Abstract
The crucial function of blood platelets in hemostasis is to prevent blood loss by stable thrombus formation. This process is driven by orchestrated mechanisms including several signal transduction cascades and morphologic transformations. The cytoplasmic microtubule modulator RanBP10 is a Ran and β1-tubulin binding protein that is essential for platelet granule release and mice lacking RanBP10 harbor a severe bleeding phenotype. In this study, we demonstrate that RanBP10-nullizygous platelets show normal adhesion on collagen and von Willebrand factor under flow conditions. However, using a ferric chloride-induced arterial thrombosis model, the formation of stable thrombi was significantly impaired, preventing vessel occlusion or leading to recanalization and thromboembolization. Delta-granule secretion was normal in mutant mice, whereas platelet shape change in aggregometry was attenuated. Lack of RanBP10 leads to increased β1-tubulin protein, which drives α-monomers into polymerized microtubules. In mutant platelets agonists failed to contract the peripheral marginal band or centralize granules. Pretreatment of wild-type platelets with taxol caused microtubule stabilization and phenocopied the attenuated shape change in response to collagen, suggesting that RanBP10 inhibits premature microtubule polymerization of β1-tubulin and plays a pivotal role in thrombus stabilization.
Introduction
Mammals share a unique mechanism to ensure that injured blood vessels are sealed and loss of blood is minimized. Although specialized cells for this purpose are even found in invertebrates, the generation of anuclear cell fragments, designated platelets, are only present in mammals where they bud off cytoplasmic protrusions emanating from the megakaryocyte periphery.1 These precursor cells form proplatelets and release nascent platelets across the endothelial barrier into the blood stream.2 Endothelial cell lesions trigger platelets to make direct cellular contact with the subcellular matrix, which leads to platelet adhesion at the site of injury. Circulating platelets bind to an adhered layer of platelets, resulting in aggregation and thrombus formation, which is restricted to the site of injury and may ultimately occlude the vessel.3 This process is tightly regulated as false activation leads to thrombosis and infarction, one of the major causes of death in the Western countries. Platelet activation is a multistep process. A hallmark of activation is the release of bioactive small molecules and proteins stored in 3 defined sets of granules present within the platelet cytoplasm: release of α-granules leads to surface expression of CD62P; CD63 is a marker for lysosomal granules; and dense granules harbor nucleotides such as ATP, ADP, amines, and bivalent cations. Although the granule markers are well-characterized, the molecular and cell biologic mechanisms underlying the process of granule release are less well understood.4-6 Platelets harbor a cortical ring of bundled microtubules. In resting platelets this “marginal band” is essential to maintain the discoid shape. After activation, the microtubule ring contracts and concentrates the granules in the cell center before their release.
RanBP10 is a cytosolic Ran and β1-tubulin binding protein originally identified in megakaryocytes (MKs) where it concentrates along polymerized microtubules.7 β1-tubulin is the most diverse tubulin isoform on the amino acid level and exclusively expressed in MKs and platelets, where it is the prominent isotype in microtubules forming the platelet marginal band.8,9 In addition to its binding properties, it harbors guanine nucleotide exchange factor (GEF) activity toward the small GTPase Ran, leading to an increased fraction of GTP-bound Ran, which is found upstream of microtubule nucleating activity.10,11 Overexpression of RanBP10 in primary MKs leads to increased bundling of microtubules,12 whereas truncated or shortened microtubule filaments are found in RanBP10 shRNA-treated MKs.7 These opposite effects have implied that RanBP10 modulates noncentrosomal microtubules and might play a role in MK maturation, platelet biogenesis, or function. Transgenic mice generated to lack RanBP10 show an overall inconspicuous phenotype with normal platelet counts. However, these mice present with a dramatically prolonged bleeding time. Although blood flow diminishes normally during the first minutes after tail cut, the blood stream intensifies again and vessels do not occlude during normal time frame. This is reflected by a reduced reactivity toward suboptimal agonists in granule secretion and aggregometry. These agonists bind their cognate receptors on the platelet surface and typically initiate intracellular signaling via G-protein coupled receptors of the G(q), G(i), and G(13) subfamilies13 and disruption of these pathways in mouse models can lead to severe bleeding phenotypes.14,15 RanBP10 might thus be a good candidate to regulate platelet granule secretion by modulating the microtubule filaments. Here, we sought to identify the role of RanBP10 for thrombus formation and to shed some light on the underlying cytoskeletal mechanism in platelet granule release.
Methods
Animal husbandry
RanBP10-deficient mice were generated as recently described.12 Animal husbandry and all experiments were performed according to institutional guidelines with the approval of the local ethics authorities.
Megakaryocyte ploidy and immunohistochemistry
Bone marrow cells were extracted from femur bones by rinsing PBS through the cavity and washed in PBS before resuspension in citrate buffer supplemented with RNase K (10 mg/μL) and propidium iodide (1 mg/mL). After 30 minutes megakaryocyte ploidy profile was evaluated flow cytometrically. For immunofluorescence staining, washed platelets were stimulated with PAR4p as indicated and the reaction stopped by adding 8% formaldehyde with subsequent centrifugation on poly-L-lysine coated coverslips. Samples were permeabilized by incubation with 0.5% Triton-X 100. Free binding sites were then blocked with 3% goat serum. β1-tubulin was stained with a generated antiserum. Cytospins were next mounted with Fluoromount-G (Southern Biotech) and dried. Analysis was performed on a Nikon A1 confocal microscope using a CFI Plan Apo VC 100× oil lens with a numerical aperture of 1.4. Platelet perimeter was measured using Nikon NIS Elements Version 3.20 software on 80 to 180 platelets of at least 3 independent fields per time point and concentration with a total of 1740 platelets.
Gene expression quantification via real-time PCR
Total RNA was extracted from cell suspension using TRIzol (Invitrogen) according to the manufacturer‘s instructions. cDNA synthesis was performed via Superscript II First-Strand Synthesis System (Invitrogen). For amplicon quantification SYBR-Green (Applied Biosystems) was used. ΔΔCT-analysis16 was performed using 7500 Fast Real-Time software (Applied Biosystems). Quantitative real-time PCR analysis was executed with the following primer-pairs (Metabion). For β1-tubulin forward 5′-AGCCAAGTTCTGGGAGGTGATCGG and reverse 5′-GGTCCACAAGGACGGCTCGC, for β5-tubulin forward 5′-CGACCTGCAGCTGGACCGAA and reverse 5′-CCTGCCCCAGACTGACCGAA, for GAPDH forward 5′-TTCACCACCATGGAGAAGG and reverse 5′-CACACCCATCACAAACATGG, for RanBP9 forward 5′-GACTGTACCGGAGCTAAAC and reverse 5′-AGTTTCTCCAACTCCATTG, and for RanBP10 forward 5′-AGGACTATATGCGGGAGTGG and reverse 5′ TGCACCAGGTAAGACGAGAC.
Platelet isolation and analysis
Platelet rich plasma (PRP) was isolated from citrated blood after differential centrifugation. For transmission electron microscopy (TEM) platelets were stimulated with 1mM or 0.1mM PAR4p, respectively. Samples were fixed with 4% glutaraldehyde in 0.1M cacodylate-buffer for 15 minutes. After pelleting (6 minutes, 400g) samples were resuspended in 0.1M cacodylate-buffer containing 2% glutaraldehyde and stored at 4°C until analysis.17 Ultragraphs were analyzed on a Zeiss EM906 (Oberkochen). Depolymerization and repolymerization studies were performed as recently described.18 Briefly, platelets were incubated at 4°C, 37°C, or 4°C with subsequent rewarming to 37°C. Samples were fixed followed by centrifugation onto polylysin coated coverslips and immunostained using an anti–tubulin-antibody (Sigma-Aldrich). Platelet life-span was determined daily over a period of 5 days by the recovery rate of a 488-Dylight–coupled anti–GPIX-IgG derivative (Emfret Analytics) injected retro-orbitally into mice. For glycoprotein expression of resting and stimulated platelets, 50 μL heparinized blood was diluted in 2mM Ca2+ containing HEPES-Tyrode buffer and incubated with 1:5 antibody-solution at RT. Reaction was stopped by adding 500 μL PBS followed by flow-cytometric analysis. Glycoproteins with their corresponding antibody were named hereinafter and purchased from Emfret Analytics unless designated otherwise. Antibodies against GPVI (JAQ1), α2-integrin (Sam.G4), β1-integrin (Becton Dickinson), CD9 (Nyn.H1), GPIb (Xia.G5), GPIX (Xia.B4), αIIbβ3 (14A3), CLEC-2 (INU1), and α-GPV (Gon.C6).
Flow-chamber, platelet spreading, and in vivo thrombus formation
Coverslips were coated over night at 37°C with either HORM-collagen (Nycomed) or anti–human VWF-antibody (A0082; Dako) followed by blocking with 1% BSA, 1 hour at RT. For adhesion on VWF coverslips were subsequently incubated with murine plasma for VWF to adhere on immobilized antibody for 2 hours at 37°C. Heparinized blood (20 U/mL) was diluted with ½ volume HEPES-Tyrode buffer with antibody (56F8 conjugated to Dylight 488) and incubated for 5 minutes at 37°C. Perfusion was performed as previously described.19,20 For platelet spreading analysis coverslips were coated at 4°C over night with fibrinogen (100 μg/mL; Sigma-Aldrich), collagen (50 μg/mL; StemCell Technologies), or BSA (1.5% in PBS) and excess matrix proteins washed away. Platelets were allowed to adhere for 45 minutes at 37°C before nonbinding platelets were washed with PBS. Samples were fixed with 4% paraformaldehyde and mounted with Fluoromount-G (Southern Biotech).21 For intravital thrombus formation mice were anesthetized via intraperitoneal application of a ketamine/xylazine mix (100:5 mg/kg; Parke-Davis and Bayer) followed by retro-orbital injection of 2 μg 488-Dylight–coupled GPIX-IgG derivative (X488; Emfret Analytics) in 150 μL PBS. Mesenteric tissue was exteriorized and arterioles injured by topic application of 20% ferric chloride solution. Thrombus formation of fluorescent platelets was visualized as previously described20 during an overall observation period of 40 minutes or until complete arteriole occlusion occurred for a period longer than 1.5 minutes.
Mepacrine assay, Modified Tubulins, Aggregometry and dense granule release
Citrated blood was diluted in HEPES-Tyrode buffer. Platelet dense-granules were labeled in suspension with 390μM mepacrine,22 and incubated in the dark at 37°C for 30 minutes. Platelets were stimulated with thrombin concentrations and time points as indicated. Granule release was followed flow cytometrically and the residual platelet granule content calculated: Nonsecreted δ-granules (%) = MFI (activated platelets) × 100/MFI (resting platelets). For protein analysis platelets were incubated with agonists as indicated. Modified tubulins were analyzed in total platelet lysates with antibodies against tyrosinated and glutamylated tubulins over night. For microtubule stabilization platelets were preincubated 5 minutes with 50μM paclitaxel in PHEM-buffer and subsequently ultracentrifuged at 150 000g for 30 minutes. The pelleted fraction was dissolved at 37°C over night and immunoblotted against α, pan-β, and β1-tubulin.23 Cell fractioning into pellet and supernatant was performed as described12 recognizing pro and antiangiogenic factors with antibodies against fibrinogen, VEGF, and thrombospondin 1.6,24
For aggregometry and ATP release platelets were washed as described25 and resuspended in Tyrode buffer containing 2mM calcium and 100 μg/mL human fibrinogen (Sigma-Aldrich) at a concentration of 50 000 per μL. Shape change and aggregometry was induced by thromboxane A2 analog U46619 (Merck), ADP, or collagen (Probe & Go) at concentrations indicated. Microtubule stabilization was performed by preincubating platelets with 0.5mM paclitaxel (Sigma-Aldrich) for 30 minutes at 37°C before adding the agonists. For ATP release blood was diluted 1:4.5 in prewarmed saline. Subsequently 50 μL luciferase (Probe & Go) was added for 2 minutes. Platelets were stimulated with indicated concentrations of thrombin and compared with an ATP standard by Chrono-Lume. Serotonin release was measured in the supernatant of resting and stimulated platelets by ELISA (IBL International) according to the manufacturer's instruction.
Statistical analysis
Data were analyzed by Student t tests where P values < .05 were defined as statistically significant.
Results
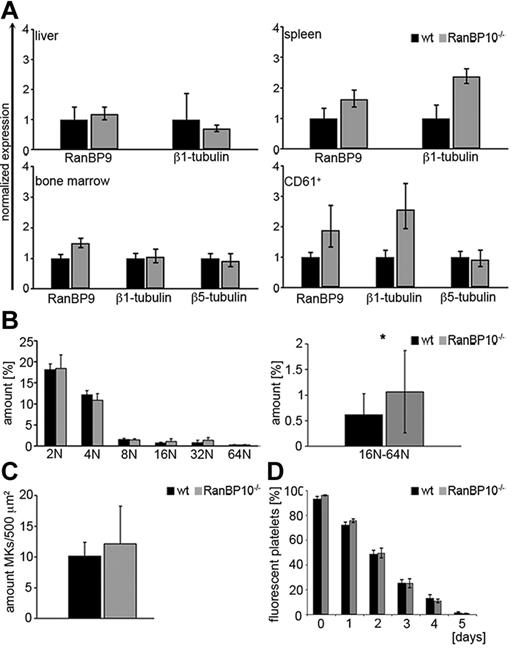
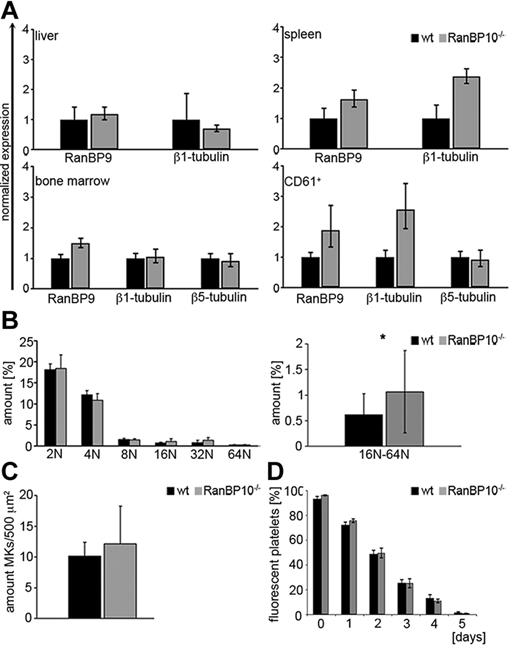
Microtubules are polymerized filaments assembled from α and β-tubulin dimers that harbor distinct functions during the cell cycle including the mitotic spindle or the aster-type fibers that emanate from the microtubule-organizing center present during interphase.26,27 Although most β-tubulin isotypes are broadly expressed and highly homologous, β1-tubulin is only expressed in MKs and platelets. TUBB1–deficient mice are thrombocytopenic and show platelet spherocytosis demonstrating the importance of β1-tubulin for platelet formation and function.9 RanBP10 binds to the C-terminus of β1-tubulin; however, RanBP10 ablation in mice does not lead to a reduced peripheral platelet count.12 As proplatelet formation was slightly reduced in MKs derived from nullizygous animals, we first asked whether compensatory mechanisms are responsible for the unaltered platelet count. RanBP10 shares a cellular homolog, designated RanBP9, which is ubiquitously expressed.28 We isolated RNA from liver, spleen, bone marrow, and purified CD61 cells and compared mRNA expression levels of RanBP9, TUBB1, and TUBB5 between RanBP10-deficient mice and wild-type controls. Although expression levels were comparable in liver and bone marrow, we found marked up-regulation of RanBP9 mRNA in CD61+ MKs and in spleen, a tissue enriched for MKs. TUBB1 was also up-regulated, whereas TUBB5 expression was unaffected (Figure 1A). Ploidy analysis of primary bone marrow MKs revealed that 2N/4N levels were normal, whereas the fraction of high ploidy (8N/16N/32N) was significantly increased in the absence of RanBP10 (Figure 1B), suggesting that these MKs might produce more platelets. Next, we quantified bone marrow MKs in sectioned femur bones from transgenic and mutant mice. As shown in Figure 1C the amount of CD41+ MKs in mutant mice was slightly elevated. Finally, we analyzed platelet life span by labeling with a fluorophore-conjugated antibody against GPIX.25 As expected the fraction of fluorescent platelets decreased during the next 5 days, but there was no difference between mutant and control animals (Figure 1D). Taken together these results imply that the reduced proplatelet formation found in RanBP10-null mice is compensated for by several mechanisms including increased MK number, ploidy and up-regulation of RanBP9 that finally result in an overall unaltered platelet count.
RanBP10 depletion is partially compensated for by overexpression of RanBP9 but does not influence platelet turnover, MK numbers or receptor expression. (A) RanBP9 mRNA expression is up-regulated in spleen and CD61+ cells of RanBP10-null mice. Expression levels were analyzed by quantitative real-time PCR and were normalized to GAPDH. (B) Bone marrow MKs were stained with PI and ploidy evaluated by flow cytometry. The amount of 16N-64N MKs is significantly elevated in RanBP10−/− mice compared with controls. (C) The amount of bone marrow MKs is slightly elevated in mutant mice. (D) Platelet life span in vivo is unaltered in mice lacking RanBP10 (*P < .05).
RanBP10 depletion is partially compensated for by overexpression of RanBP9 but does not influence platelet turnover, MK numbers or receptor expression. (A) RanBP9 mRNA expression is up-regulated in spleen and CD61+ cells of RanBP10-null mice. Expression levels were analyzed by quantitative real-time PCR and were normalized to GAPDH. (B) Bone marrow MKs were stained with PI and ploidy evaluated by flow cytometry. The amount of 16N-64N MKs is significantly elevated in RanBP10−/− mice compared with controls. (C) The amount of bone marrow MKs is slightly elevated in mutant mice. (D) Platelet life span in vivo is unaltered in mice lacking RanBP10 (*P < .05).
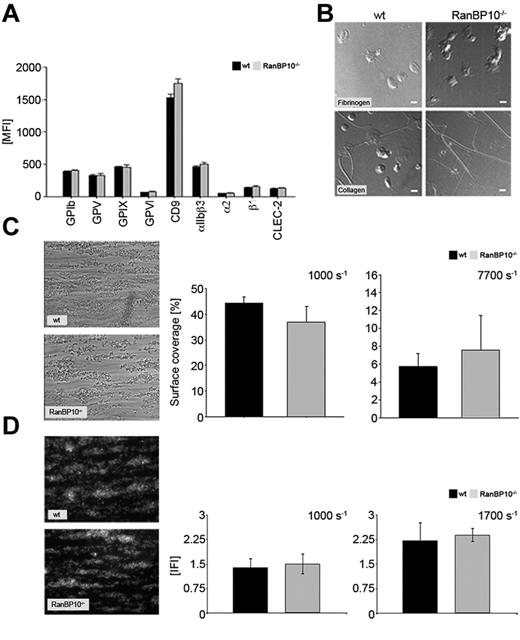
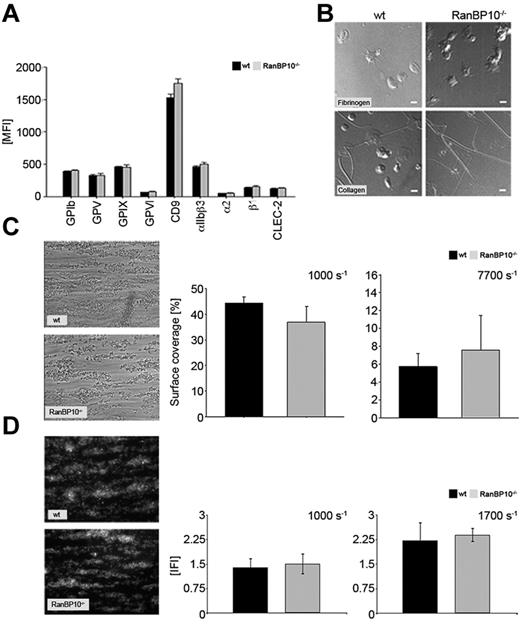
To exclude the possibility that the platelets produced in the absence of RanBP10 have altered surface receptors we stained resting platelets with a series of specific fluorophore-labeled antibodies. Platelets lacking RanBP10 harbor the same density for fibrinogen receptor (αIIb/β3), von Willebrand receptor (GPIbβ, GPV, GPIX), collagen receptors (GPVI, α2β1), as well as CD9 and CLEC-2 on their surface as platelets of control mice (Figure 2A). Although the flow cytometric expression analysis provides important information on density and composition of platelet surface receptors, it does not address their binding properties to matrix proteins under more physiologic conditions. Therefore, we analyzed spreading of platelets adhering to fibrinogen or collagen and found no alterations in RanBP10−/− mice (Figure 2B). Adhesion of mutant platelets to collagen under flow tended to be slightly increased under high shear (7700 seconds−1), but was rather normal under intermediate shear (1000 seconds−1, Figure 2C) or when the chamber was coated with VWF (data not shown). In addition, we calculated the thrombus volume and could not observe any differences between wild-type and RanBP10–null platelets adhering to collagen under intermediate shear rates of 1000 seconds−1 and 1700 seconds−1 (Figure 2D). These data together imply that binding mechanisms to matrix proteins under flow conditions are overall unaffected in the absence of RanBP10.
RanBP10−/− platelets show modestly altered adhesion under shear but spread normally. (A) Regular receptor density of platelet surface markers in RanBP10-null and control mice. (B) Platelet spreading on fibrinogen (top row) and collagen (bottom row) is unaffected in RanBP10-null platelets, showing the typical flat “fried-egg” appearance. (C) Phase contrast microscopy and quantitative analysis of surface coverage of wild-type and RanBP10−/− platelets aggregating on collagen under intermediate flow (1000 seconds−1 left panel) and high flow (7700 seconds−1 right panel) show overall unaltered adhesion. (D) Analysis of thrombus volume in integrative fluorescence intensities (IFI) and quantification shows that wild-type and RanBP10−/− platelets form equal-sized thrombi at each shear rates as depicted for 1000 seconds−1 left panel and 1700 seconds−1 right panel.
RanBP10−/− platelets show modestly altered adhesion under shear but spread normally. (A) Regular receptor density of platelet surface markers in RanBP10-null and control mice. (B) Platelet spreading on fibrinogen (top row) and collagen (bottom row) is unaffected in RanBP10-null platelets, showing the typical flat “fried-egg” appearance. (C) Phase contrast microscopy and quantitative analysis of surface coverage of wild-type and RanBP10−/− platelets aggregating on collagen under intermediate flow (1000 seconds−1 left panel) and high flow (7700 seconds−1 right panel) show overall unaltered adhesion. (D) Analysis of thrombus volume in integrative fluorescence intensities (IFI) and quantification shows that wild-type and RanBP10−/− platelets form equal-sized thrombi at each shear rates as depicted for 1000 seconds−1 left panel and 1700 seconds−1 right panel.
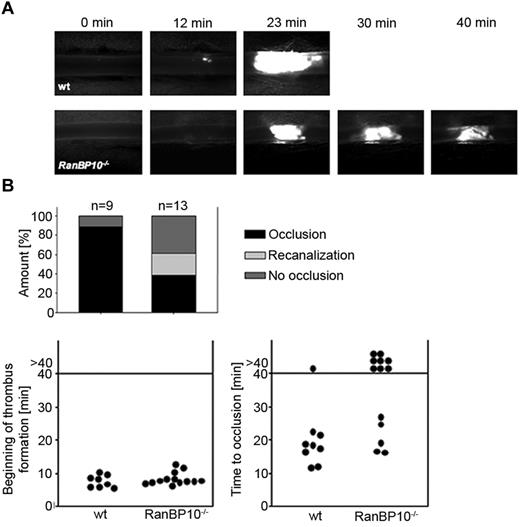
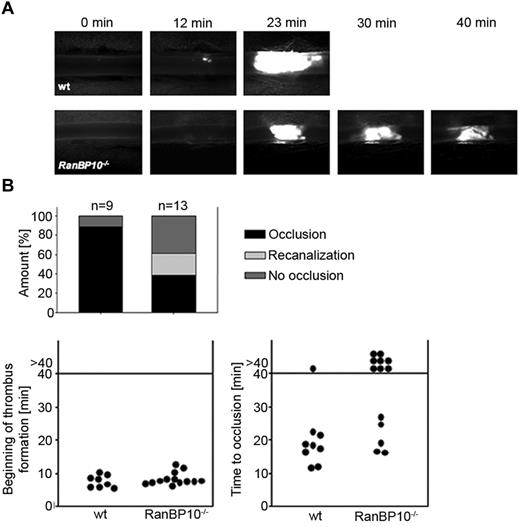
As RanBP10–null mice display a severe bleeding phenotype in a tail bleeding assay,12 we sought to determine whether thrombus formation is affected in vivo in mice lacking RanBP10. We used an intravital mouse thrombosis model where thrombus formation in mesenterial arterioles is initiated by ferric chloride and visualized by intravital fluorescence microscopy. In wild-type controls (n = 9), initial thrombus formation at the vessel wall was first detectable 8 minutes after injury, followed by thrombus growth. Typically within 20 to 25 minutes the vessel was obstructed (Figure 3A top row, and supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In RanBP10-null mice, platelet adhesion at the site of injury was normal and we could observe the beginning of thrombus formation after 7.5 minutes (n = 13). Intriguingly, the growing thrombus was unstable and continued to disintegrate during the overall observation period of up to 40 minutes (Figure 3A bottom row; supplemental Video 2). Quantification indicates that recanalization in arterioles was only found in RanBP10-null mice. Onset of thrombus formation was within the same time range as in wild-type controls (Figure 3B left panel), but 8/13 arterioles failed to occlude within the overall observation time of 40 minutes in mutant mice compared with 1/9 wild-type arterioles (Figure 3B right panel). These findings together imply that RanBP10 plays an essential role to ensure thrombus stability in vivo, which may also provide an explanation for the severe bleeding phenotype found in the tail-bleeding assay in the mutant mice.12
RanBP10−/− mice show impaired thrombus formation in an arterial thrombosis model. (A) The onset of thrombus formation after FeCl3-induced injury is unaffected (top panel). In wild-type mice vessels are occluded within 20 minutes whereas in mutant animals no stable clot is formed within 40 minutes observation time (bottom panel). (B) Quantification reveals that in wild-type mice 8 of 9 injured arterioles show normal occlusion, although this was only present in 5 of 13 arterioles of RanBP10−/− mice. In 8 of 13 arterioles we found no occlusion or recanalization within the observation time.
RanBP10−/− mice show impaired thrombus formation in an arterial thrombosis model. (A) The onset of thrombus formation after FeCl3-induced injury is unaffected (top panel). In wild-type mice vessels are occluded within 20 minutes whereas in mutant animals no stable clot is formed within 40 minutes observation time (bottom panel). (B) Quantification reveals that in wild-type mice 8 of 9 injured arterioles show normal occlusion, although this was only present in 5 of 13 arterioles of RanBP10−/− mice. In 8 of 13 arterioles we found no occlusion or recanalization within the observation time.
We recently showed reduced release of α and lysosomal granules in RanBP10−/− platelets in response to suboptimal agonist concentrations by impaired expression of P-selectin (CD62P), or CD63 by flow cytometry. Furthermore, exocytosis of antiangiogenic factor PF4 was diminished in response to PAR4p in mutant platelets.12 As α-granules harbor either pro or antiangiogenic proteins that can be released differentially,6,29 we analyzed whether there is any bias in release between both genotypes. Platelets were stimulated with ADP for release of proangiogenic factors VEGF and fibrinogen and with thromboxane A2 mimetic U46619 for antiangiogenic factor thrombospondin. Overall, we found less granule factors of either type in the supernatant of mutant platelets, indicating that there is no difference between pro or antiangiogenic factors (data not shown), which implies that RanBP10 causes a general defect in granule release. However, we found that α-granules in mutant platelets harbor slightly less VEGF and fibrinogen than in wild-type controls (data not shown).
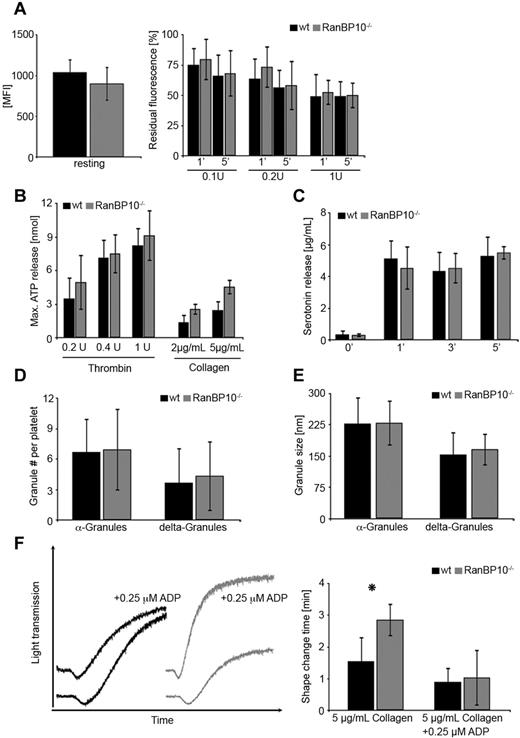
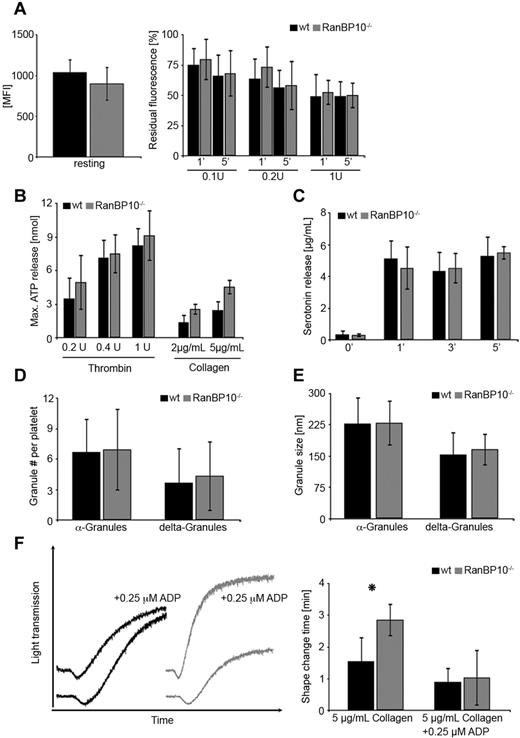
Dense granule release can be monitored by different methods: the most convenient way is to stain resting platelets with the fluorescent dye mepacrine and document the decline of fluorescence after agonist treatment. The extent of fluorescence retained after stimulation is a measure of dense granules that failed to be released. Platelets from either strain labeled equally well for mepacrine (Figure 4A left panel). When suboptimal concentrations of thrombin (0.1-0.2 U/mL) were applied, slightly more fluorescence was retained in mutant platelets compared with wild-type controls after 1 minute. This was normalized after 5 minutes. After stimulation with 1 U/mL thrombin, dense granule release was identical in both strains (Figure 4A right panel). As mepacrin release is an indirect marker for dense granule release, we next determined the ATP release in response to suboptimal thrombin and collagen by aggregoluminometry. Using this technique in whole blood we encountered some variability between different samples of either genotype but did not find any significant difference in maximal (Figure 4B) or cumulative (data not shown) ATP release between transgenic animals and wild-type controls. In addition, we determined the release of the dense granule constituent serotonin. Washed platelets were stimulated with PAR4p and pelleted and serotonin concentrations measured after 1, 3, and 5 minutes in the supernatant. We found no difference between wild-type and mutant platelets indicating that serotonin release is overall unaltered (Figure 4C). The small discrepancy between mepacrine assay, maximal ATP release, and serotonin release is probably because of the different methods in platelet preparations and readouts and also found in other studies.25 Therefore, we refined our analysis on numbers and size of α and dense-granules. On electromicrographs of wild-type and mutant platelets there was no significant difference between granule numbers (Figure 4D) or diameter (Figure 4E), although in mutant platelets dense granule number was slightly increased by 18%, probably because of the increased size. It is worth mentioning that platelet fragments after complete granule release are underrepresented in the ultramicrographs because of their markedly reduced density compared with intact platelets. We thus cannot determine the amount of granules in completely degranulated platelets. Next, we asked whether exogenous ADP can at least partially rescue the aggregation phenotype in RanBP10-null platelets. We added 0.25μM ADP to platelets from either genotype before stimulation with 5 μg/mL collagen. This threshold of ADP concentration alone had no impact on aggregation. When added to collagen in wild-type platelets, shape change, and maximal aggregation were not significantly altered. In contrast, ADP preincubation of RanBP10-null platelets normalized the shape change, and in part, the maximal aggregation (Figure 4F left panel). We measured the duration of shape change in response to collagen and found a statistically significant attenuation in mutant platelets. In the presence of ADP this attenuation was restored to normal levels (Figure 4F right panel). These findings together imply that lack of RanBP10 does not affect dense granule number, composition or release, although the release is attenuated somewhat in response to low agonist concentrations, but that RanBP10 is important for optimal platelet shape change when suboptimal or threshold concentrations are used.
Release of α and δ-granule contents of platelets in RanBP10−/− mice. (A) Similar mepacrine uptake in platelets of either genotype (MFI = mean fluorescence intensity (left panel). Mepacrine release after 5 minutes is overall unaltered between wild-type and RanBP10-null platelets, whereas it is slightly slower in mutant platelets after 1 minute when suboptimal thrombin concentrations (0.1 or 0.2 U/mL) are used. Data show an average of 6 independent experiments. Error bars depict the standard deviation. (B) Maximal ATP release in whole blood of RanBP10-null animals and controls was determined by aggregoluminometry revealing a slightly increased ATP release after treatment with 0.2, 0.4, or 1 U/mL thrombin or with 2 or 5 μg/mL collagen. (C) Serotonin release in response to 0.5μM PAR4p treatment showed no difference after 1, 3, or 5 minutes between wild-type and mutant platelets. (D) α and dense granules were counted on electron micrographs showing no difference in intact platelets. (E) Measurements of the diameter of α and dense-granules show that there is no difference between wild-type and RanBP10-null platelets. (F) Wild-type and RanBP10-null platelets were activated with 5 μg/mL collagen in the presence or absence of 0.25μM ADP. This concentration had little impact on collagen-induced aggregation in wild-type platelets (black), but restored attenuated shape change and aggregation in knockout platelets (gray curves) almost to wild-type levels (left panel). Time of shape change is significantly prolonged in mutant animals, but can be restored in the presence of threshold ADP concentrations (right panel). Mean of 5 experiments is shown, error bars indicate standard deviation (*P < .05).
Release of α and δ-granule contents of platelets in RanBP10−/− mice. (A) Similar mepacrine uptake in platelets of either genotype (MFI = mean fluorescence intensity (left panel). Mepacrine release after 5 minutes is overall unaltered between wild-type and RanBP10-null platelets, whereas it is slightly slower in mutant platelets after 1 minute when suboptimal thrombin concentrations (0.1 or 0.2 U/mL) are used. Data show an average of 6 independent experiments. Error bars depict the standard deviation. (B) Maximal ATP release in whole blood of RanBP10-null animals and controls was determined by aggregoluminometry revealing a slightly increased ATP release after treatment with 0.2, 0.4, or 1 U/mL thrombin or with 2 or 5 μg/mL collagen. (C) Serotonin release in response to 0.5μM PAR4p treatment showed no difference after 1, 3, or 5 minutes between wild-type and mutant platelets. (D) α and dense granules were counted on electron micrographs showing no difference in intact platelets. (E) Measurements of the diameter of α and dense-granules show that there is no difference between wild-type and RanBP10-null platelets. (F) Wild-type and RanBP10-null platelets were activated with 5 μg/mL collagen in the presence or absence of 0.25μM ADP. This concentration had little impact on collagen-induced aggregation in wild-type platelets (black), but restored attenuated shape change and aggregation in knockout platelets (gray curves) almost to wild-type levels (left panel). Time of shape change is significantly prolonged in mutant animals, but can be restored in the presence of threshold ADP concentrations (right panel). Mean of 5 experiments is shown, error bars indicate standard deviation (*P < .05).
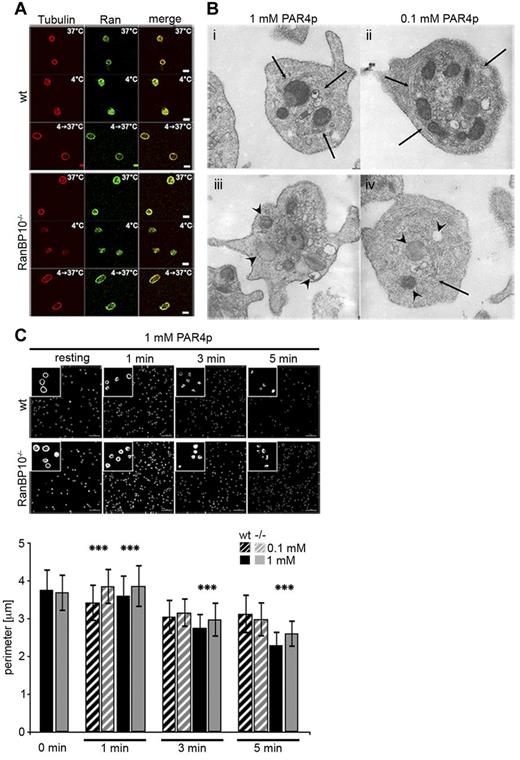
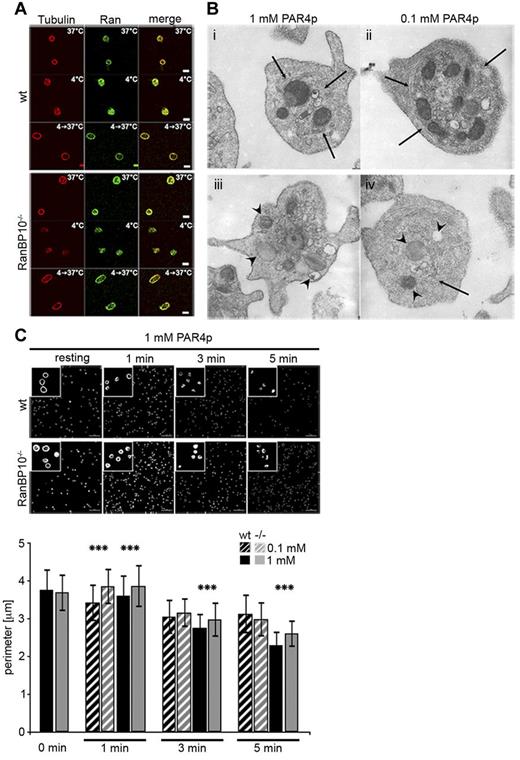
RanBP10 binds to both Ran and β1-tubulin. The distribution of these proteins offers mechanistic insight into how RanBP10 might modulate noncentrosomal microtubules: De novo nucleation of microtubule filaments can be induced by cooling and rewarming of platelets and cold-induced depolymerization is reversed when platelets are rewarmed again.17 We stained wild-type and RanBP10−/− platelets after cooling (4°C) and after rewarming (4°C- > 37°C) for β-tubulin and did not detect any differences in the staining pattern between the 2 genotypes, demonstrating that RanBP10 is not required for de novo nucleation of microtubule filaments from dimeric tubulins. Ran, colocalizing with tubulin in resting platelets, is also found throughout the cell in cooled platelets and redistributes with newly polymerized cortical microtubules after rewarming, indicating that the binding properties of RanBP10 are unaffected (Figure 5A). We recently found that in many activated RanBP10-null platelets the microtubule coil remained larger in size.12 We thus asked whether RanBP10 affects the centralization of granules by impaired microtubule ring contraction by performing TEM on stimulated platelets. In most wild-type platelets treated with 1 or 0.1mM PAR4p, granules were found in the platelet center, whereas they were scattered throughout the complete cytoplasm in RanBP10−/− platelets (Figure 5B). Contracted microtubule filaments were visible in wild-type platelets stimulated with either concentration (Figure 5Bi and ii arrow), whereas in many RanBP10−/− platelets the marginal band was still detected in the periphery. It showed an irregular distribution pattern forming longitudinal and cross-sectioned coils or had spiral instead of circular coils (Figure 5Biii and iv) giving independent evidence that microtubule ring contraction is impaired in the absence of RanBP10. In platelets of either genotype agonist treatment led to formation of pseudopodia, indicating that redistribution of actin filaments occurs independently of tubulin dynamics. Staining of platelets with fluorophore-labeled phalloidin also showed no difference in actin filament morphology (data not shown). To gain better insight into the kinetics of marginal band contraction we performed β1-tubulin immunostaining. In resting platelets the marginal band size was comparable between the genotypes. However, already 1 minute after stimulation with PAR4p, we found contraction in wild-type, but not in knockout platelets (Figure 5C). After 5 minutes the average diameter converged toward the wild-type. Thus, in RanBP10-null platelets the attenuated or incomplete marginal band contraction occurs shortly after stimulation.
RanBP10 is dispensable for spontaneous microtubule polymerization but is essential for marginal band contraction after platelet activation. (A) Microtubule depolymerization and repolymerization was normal after platelet cooling and rewarming. Tubulin is stained in red (Alexa647) and Ran in green (Alexa488). (B) TEM ultragraphs of platelets treated with 1mM or 0.1mM PAR4p show that in wild-type platelets the microtubule coil contracts (arrows, i and ii) and granules become centralized. In RanBP10−/− platelets (iii and iv) the marginal microtubule band fails to contract and granules are no longer centralized (arrowheads). Normal platelet activation is shown by protrusions in either strain. Subpanel iv shows an example of disorganized microtubule filaments (arrow). (C) Immunofluorescence staining with anti–β1-tubulin antibody and quantification of fixed platelets at time-points indicated after stimulation with PAR4p (top panel). Quantification of wild-type (black) and RanBP10−/− platelets (gray) after stimulation with 0.1mM PARp (hatched bars) and 1mM PAR4p (filled bars; ***P < .001, double-sided Student t test with independent variances).
RanBP10 is dispensable for spontaneous microtubule polymerization but is essential for marginal band contraction after platelet activation. (A) Microtubule depolymerization and repolymerization was normal after platelet cooling and rewarming. Tubulin is stained in red (Alexa647) and Ran in green (Alexa488). (B) TEM ultragraphs of platelets treated with 1mM or 0.1mM PAR4p show that in wild-type platelets the microtubule coil contracts (arrows, i and ii) and granules become centralized. In RanBP10−/− platelets (iii and iv) the marginal microtubule band fails to contract and granules are no longer centralized (arrowheads). Normal platelet activation is shown by protrusions in either strain. Subpanel iv shows an example of disorganized microtubule filaments (arrow). (C) Immunofluorescence staining with anti–β1-tubulin antibody and quantification of fixed platelets at time-points indicated after stimulation with PAR4p (top panel). Quantification of wild-type (black) and RanBP10−/− platelets (gray) after stimulation with 0.1mM PARp (hatched bars) and 1mM PAR4p (filled bars; ***P < .001, double-sided Student t test with independent variances).
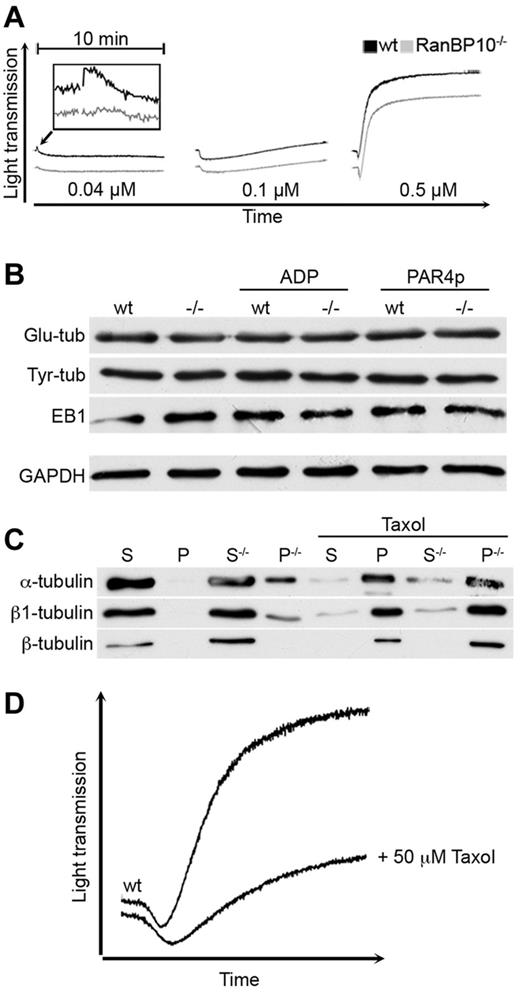
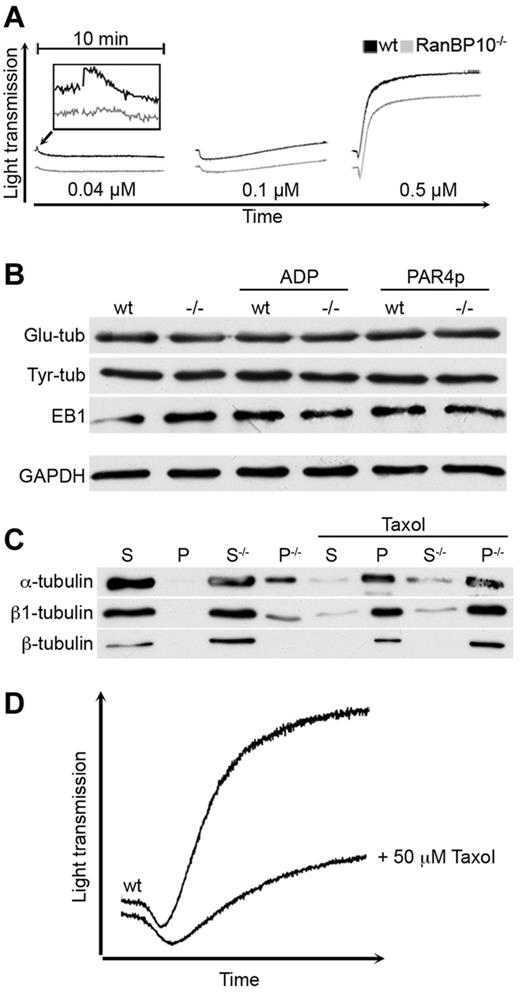
If RanBP10 alters the dynamic of the microtubule ring contraction in low agonist concentrations, lack of RanBP10 might best be detected using the initial shape change in Born aggregometry. The murine thromboxan A2 (TxA2) receptor couples to the more sensitive Gα12/13 subunits and at higher agonist concentrations additionally to the Gαq subunit, leading to RhoA activation upstream of platelet shape change, granule centralization and release.15 At high concentrations of TxA2 mimetic U46619 (0.5μM) mutant and wild-type platelets showed the same shape change and aggregation as expected. Intermediate agonist concentrations (0.1μM) caused shape change without aggregation in either strain, whereas threshold concentrations (0.04μM) led to shape change only in wild-type animals, although it was abrogated in RanBP10−/− platelets (Figure 6A). Lower concentrations did not induce any shape change (data not shown). Posttranslational tubulin modifications are indicators of microtubule filament stability and turnover.30 Antibodies recognizing glutamylated or tyrosinated tubulin showed normal expression in resting platelets of both strains or when platelets of either strain were stimulated with ADP or PAR4p (Figure 6B). Platelets also contain EB1, an end binding protein that binds selectively to the plus-end of filaments.31 Again, there was no difference between wild-type and knockout platelets, indicating that these properties are not affected by RanBP10. Finally, we sought to analyze the effect of RanBP10 ablation on tubulin polymerization biochemically. Polymerized microtubules were isolated from fetal liver-derived MKs after ultracentrifugation and the pelleted fraction compared with the soluble supernatant. Total β1-tubulin protein was up-regulated in RanBP10-null MKs and a substantial part found in the pellet fraction enriched for polymerized microtubules (Figure 6C). α-tubulin was shifted in a comparable amount into the polymerized form. To our surprise, β2 and β5-tubulin that are ubiquitously expressed, are not affected by RanBP10 ablation, as the commercial β-tubulin antibody has a low affinity for the β1-tubulin isotype. When taxol was added before ultracentrifugation, the majority of all tubulin isotypes was found in the polymerized fraction as described,9 whereas GAPDH remained in the soluble fraction (data not shown). These data imply that RanBP10 might prevent α/β1-tubulin dimers from premature polymerization. As this interaction is specific for β1-tubulin, RanBP10 function might be more profound in MKs and platelets where β1-tubulin is highly expressed.32 To test whether this altered microtubule equilibrium causes a delayed shape change, we preincubated wild-type platelets with taxol before activation with collagen. As shown in Figure 6D taxol-stabilized platelets had a delayed shape change and showed less aggregation, partially phenocopying the RanBP10-phenotype. Taken together, our data suggest that RanBP10 acts more on the β1-tubulin monomer-polymer equilibrium than on a direct bundling of MT filaments.
RanBP10 modulates the tubulin-microtubule equilibrium and is essential for the platelet shape change. (A) Platelet activation with 0.04μM U46619 (left panel) still caused a shape change in wild-type (black curve), but not in RanBP10−/− platelets (gray curve). Intermediate concentrations (0.1μM, middle panel) induced shape change without aggregation in both strains, whereas high concentrations led to similar shape changes in platelets of wild-type and mutant platelets with full aggregation (right panel). (B) The amount of glutamylated (Glu-tub) or tyrosinated tubulin (Tyr-Tub) as well as EB1 proteins were unaffected in resting, ADP, or PAR4p-stimulated knockout platelets (−/−) compared with wild-type (WT) controls. (C) RanBP10 prevents premature polymerization of α/β1–tubulin dimers. Megakaryocytes lacking RanBP10 (−/−) show an increased fraction of β1 and α-tubulin in the pelleted fraction (P) representing polymerized microtubules compared with the soluble (S) fraction isolated from the supernatant. Other β-tubulins recognized by a commerical β-tubulin antibody remain unaffected. In the presence of taxol filaments polymerize independent of RanBP10. (D) Platelets were incubated with either 0.5mM taxol or PBS before aggregometry with 5 μg/mL collagen. When microtubules were stabilized with taxol, the shape change is delayed and aggregation diminished, mimicking the RanBP10-null phenotype.
RanBP10 modulates the tubulin-microtubule equilibrium and is essential for the platelet shape change. (A) Platelet activation with 0.04μM U46619 (left panel) still caused a shape change in wild-type (black curve), but not in RanBP10−/− platelets (gray curve). Intermediate concentrations (0.1μM, middle panel) induced shape change without aggregation in both strains, whereas high concentrations led to similar shape changes in platelets of wild-type and mutant platelets with full aggregation (right panel). (B) The amount of glutamylated (Glu-tub) or tyrosinated tubulin (Tyr-Tub) as well as EB1 proteins were unaffected in resting, ADP, or PAR4p-stimulated knockout platelets (−/−) compared with wild-type (WT) controls. (C) RanBP10 prevents premature polymerization of α/β1–tubulin dimers. Megakaryocytes lacking RanBP10 (−/−) show an increased fraction of β1 and α-tubulin in the pelleted fraction (P) representing polymerized microtubules compared with the soluble (S) fraction isolated from the supernatant. Other β-tubulins recognized by a commerical β-tubulin antibody remain unaffected. In the presence of taxol filaments polymerize independent of RanBP10. (D) Platelets were incubated with either 0.5mM taxol or PBS before aggregometry with 5 μg/mL collagen. When microtubules were stabilized with taxol, the shape change is delayed and aggregation diminished, mimicking the RanBP10-null phenotype.
Discussion
The transition from hemostatic thrombocytes in other vertebrates to anucleate blood platelets in mammals and humans has come with a specialized set of proteins like β1-tubulin. In the absence of this most diverse β-tubulin isotype, mice are thrombocytopenic and the platelets formed are spheric with reduced reactivity toward thrombin. Although other tubulins including β2 and β5-tubulin are up-regulated, the equilibrium of tubulin monomers and polymerized microtubules is shifted toward the monomeric state.9 Schwer and coworkers concluded that β1-tubulin helps to polymerize microtubules in MKs and platelets.9 The divergent C-terminus of β1-tubulin including the helices α10 and α11 that protrude from the globular core of tubulin and are known to bind microtubule-associated proteins. This domain has been used as bait in a yeast 2-hybrid screen and we identified RanBP10, a novel protein whose expression is restricted to bone marrow and spleen and enriched in MKs.7 Several compensatory mechanisms might contribute to compensate for slightly impaired proplatelet formation seen in MKs that lack RanBP10: an overall increase in bone marrow MKs, higher ploidy, and the up-regulation of RanBP9, its only known paralog. Although both proteins have 68% sequence homology on the amino acid level33 and share a SPRY, LisH, and CTLH domains, the RhoGEF-consensus domain has not been identified in RanBP9. Although both proteins bind to Ran, it is unclear whether RanBP9 binds to β1-tubulin. None of the original clones in the 2-hybrid screen encoded for RanBP9, although it is expressed in MKs and has been found in many screens using different baits.34
The severe bleeding phenotype in RanBP10–null mice was originally attributed to impaired granule release in response to suboptimal concentrations of agonists12 rather than to platelet spherocytosis, which has a weaker impact on platelet function.18 Our data presented in this study rather suggest that lack of RanBP10 leads to a delayed contraction of the circumferential microtubule coil in response to threshold concentrations of platelet agonists that attenuates granule centralization and release (Figure 5B-C). In RanBP10−/− animals the average microtubule coil consists of 12 filaments compared with 10 in wild-type mice.12 This is independently reflected by up-regulation of β1-tubulin mRNA and protein found in this study (Figures 1A and 6C). Although contraction of the peripheral microtubule coil precedes granule centralization and release, microtubule stabilizing agents do not prevent the release of granules,35 suggesting that parallel pathways become activated in response to strong agonists.
The actin cytoskeleton seems to remain unaffected during activation of RanBP10-mutant platelets, as shown by pseudopodia formation (Figure 5B) and phalloidin staining. Actin-fibers prevent premature granule release and differentially regulate α and dense-granule secretion in response to agonists.5
Early secretion analysis revealed that dense granules are released first, 10 seconds after agonist supplementation,36 followed by α-granules and lysosomes.37 The release of dense granules is MT-independent. In contrast, several reports describe that although MT interfering agents do not completely abrogate granule centralization, they clearly can inhibit secretion (reviewed in Ref. 38).38 Probably, the marginal band contraction supports granule centralization by organizing the contractile microfilaments.39 In mice lacking RanBP10 the release defect is more affecting α then dense granule exocytosis, which is in concordance with the kinetics observed for the attenuated MT coil contraction. However, this defect is indeed rather subtle, suggesting that the attenuated shape change seen in aggregometry (Figure 4F) occurs in a small time window. This slow agonist response reaction present for multiple agonists in threshold concentrations might reflect best the in vivo situation. It is striking that most assays, in which platelet function was addressed under flow conditions, were completely normal and even the intravital thrombosis model revealed an effect only in the late phase of thrombus stabilization (Figure 3), affecting the outer thrombus shell, whereas platelet adhesion and the inner center remained stable.
Mouse RanBP10 is mainly expressed in the liver and the spleen, an organ in which MKs are enriched. In contrast, protein levels were virtually absent in neutrophils compared with MKs,12 excluding a role of RanBP10 in leukocytes for defective thrombus formation. Although our data on aggregometry and flow cytometry has been performed with isolated platelets, indicating the defect to be intrinsic to platelets, we cannot completely rule out the possibility that RanBP10 expression in endothelial cells contributes to the defective thrombus formation. RanBP10 mRNA expression levels in endothelial cells were as low as in 3T3-fibroblasts, compared with 3-fold higher expression in primary MKs (data not shown), thus making it highly unlikely that RanBP10 in the endothelial tissue contributes significantly to the impaired thrombus formation. Interestingly, the pattern of reduced platelet reactivity, bleeding phenotype and impaired thrombus formation is partially phenocopied in transgenic mouse models lacking the G-protein receptor-associated subunits Gα11/q and Gα12/13, which transduce their signals to the small GTPase RhoA. Several models imply that Gα11/q is essential for granule release and aggregation, but dispensable for shape change.40 In contrast, both Gα13 and RhoA are essential for proper platelet shape change preceding granule concentration and release after activation.15,41,42 Mice lacking RanBP10 show normal reactivity to thrombin or high concentrations of U46619 or ADP, suggesting that the Gq pathway is unaffected in these mice, whereas shape change and MT ring contraction in RanBP10−/− platelets is attenuated when lower agonist concentrations are used (Ref. 12; Figures 4F, 5C, and 6A) similar to mice lacking Gα1315 and platelets of RhoA−/− mice that are also more spherical and increased in size.15,41,42 RanBP10 harbors a RhoGEF consensus domain, which might have a so far uncharacterized enzymatic or binding activity to RhoA. Although the nonmammalian homolog Rho1 was not a substrate for the GEF-activity of RanBP10, we cannot exclude that this domain might modulate RhoA function. The attenuated marginal band contraction identified in RanBP10−/− platelets could thus be a mechanistic link between impaired cellular signaling and the microtubule dynamics underlying granule centralization and release. Several G-protein coupled receptors have been considered targets for influencing platelet activity43 ; however, more downstream targets are an alternate strategy, especially when their expression is overall restricted to the megakaryocytic lineage. RanBP10 might become one of these targets in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Petra Schrade for excellent technical assistance with electron microscopy.
This work was supported by the Deutsche Forschungsgemeinschaft (SCHU 1421/5-1 and 5-2).
Authorship
Contribution: I.M., S.K., S.S., I.H., S.D., and H.S. performed experiments; H.S. and B.N. designed experiments, and analyzed and interpreted data; I.M., S.K., J.E.I., S.B., and I.H. analyzed data; J.E.I. provided reagents; H.S. is the primary investigator of this study and takes full responsibility; and I.M. and H.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harald Schulze, Charité, Labor für Pädiatrische Molekularbiologie, Charité Universitätsmedizin Berlin, Ziegelstrasse 5-9, 10098 Berlin, Germany; e-mail: harald.schulze@charite.de.