organization and suppressed microtubule dynamic instability in T-DM1–treated megakaryocytes was similar to that observed in MCF7 breast cancer cells treated with both conjugate and free maytanisinoids,16 which suggested that DM1 disrupted proplatelet formation.
T-DM1 inhibits megakaryocyte differentiation and proplatelet production by disrupting microtubule organization. The rate/extent of megakaryocyte differentiation and proplatelet production was examined in at least 3 different primary cultures after incubation with 100 μg/mL of T-DM1, 5B6-DM1 (trastuzumab specificity control), and trastuzumab Ab control. Quantitative analysis of fetal liver cell and round megakaryocyte cultures was performed on the IncuCyte system using investigator-coded software. (A) Quantification of the rate and extent of megakaryocyte differentiation from mouse fetal liver cell culture over 96 hours. Inserts demonstrate representative images taken on day 4 of culture. (B) Quantification of the rate and extent of proplatelet production by mature megakaryocytes over 48 hours. Inserts demonstrate representative images taken after 10 hours of culture. (C) Megakaryocytes incubated with T-DM1 showed fewer individual proplatelet extensions per megakaryocyte and reduced proplatelet branching relative to trastuzumab control. Quantification of proplatelet extensions per megakaryocyte was performed at 6 hours for trastuzumab culture and at 10 hours for T-DM1 culture to account for the delay in proplatelet production. Quantification of proplatelet length before branching was performed at 24 hours for both cultures. (D) Mouse culture megakaryocytes were retrovirally transfected to express EGFP–β1-tubulin. Inserts demonstrate representative images taken before and after proplatelet production. Megakaryocytes incubated with T-DM1 showed thicker microtubules relative to trastuzumab control as demonstrated by quantification of mean fluorescence intensity across the shaft (t = 24 hours). Statistical significance was established using a 1-tailed Student t test for paired samples. *P < .05; **P < .01. Error bars represent 1 SD about the mean for at least 28 independent samples.
T-DM1 inhibits megakaryocyte differentiation and proplatelet production by disrupting microtubule organization. The rate/extent of megakaryocyte differentiation and proplatelet production was examined in at least 3 different primary cultures after incubation with 100 μg/mL of T-DM1, 5B6-DM1 (trastuzumab specificity control), and trastuzumab Ab control. Quantitative analysis of fetal liver cell and round megakaryocyte cultures was performed on the IncuCyte system using investigator-coded software. (A) Quantification of the rate and extent of megakaryocyte differentiation from mouse fetal liver cell culture over 96 hours. Inserts demonstrate representative images taken on day 4 of culture. (B) Quantification of the rate and extent of proplatelet production by mature megakaryocytes over 48 hours. Inserts demonstrate representative images taken after 10 hours of culture. (C) Megakaryocytes incubated with T-DM1 showed fewer individual proplatelet extensions per megakaryocyte and reduced proplatelet branching relative to trastuzumab control. Quantification of proplatelet extensions per megakaryocyte was performed at 6 hours for trastuzumab culture and at 10 hours for T-DM1 culture to account for the delay in proplatelet production. Quantification of proplatelet length before branching was performed at 24 hours for both cultures. (D) Mouse culture megakaryocytes were retrovirally transfected to express EGFP–β1-tubulin. Inserts demonstrate representative images taken before and after proplatelet production. Megakaryocytes incubated with T-DM1 showed thicker microtubules relative to trastuzumab control as demonstrated by quantification of mean fluorescence intensity across the shaft (t = 24 hours). Statistical significance was established using a 1-tailed Student t test for paired samples. *P < .05; **P < .01. Error bars represent 1 SD about the mean for at least 28 independent samples.
T-DM1 is taken up by mouse megakaryocytes and mouse/human platelets
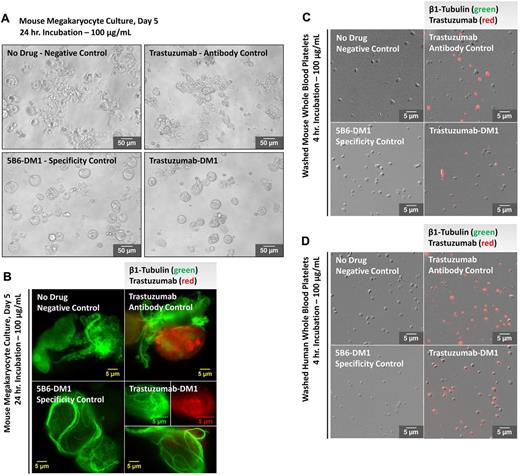
Megakaryocytes/platelets do not express the HER2 receptor and mice do not contain the FcγRIIA receptor for human IgG.17,18 Although trastuzumab is a humanized mAb directed against the extracellular domain of HER2, our observation that 5B6-DM1 has similar effects to T-DM1 in mouse hematopoietic stem cells and megakaryocyte cultures suggests that drug uptake is HER2 and FcγRIIA receptor independent. To demonstrate that trastuzumab is taken up by mouse megakaryocytes in culture, samples were incubated with 100 μg/mL of T-DM1, 5B6-DM1, trastuzumab, or a vehicle control for up to 24 hours (Figure 4A). Megakaryocytes were permeabilized and probed for β1-tubulin (green) and trastuzumab (red) and analyzed at 63× magnification by immunofluorescence microscopy (Figure 4B). Trastuzumab entered the megakaryocytes and was detected in both T-DM1– and trastuzumab-treated samples. Conversely, T-DM1 and 5B6-DM1 induced microtubule thickening and reduced proplatelet elongation in megakaryocytes, which confirms that DM1 affects microtubule organization in these cells.
T-DM1 permeates cultured mouse megakaryocytes and mouse/human platelets. Trastuzumab incorporation was examined in megakaryocytes from at least 3 different cultures and washed platelets from at least 3 different mouse/human donors after incubation with 100 μg/mL of T-DM1, 5B6-DM1 (trastuzumab specificity control), and trastuzumab Ab control. (A) Representative differential interference contrast image. T-DM1 and 5B6-DM1 treatment resulted in inhibition of proplatelet production 24 hours after treatment. Immunofluorescence microscopy reveals that both T-DM1 and trastuzumab (red) permeate cultured mouse megakaryocytes (B) and mouse/human platelets (C-D) and localize cytoplasmically. T-DM1 disrupts microtubule organization (green).
T-DM1 permeates cultured mouse megakaryocytes and mouse/human platelets. Trastuzumab incorporation was examined in megakaryocytes from at least 3 different cultures and washed platelets from at least 3 different mouse/human donors after incubation with 100 μg/mL of T-DM1, 5B6-DM1 (trastuzumab specificity control), and trastuzumab Ab control. (A) Representative differential interference contrast image. T-DM1 and 5B6-DM1 treatment resulted in inhibition of proplatelet production 24 hours after treatment. Immunofluorescence microscopy reveals that both T-DM1 and trastuzumab (red) permeate cultured mouse megakaryocytes (B) and mouse/human platelets (C-D) and localize cytoplasmically. T-DM1 disrupts microtubule organization (green).
Because T-DM1 may also be affecting circulating platelets, mouse and human platelets were isolated from whole blood and incubated with 100 μg/mL of T-DM1, 5B6-DM1, trastuzumab, or a vehicle control for up to 4 hours. Platelets were fixed, permeabilized, and probed for trastuzumab (red) and analyzed at 20× magnification by immunofluorescence microscopy. Composite differential interference contrast/immunofluorescence microscopy images are shown in Figure 4C and D and confirm that the Ab-drug conjugate permeates megakaryocytes and platelets through an HER2- and FcγRIIA receptor–independent pathway.
DM1 disrupts the microtubule cytoskeleton of both megakaryocytes and platelets
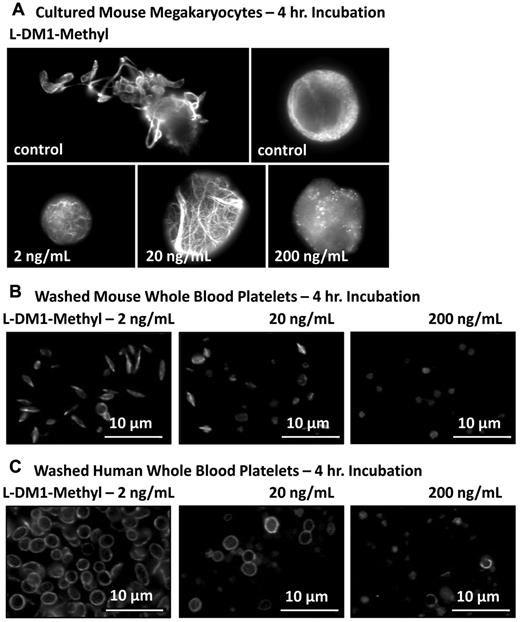
On endocytosis, T-DM1 is catabolized, resulting in the release of the active catabolite, Lys-MCC-DM1.19,20 To establish the potential effect of DM1 on cultured mouse megakaryocytes and mouse or human washed platelets independent of trastuzumab, cells were incubated with increasing concentrations of L-DM1-methyl, a more potent analog of Lys-MCC-DM1. These data revealed microtubule thickening/elongation and reduced proplatelet extension (Figure 5), which are characteristic of T-DM1 treatment and confirm that DM1 can disrupt microtubule organization at the earliest stage of platelet production.
L-DM1-methyl disrupts microtubule organization in mouse culture megakaryocyte and mouse/human platelets. The effect of 2, 20, and 200 ng/mL of L-DM1-methyl on cultured mouse megakaryocytes and mouse/human washed platelets was examined. Samples were probed for β1-tubulin to delineate their microtubule cytoskeleton. Cultured mouse megakaryocytes (A) and mouse/human platelets (B-C) demonstrated reduced proplatelet elongation and microtubule disruption characteristic of T-DM1 treatment.
L-DM1-methyl disrupts microtubule organization in mouse culture megakaryocyte and mouse/human platelets. The effect of 2, 20, and 200 ng/mL of L-DM1-methyl on cultured mouse megakaryocytes and mouse/human washed platelets was examined. Samples were probed for β1-tubulin to delineate their microtubule cytoskeleton. Cultured mouse megakaryocytes (A) and mouse/human platelets (B-C) demonstrated reduced proplatelet elongation and microtubule disruption characteristic of T-DM1 treatment.
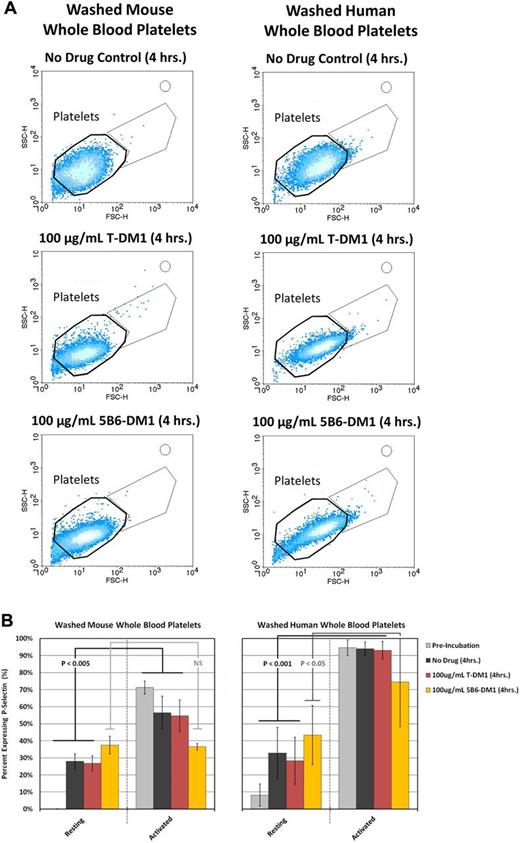
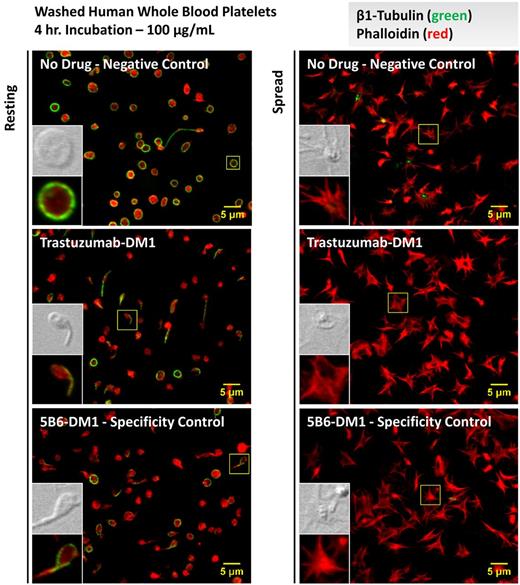
To determine whether physiologic plasma concentrations of T-DM1 activate platelets, mouse and human washed platelets were treated with 100 μg/mL of T-DM1, 5B6-DM1, or a vehicle control for 4 hours and examined by flow cytometry. Whereas platelets treated with the vehicle control remained discoid, both T-DM1 and 5B6-DM1–treated platelets showed aberrant morphologies, as demonstrated by their forward- and side-scatter parameters by flow cytometry (Figure 6A). P-selectin surface expression is a classic marker of platelet activation that marks them for clearance by professional phagocytes in the blood. Platelets treated with either T-DM1 or 5B6-DM1 ex vivo demonstrated normal surface expression of P-selectin under resting conditions and on activation with thrombin (Figure 6B). Because the parent molecule of DM1, maytansine, has been shown to bind microtubules in a manner similar to that of vinca alkaloids,21 we examined platelets from healthy donors for microtubule defects under resting conditions and on contact activation with glass by immunofluorescence microscopy. Interestingly, human platelets formed tennis racket shapes, and revealed less uniformity in platelet diameter when incubated with either T-DM1 or 5B6-DM1 relative to the vehicle control (Figure 7). Although platelet clearance may also contribute to thrombocytopenia, mouse and human platelets did not express classic biomarkers of activation after incubation with T-DM1 (Figure 6B). Moreover, platelets showed normal spreading and microtubule depolymerization on contact with glass, suggesting that T-DM1 does not affect activation. These data suggest that T-DM1 decreases platelet counts by targeting proplatelet production and elucidate a mechanism by which thrombocytopoiesis is inhibited.
T-DM1 does not increase surface expression of P-selectin in platelets. P-selectin surface expression was examined in resting and thrombin (1 mU/μL)–activated washed platelets from at least 3 different mouse/human donors before and after incubation with a vehicle control or 100 μg/mL of T-DM1 or 5B6-DM1 (trastuzumab specificity control) for 4 hours. (A) Mouse/human platelets exhibit a change in morphology during incubation with T-DM1 and 5B6-DM1 as is evidenced by their forward- and side-scatter distribution relative to the vehicle control. (B) Mouse/human platelets treated with the vehicle control or T-DM1 show normal surface expression of P-selectin under resting and thrombin-activated conditions. Platelets treated with 5B6-DM1 show increased surface expression of P-selectin under resting conditions that is not increased further on treatment with thrombin.
T-DM1 does not increase surface expression of P-selectin in platelets. P-selectin surface expression was examined in resting and thrombin (1 mU/μL)–activated washed platelets from at least 3 different mouse/human donors before and after incubation with a vehicle control or 100 μg/mL of T-DM1 or 5B6-DM1 (trastuzumab specificity control) for 4 hours. (A) Mouse/human platelets exhibit a change in morphology during incubation with T-DM1 and 5B6-DM1 as is evidenced by their forward- and side-scatter distribution relative to the vehicle control. (B) Mouse/human platelets treated with the vehicle control or T-DM1 show normal surface expression of P-selectin under resting and thrombin-activated conditions. Platelets treated with 5B6-DM1 show increased surface expression of P-selectin under resting conditions that is not increased further on treatment with thrombin.
T-DM1 disrupts the platelet microtubule cytoskeleton but does not affect spreading. Resting and spread (glass-activated) platelet cytoskeletal structure was examined in washed platelets from at least 3 different human donors before and after incubation with a vehicle control or 100 μg/mL of T-DM1 or 5B6-DM1 (trastuzumab specificity control) for 4 hours. Platelets were permeabilized and probed for β1-tubulin (green) and phalloidin (red). Resting platelets showed aberrant morphologies (ie, diffuse tubulin staining, tennis racket forms, less uniformity in platelet diameter, and smaller platelets) in the presence of T-DM1 and 5B6-DM1, but normal spreading on glass.
T-DM1 disrupts the platelet microtubule cytoskeleton but does not affect spreading. Resting and spread (glass-activated) platelet cytoskeletal structure was examined in washed platelets from at least 3 different human donors before and after incubation with a vehicle control or 100 μg/mL of T-DM1 or 5B6-DM1 (trastuzumab specificity control) for 4 hours. Platelets were permeabilized and probed for β1-tubulin (green) and phalloidin (red). Resting platelets showed aberrant morphologies (ie, diffuse tubulin staining, tennis racket forms, less uniformity in platelet diameter, and smaller platelets) in the presence of T-DM1 and 5B6-DM1, but normal spreading on glass.
Discussion
The results of the present study establishe a novel high-content, quantitative, live-cell imaging assay using the IncuCyte system to measure the rate and extent of proplatelet production under live-culture conditions for extended periods of time. As proof of concept, we applied this assay to a clinically relevant case of drug-induced thrombocytopenia caused by the HER2+ breast cancer drug T-DM1 to elucidate the mechanism by which platelet production is inhibited. Whereas drug-induced thrombocytopenia is common, most studies to date have focused on the platelet clearance effect of drugs and have ignored their role in platelet production. This has been due to significant limitations that have made thresholding individual cells and high-throughput quantification of key intermediate stages in platelet production impractical for live-cell cultures across multiple sample replicates. Our assay overcomes the major limitations of image-based analysis using low-energy incident light and software-driven focus provided by the IncuCyte HD system to enable hourly live-cell imaging over 4 days. A combination of changes to the optical path of light and background correction eliminate the visual effect of light refraction through the meniscus and enable accurate thresholding of megakaryocytes throughout differentiation and scoring of cells based on relevant morphologic parameters.
One major advantage of this imaging platform is its capacity to collect information from individual cells. For example, the kinetics of proplatelet production are often hidden in whole population measurements, for which the absence of relevant biomarkers makes the identification of intermediate stages over time not possible by classic flow cytometric approaches. Another significant advancement is the ability to multiplex analysis using a secondary fluorescent label for β1-tubulin. This enabled visualization of the microtubule-based cytoskeleton during megakaryocyte maturation and throughout proplatelet production—a technically challenging experiment not otherwise amenable to biologic replicate analysis. Lastly, by storing and harvesting normally discarded useful information for future elaboration, this assay permits virtual secondary screens for unexpected readouts to be performed after the initial screen. From the same dataset, we were able to measure the extent of microtubule branching and thickness using images collected in both bright-field and fluorescent channels after observing disrupted proplatelet production by morphologic analysis. These advances have allowed us to study the effect of drug-induced thrombocytopenia on platelet production in multiple sample replicates under high-throughput experimental conditions.
An innovative approach to the treatment of cancer is the use of targeted therapies to preferentially deliver cytotoxic chemotherapy to malignant cells.22 T-DM1 is an Ab-drug conjugate composed of the cytotoxic DM1 (a maytanisinoid antimicrotubule compound) linked to trastuzumab via lysine side chains.23 This agent combines the intracellular delivery of DM1 with the HER2-targeted antitumor properties of the humanized mAb trastuzumab.15,24 HER2 is a member of the epidermal growth factor receptor family, and approximately 20% of breast cancers are HER2+.14 It is thought that after the trastuzumab portion of the Ab-drug complex attaches to the malignant cell, T-DM1 undergoes receptor-mediated internalization. Intracellular release of DM1-containing catabolites requires complete hydrolysis of the polypeptide backbone of the Ab component in the lysosomes of cells.20,25 Whereas T-DM1 is showing promise at shrinking tumors in patients with HER2+ cancer and has the potential to help nearly 300 000 women worldwide, side effects such as lowered platelet counts are of significant concern.
Declines in platelet levels occurred in almost all patients receiving T-DM1 at doses > 1.2 mg/kg beginning as soon as 1 day after administration and reaching their nadir by approximately day 8, suggesting that T-DM1 treatment may affect platelet production. Indeed, T-DM1 was taken up by cultured mouse megakaryocytes and mouse/human platelets, and DM1 was shown to target microtubule organization in these cells. Platelet activation was not measurably affected, and patients receiving T-DM1 have not presented with clinically significant bleeding events, which supports these observations.15,26 Whereas megakaryocytes and platelets do not express EGFR, HER2, or HER3 surface receptors,24,27 both take up T-DM1 despite also lacking the FcγRIIA receptor, suggesting that T-DM1 is endocytosed through an alternative pathway.18,28,29 On entering mouse megakaryocytes, DM1 was shown to disrupt the microtubule cytoskeleton and completely inhibit proplatelet production, possibly contributing to thrombocytopenia in patients.
Defining the pathways by which therapeutics such as T-DM1 affect megakaryocyte differentiation and proplatelet production may yield strategies to manage drug-induced thrombocytopenias. Whereas the ability to quickly assess drug-induced inhibition of platelet production in biologic replicates before the commencement of clinical trials is of clear medical importance, the capacity to screen small-molecule libraries for regulators of thrombopoiesis represents a major paradigm shift in this field. Indeed, the combined area necessary to quantify 120 megakaryocytes per replicate was 7.22 × 105 μm2, or only 1.5% of the total surface area of a 24-well plate well (1.9 cm2). High-content live-cell imaging of megakaryocyte cultures can therefore be extended to a 384-well plate format (0.031 cm2) to perform high-throughput analysis of proplatelet production for drug screening. Moreover, fluorescence microscopy can be used to track expression of fluorescently labeled proteins in megakaryocytes or as a method of both detecting and following transfected cells. Future studies will use high-throughput, quantitative, live-cell imaging of megakaryocyte differentiation and proplatelet production to identify putative drugs that stimulate platelet release directly, establishing therapeutic autotransfusion for clinical thrombocytopenias.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Genentech. J.N.T. is an American Society of Hematology Scholar. J.E.I. is an American Society of Hematology Junior Faculty Scholar.
National Institutes of Health
Authorship
Contribution: J.N.T. was the primary author of the paper, designed and performed the experiments, analyzed the data, and interpreted the results; M.T.D. performed the experiments; A.J.B. constructed the retroviral vector for EGFP–β1-tubulin transfections; J.T. (Genentech) provided the reagents, assisted in designing the experiments, and participated in discussions; and J.E.I. designed the experiments, interpreted the results, participated in discussions, and assisted in manuscript preparation and editing.
Conflict-of-interest disclosure: J.T. is a Genentech employee. J.E.I. received research funding from Genentech and served as a research consultant for Genentech from 2008-2009. The remaining authors declare no competing financial interests.
Correspondence: Joseph E. Italiano Jr, Hematology Division, Department of Medicine, Brigham and Women's Hospital, 1 Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: jitaliano@rics.bwh.harvard.edu.