Abstract
Gene therapy (GT) for adenosine deaminase–deficient severe combined immune deficiency (ADA-SCID) can provide significant long-term benefit when patients are given nonmyeloablative conditioning and ADA enzyme-replacement therapy (ERT) is withheld before autologous transplantation of γ-retroviral vector-transduced BM CD34+ cells. To determine the contributions of conditioning and discontinuation of ERT to the therapeutic effects, we analyzed these factors in Ada gene knockout mice (Ada−/−). Mice were transplanted with ADA-deficient marrow transduced with an ADA-expressing γ-retroviral vector without preconditioning or after 200 cGy or 900 cGy total-body irradiation and evaluated after 4 months. In all tissues analyzed, vector copy numbers (VCNs) were 100- to 1000-fold greater in mice receiving 900 cGy compared with 200 cGy (P < .05). In mice receiving 200 cGy, VCN was similar whether ERT was stopped or given for 1 or 4 months after GT. In unconditioned mice, there was decreased survival with and without ERT, and VCN was very low to undetectable. When recipients were conditioned with 200 cGy and received transduced lineage-depleted marrow, only recipients receiving ERT (1 or 4 months) had detectable vector sequences in thymocytes. In conclusion, cytoreduction is important for the engraftment of gene-transduced HSC, and short-term ERT after GT did not diminish the capacity of gene-corrected cells to engraft and persist.
Introduction
In humans, a deficiency of adenosine deaminase (ADA) results in a severe combined immunodeficiency (SCID), and, if left untreated in infants, results in mortality.1 ADA is responsible for the deamination of adenosine (Ado) and 2′-deoxyadenosine (dAdo) to form inosine and 2′-deoxyinosine, respectively, and is essential to purine salvage. Without ADA, Ado and dAdo both accumulate; however, it is primarily the accumulation of dAdo and subsequent formation of dATP that cause cytotoxicity in immature T lymphocytes.2 The preferred treatment for ADA-deficient SCID patients (ADA-SCID) is HSCT with a matched sibling donor. ADA-SCID patients who undergo transplantation via the use of matched-unrelated or haploidentical donors have lower rates of survival.3 When a suitable donor is not available or the patient is too ill to undergo transplantation, the patient may be started on enzyme-replacement therapy (ERT) with pegylated bovine ADA (Adagen; Sigma-Tau Pharmaceuticals Inc). ERT does increase lymphocyte counts and immune function; however, absolute lymphocyte counts remain below normal, and immune function decreases over time.4,5
Because the current clinical options are suboptimal, ADA-deficient SCID patients are ideal candidates for gene therapy (GT) approaches. It has been hypothesized that correction of a few autologous HSCs could result in corrected T-cell progeny that would have a strong selective advantage and would eventually fill the T-cell compartment.6 The selective advantage of corrected T cells in ADA-SCID has been observed in patients who had a spontaneous reversion of the mutation in one T-cell precursor where, over time, the T-cell compartment was entirely composed of corrected T-cell progeny.7,8
The earliest GT clinical trials for ADA deficiency used murine γ-retroviral vectors to transduce peripheral blood T lymphocytes and CD34+ isolated from bone marrow and from cord blood.9-12 In these studies, patients were not given cytoreductive conditioning before receiving transduced cells, and all patients remained on ERT. These approaches did not provide clinical benefit, and the frequency of gene-marked peripheral blood cells remained very low. However, in some studies, the frequency of gene-corrected peripheral blood T cells increased when the ERT dose was either decreased or discontinued.13,14 It was hypothesized that the survival advantage of gene-corrected cells was blunted by the ERT. Long-term follow-up studies have indicated gene-corrected cells can persists for more than 10 years, contain different vector integration sites, and are not the result of insertional oligoclonal expansion.15,16
In a subsequent trial with improved γ-retroviral vectors and transduction conditions, unconditioned patients remaining on ERT did not have clinical benefit.17 In 2002, investigators in Milan reported that cytoreductive conditioning with a moderate dose of busulfan chemotherapy before reinfusion of transduced cells and cessation of ERT resulted in multilineage engraftment of transduced cells and immune reconstitution.18 ADA activity was detected across lineages, which was associated with a decrease in adenine metabolites. In a subsequent publication, investigators analyzed the long-term follow-up (1.8-10 years) of 10 patients treated with this approach and reported significant, long-term, clinical benefit.19 Since then, groups in the United Kingdom and the United States have demonstrated efficacy with this approach as well.20-22
Although GT for ADA-SCID has met success with clinical benefit for many patients (∼ 70%), several patients in the ongoing clinical trials have experienced events that necessitated the restarting of ERT,19-21 suggesting there could be improvements in outcomes. Thus, the primary goal of this study was to delimit the roles of ERT and cytoreductive conditioning in GT ex vivo for ADA-SCID by use of the ADA-deficient mouse and a γ-retroviral vector currently being evaluated in a clinical GT trial. We also investigated the efficacy of a short-term course of ERT after GT to enhance engraftment of transduced cells. The results of these experiments clearly demonstrate the importance of cytoreductive conditioning and the potential to enhance thymic engraftment with short-term ERT after γ-retroviral–mediated GT for the clinical treatment of ADA-SCID.
Methods
Vector, packaging, and stability
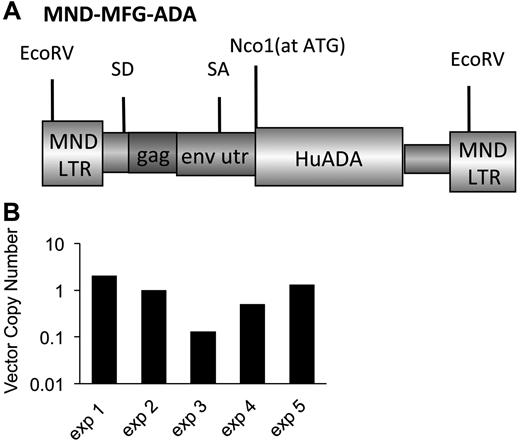
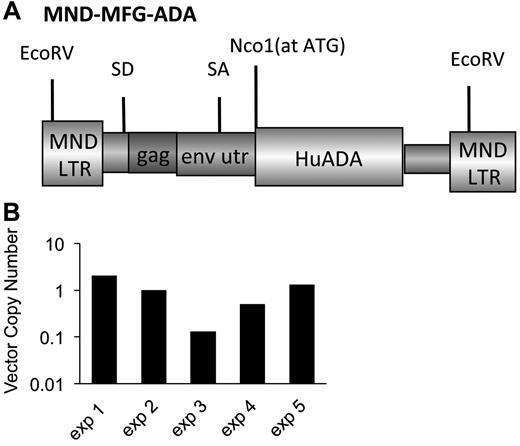
The γ-retroviral vector, MND-MFG-huAda (MMA), based on the murine leukemia virus retrovirus, as was modified by use of the MND LTR enhancer/promoter to drive expression of the human Ada cDNA and includes the splice acceptor site from the 5′-untranslated region of murine leukemia virus envelope gene (env).23-25 The ATG transcription start site of the human Ada cDNA is aligned at the ATG transcription start site of env to enhance transgene expression (Figure 1A).26 MMA was packaged by transient transfection in 293T human embryonic kidney cells in D10 for 10-14 days (D10-Dulbecco modified essential medium, 10% FCS, 100 U penicillin/streptomycin), and pseudotyped with vesicular stomatitis virus capsid protein. MMA/virus capsid protein was used to transduce GP + E + 86 cells27 and repackage the vector with the murine ecotropic ENV protein. Cellular GP + E + 86 clones were made by limited dilution of the transduced pool. Titer was determined on National Institutes of Health 3T3 fibroblasts, and a producer clone was determined by the amount of ADA enzyme activity (∼ 120 U/mg/min).
Mice
A 2-stage murine model of ADA-deficient SCID was generated and described previously (FVB;129-Ada tm1Mw Tg(PLADA)4118Rkmb/J; The Jackson Laboratory).28,29 ERT was administered by weekly intramuscular injection of 250 U/kg of Adagen (Sigma-Tau Pharmaceuticals Inc). Mice were housed in accordance with the Institutional Animal Care and Use Committees (Saban Research Institute at Children's Hospital Los Angeles) and the National Institutes of Health guidelines. All animals were handled in laminar flow hoods and housed in microinsulator cages in a pathogen-free colony.
BM harvest, lineage depletion, and HSCT
Ada−/− donor mice (8-10 weeks) were euthanized, and marrow was harvested from the femur, tibia, and humeral bones; pooled; and centrifuged for 10 minutes at 400g at 10°C. Pooled marrow was plated on fibronectin-fragment CH-296 (20 mg/mL, Retronectin; Takara Bio Inc)–coated plates at 1 × 106 cells/mL in basal bone marrow media (Iscove modified Dulbecco medium supplemented with 30% FCS, 1% BSA [StemCell Technologies], 100μM 2-β-mercaptoethanol, 2mM glutamine, 100 U/mL penicillin/streptomycin) freshly supplemented with murine cytokines (IL-3: 10 ng/mL; IL-6: 25 ng/mL, and c-kit ligand: 2.5 ng/mL; Biosource International, Inc) and cultured at 37°C for 48 hours before stimulation. Pooled marrow cells were lineage-depleted with antimurine, biotin-conjugated, antibodies: CD5, CD45R (B220), CD11b, and Anti–Gr-1 (Ly-6G/C, 7-4, and Ter-119) according to the manufacturer's instructions (Murine Lineage Depletion Kit; Miltenyi Biotec). Lineage-depleted cells (lin−) were resuspended in basal bone marrow media at 1 × 106 cells/mL and plated, as described previously, for prestimulation for 48 hours. Congenic (Ada+/+) female donor mice (8-10 weeks) were used as controls for transplantation of fully ADA-replete marrow, with 5 × 106 unprocessed marrow cells suspended in injectable normal saline (USP; APP) injected via the tail vein into male Ada−/− mice (8-10 weeks).30
Transduction
After 48 hours, one-half of the medium plus nonadherent cells in the flask were collected and centrifuged at 400g for 10 minutes. The supernatant was discarded, and the cells were resuspended in the same volume of MMA vector supernatant, supplemented with 4 μg/mL polybrene (Sigma-Aldrich), and returned to the flasks. After 24 hours, the transduction procedure was repeated with fresh vector supernatant.
Cytoablative conditioning and transplantation
Ada−/− recipient mice (8-10 weeks) were conditioned with total body irradiation (TBI) on the day of BM transplantation (0, 200, or 900 cGy) from a 137Cesium source at a dose rate of 95 cGy/min. The nonadherent transduced cells were transferred to a 50-mL conical tube, and the adherent cells were harvested with cell-dissociation buffer (Invitrogen) for 15 minutes at 37°C. Transduced marrow cells were resuspended in 0.9% injectable sodium chloride at 5.0 × 107 cells/mL, and transduced lin− marrow cells were resuspended at 5.0 × 106 cells/mL. Mice were injected via the tail vein with either 5 × 106 whole marrow (wm) cells or 5.0 × 105 lin− marrow cells and were maintained on 50 mg/mL (in drinking water) Maxim 200 (orthotetracyclin supplied as 200 mg/mL; Phoenix Pharmaceuticals) for 21 days.
Necropsy, tissue harvest, and immunohistochemical analysis
Mice were killed and perfused via cardiac puncture with 15 mL of PBS. The thymus, spleen, liver, and right lungs were harvested into ice-cold PBS with 1.0% FCS (PBS-1%FCS) in a 6-well plate. Fragments of thymus, spleen, and liver were fixed, and the left lung was perfused and fixed with 2% paraformaldehyde. Immunohistochemistry was performed as described with an antihuman ADA monoclonal antibody (N-19; Santa Cruz Biotechnology).31
Immunophenotype and function assays
All immunophenotype and functional assays were performed as previously described.30,32 Single-cell suspensions were made from thymus, spleen, and BM and immunophenotyped by flow cytometry. T-cell function was assessed by an in vitro mitogenic stimulation assay, using concanavalin A. B-cell function was assessed in vivo by vaccination with Pneumovax 23 (Merck; 10 μL in 100 μL of injectable saline) followed by measurement of serum antibodies after 10 days by ELISA32 ).
VCN determination
DNA was extracted with phenol/chloroform, resuspended in TE, and quantified using DNA-specific fluorometry (Hoechst dye, DNA Quantification Kit; Sigma-Aldrich). Vector copy number (VCN) was determined by real-time quantitative PCR (qPCR). Primer/probe set was vector specific to MMA: sense primer (5′-tcaatgcggccaaatctagtt), antisense primer (5′-tggactaatcgataccgtcgac-3′), and the TAMRA probe sequence (6FAM-tcgacctgctctataaagcctatgggatgc-TAMRA). The final concentration of the primers was 400nM, and the probe was 50nM. All reactions used Universal Master Mix (Applied Biosystems Inc [ABI]) and were run under default conditions in the 7900 Sequence Detector System (ABI). For each sample, 350 ng of DNA were interrogated for vector sequence (in duplicate) and compared with a standard curve produced by serially diluting (2.0-0.00002 vector copies) the DNA of a cellular clone that contained 2 copies of the MMA γ-retroviral vector as determined by Southern blot.
Engraftment
The TSYR gene, located on the Y chromosome, was used to determine the amount of chimerism or engraftment in sex-mismatched transplants. To quantify the percentage of male DNA, qPCR was performed with a standard curve constructed from male murine tail DNA diluted into female murine tail DNA to form mixtures between 0.3% and 100% male DNA.33
ADA enzyme activity and substrate concentration in tissues
Tissues were harvested as parts of whole organs and flash frozen and stored at −80°C until processed. ADA enzyme assay was performed as described.29 Perchloric acid was used to extract adenine nucleosides from frozen tissue, and Ado and dAdo were separated and quantified by the use of reverse-phase high-pressure liquid chromatography as described.34
Statistical analysis
Descriptive statistics of continuous outcome variables, such as the means and SE by experimental groups, are presented in figures. For each experiment, overall group difference was identified by the ANOVA approach.35 If normality assumption is violated, nonparametric Kruskal-Wallis analysis of variance was used.36 Within the ANOVA/Kruskal-Wallis test framework, we performed estimation and hypothesis testing by comparing group differences under various experimental conditions. For all statistical investigations, tests for significance are 2-tailed, with a statistically significant P value threshold of .05. Statistical analyses were carried out with SAS Version 9.2.37
Results
In mice, ADA deficiency results in profound multisystem defects, including SCID. If untreated, Ada−/− pups succumb to a noninfectious, pulmonary insufficiency by postnatal d20; thus, survival after GT is a potent test for efficacy. In a series of sequential experiments, BM from Ada−/− mice was transduced ex vivo with the MMA γ-retroviral vector and transplanted into syngeneic Ada−/− recipients. The first study assessed the role of cytoreduction before GT and the role of ERT after GT, the second study assessed efficacy of a short-term course of ERT after GT compared with a long-term course, and the third study assessed differences between transplanting transduced wm compared with transduced lin− marrow cells with and without mild cytoreductive conditioning and/or ERT.
Study 1: cytoreductive conditioning and ERT cessation
Transduction efficiency and survival.
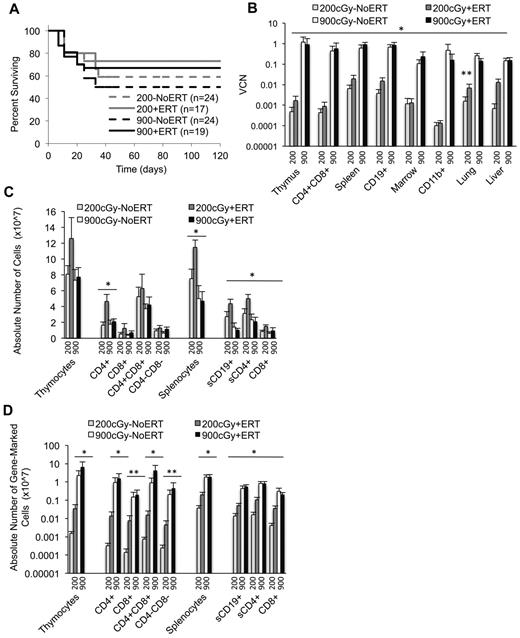
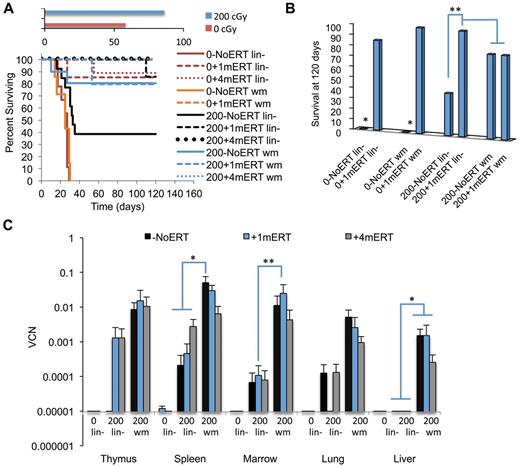
Marrow was harvested from Ada−/− male donors (8 weeks) and transduced with the MMA γ-retroviral vector (Figure 1A). Recipient Ada−/− mice (8-10 weeks) were conditioned with 200 or 900 cGy TBI and then each received 5 × 106 transduced wm cells. Recipient mice were maintained on ERT (200+ERT and 900+ERT) or not maintained on ERT (200−NoERT or 900−NoERT) and analyzed at 4 months. VCN at the end of the transduction period was determined by qPCR and ranged from 0.1-1.2 copies/cell in 5 different experiments (Figure 1B). There were no significant differences in survival between mice receiving 900 cGy or 200 cGy, regardless of whether they were maintained on ERT after GT (Figure 2A).
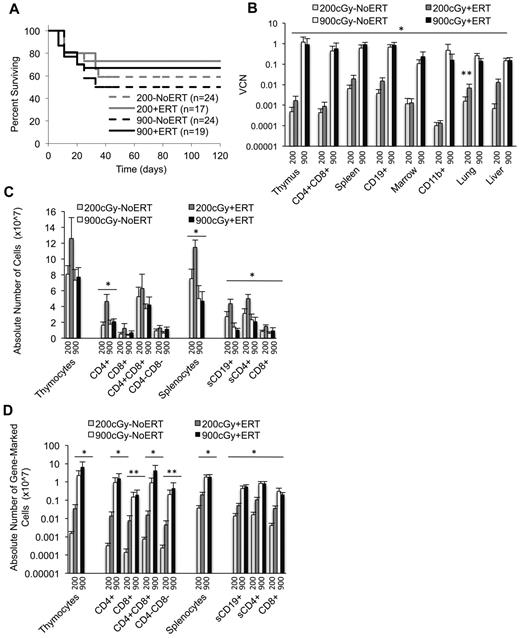
γ-retroviral−mediated gene transfer into murine ADA-deficient marrow cells. BM cells from Ada−/− donor mice were transduced with the MMA γ-retroviral vector and administered to age-matched ADA-deficient recipients conditioned either with 200 or 900 cGy of TBI. After transplantation, the recipients were either maintained on PEG-ADA ERT (+ERT) or not (−ERT). (A) Map of the MMA γ-retroviral vector construct carrying a normal human Ada cDNA (HuAda). EcoRV restriction sites in the long-terminal repeats are indicated. SA (splice acceptor site), SD (splice donor site), and residual sequences from the Moloney Murine Leukemia Virus gag and env 5′-untranslated region (env utr) are indicated. (B) VCN by qPCR of BM harvested from Ada−/− donors after transduction with the MMA vector for 5 separate transplantation experiments.
γ-retroviral−mediated gene transfer into murine ADA-deficient marrow cells. BM cells from Ada−/− donor mice were transduced with the MMA γ-retroviral vector and administered to age-matched ADA-deficient recipients conditioned either with 200 or 900 cGy of TBI. After transplantation, the recipients were either maintained on PEG-ADA ERT (+ERT) or not (−ERT). (A) Map of the MMA γ-retroviral vector construct carrying a normal human Ada cDNA (HuAda). EcoRV restriction sites in the long-terminal repeats are indicated. SA (splice acceptor site), SD (splice donor site), and residual sequences from the Moloney Murine Leukemia Virus gag and env 5′-untranslated region (env utr) are indicated. (B) VCN by qPCR of BM harvested from Ada−/− donors after transduction with the MMA vector for 5 separate transplantation experiments.
Survival, VCNs, and absolute numbers of lymphocytes (total and gene-marked) after conditioning with 200 cGy and 900 cGy of TBI. Tissues were harvested 4 months after recipients received transduced cells. VCN was determined via qPCR. Thymic subpopulations and splenic subpopulations were determined by flow cytometry. The absolute numbers of lymphocytes were determined by multiplying the percentage of cells in a subpopulation by the total number of lymphocytes in the lymphoid tissue. The absolute numbers of gene-marked cells were determined by multiplying the absolute numbers of thymocytes or splenocytes by the average VCN in thymus or spleen, respectively. (A) Survival of recipients. Mice were killed and analyzed at day 120. Total mice in 5 experiments. (B) VCN in tissue cell suspensions and isolated cell populations from the thymus (CD4/8), spleen (CD19), and marrow (CD11b) determined by qPCR for vector sequence. (900+ERT, n = 14; 900−ERT, n = 12; 200+ERT, n = 12; 200−NoERT, n = 8; mean ± SEM). *Significantly greater marking with 900 cGy compared with 200 cGy (P < .05). **Significantly greater marking with ERT compared with without ERT (P < .05). (C) Absolute numbers of thymocyte and splenocyte populations (mean ± SEM). *Significantly greater number of cells with 200+/ERT compared with 900 ± ERT (P < .05). (D) Absolute numbers of gene-marked thymocyte and splenocyte populations (mean ± SEM). *Significantly greater number of marked cells with 900+ERT compared with 200+ERT and 900−NoERT compared with 200−NoERT (P < .05). **Significantly greater number of gene-marked cells with 900+ERT compared with 200+ERT only (P < .05).
Survival, VCNs, and absolute numbers of lymphocytes (total and gene-marked) after conditioning with 200 cGy and 900 cGy of TBI. Tissues were harvested 4 months after recipients received transduced cells. VCN was determined via qPCR. Thymic subpopulations and splenic subpopulations were determined by flow cytometry. The absolute numbers of lymphocytes were determined by multiplying the percentage of cells in a subpopulation by the total number of lymphocytes in the lymphoid tissue. The absolute numbers of gene-marked cells were determined by multiplying the absolute numbers of thymocytes or splenocytes by the average VCN in thymus or spleen, respectively. (A) Survival of recipients. Mice were killed and analyzed at day 120. Total mice in 5 experiments. (B) VCN in tissue cell suspensions and isolated cell populations from the thymus (CD4/8), spleen (CD19), and marrow (CD11b) determined by qPCR for vector sequence. (900+ERT, n = 14; 900−ERT, n = 12; 200+ERT, n = 12; 200−NoERT, n = 8; mean ± SEM). *Significantly greater marking with 900 cGy compared with 200 cGy (P < .05). **Significantly greater marking with ERT compared with without ERT (P < .05). (C) Absolute numbers of thymocyte and splenocyte populations (mean ± SEM). *Significantly greater number of cells with 200+/ERT compared with 900 ± ERT (P < .05). (D) Absolute numbers of gene-marked thymocyte and splenocyte populations (mean ± SEM). *Significantly greater number of marked cells with 900+ERT compared with 200+ERT and 900−NoERT compared with 200−NoERT (P < .05). **Significantly greater number of gene-marked cells with 900+ERT compared with 200+ERT only (P < .05).
Cytoreduction.
After 4 months, the mean VCN was 100- to 1000-fold greater in mice conditioned with 900 cGy (0.11-1.2 copies/cell) compared with mice conditioned with 200 cGy (0.0007-0.006 copies/cell) for all tissues analyzed (P < .05; Figure 2B). Mean thymus VCN was approximately 1000-fold greater with 900 cGy (0.9 +ERT, 1.2 NoERT) compared with 200 cGy (0.002 +ERT, 0.0005 NoERT), and the mean spleen VCN was 100-fold greater with 900 cGy (0.9 +ERT, 0.62 NoERT) compared with 200 cGy (0.02 +ERT, 0.006 NoERT). To assess the effect of TBI dosage on the numbers of gene-corrected cells, the absolute numbers of gene-marked cells were calculated by multiplying the absolute numbers of cells by the VCN (expressed as percent transduced; Figure 2C-D). By this measure, the mean absolute numbers of gene-marked lymphocytes were significantly greater in mice conditioned with 900 cGy compared with mice conditioned with 200 cGy regardless of ERT treatment (P < .05). When there was a sex-mismatched donor, qPCR was used to quantify donor male Y-chromosome sequences in the recipient marrow. Donor engraftment was 30% in mice conditioned with 900 cGy compared with < 1% in those receiving 200 cGy, indicating that cytoreductive conditioning is very important for donor cell engraftment.
ERT.
Ada−/− mice conditioned with 900 cGy or 200 cGy had no significant differences in VCN or in the absolute numbers of gene-marked cells in the thymus, whether or not they received ERT (Figure 2B-D). However 200+ERT mice had greater numbers of lymphocytes (CD4+, CD8+, CD19+) compared with 200−NoERT mice (P < .05), presumably because of rescue of uncorrected lymphocytes. However, because neither VCN nor the absolute numbers of gene-marked cells decreased with continued ERT, ERT was not detrimental to the survival and expansion of gene-corrected cells despite dilution of the vector sequences with uncorrected cell DNA. As seen in our previous studies with Ada−/− mice, mitogenic proliferation assays showed that mature lymphocytes (corrected or uncorrected) were functional in vitro (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).30,32
Study 2: long-course ERT and short-course ERT
Experimental design.
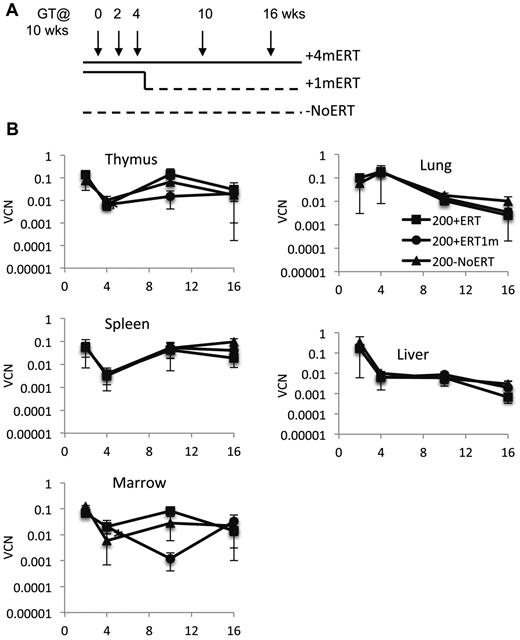
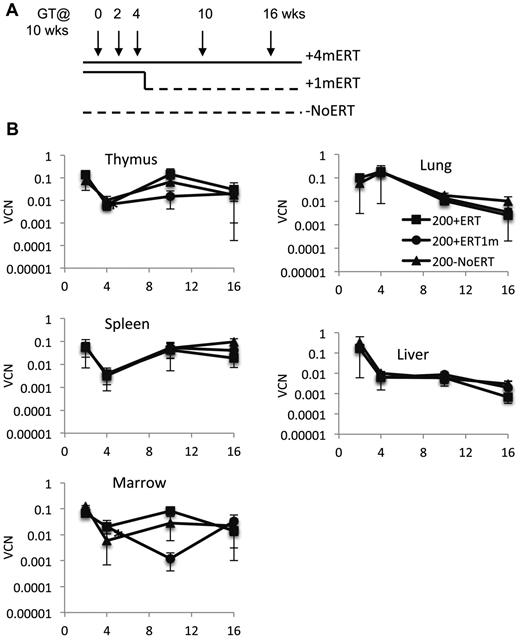
Because long-term ERT after GT did not appear to be detrimental to the engraftment, survival, and/or expansion of the corrected cells, we evaluated the effects of short-term ERT compared with long-term ERT. Recipient mice were conditioned with 200 cGy TBI and received γ-retrovirally transduced ADA-deficient wm cells and maintained on ERT for 4 months after GT (200 + 4mERT), or for only the first month after GT (200 + 1mERT), or not at all after GT (200−NoERT; Figure 3A). Treated mice were analyzed at 2, 4, 10, and 16 weeks.
Effects of ERT on survival, VCN, absolute numbers of lymphocytes and gene-marked lymphocytes, and their function after GT. (A) Experimental schema: Ada−/− were conditioned with 200 cGy of TBI, transplanted with transduced marrow, and either remained on ERT for the duration of the experiment (200 + 4mERT: solid line), remained on ERT for 1 month after GT (200 + 1mERT: solid line to dotted line), or did not remain on ERT after GT (200−NoERT: dotted line). Recipients were analyzed at 2, 4, 10, and 16 weeks (per arm: 2 weeks; n = 2, 4 weeks; n = 3, 10 weeks; n = 4, 16 weeks, n = 4). See Figure 2 for lymphocyte subpopulation analysis and calculation of absolute values (mean ± SEM). (B) VCN. VCN in tissue cell suspensions were measured by qPCR for vector sequence (mean ± SEM). *Significantly lower at 4 weeks than at 2 weeks (P < .05).
Effects of ERT on survival, VCN, absolute numbers of lymphocytes and gene-marked lymphocytes, and their function after GT. (A) Experimental schema: Ada−/− were conditioned with 200 cGy of TBI, transplanted with transduced marrow, and either remained on ERT for the duration of the experiment (200 + 4mERT: solid line), remained on ERT for 1 month after GT (200 + 1mERT: solid line to dotted line), or did not remain on ERT after GT (200−NoERT: dotted line). Recipients were analyzed at 2, 4, 10, and 16 weeks (per arm: 2 weeks; n = 2, 4 weeks; n = 3, 10 weeks; n = 4, 16 weeks, n = 4). See Figure 2 for lymphocyte subpopulation analysis and calculation of absolute values (mean ± SEM). (B) VCN. VCN in tissue cell suspensions were measured by qPCR for vector sequence (mean ± SEM). *Significantly lower at 4 weeks than at 2 weeks (P < .05).
Vector marking.
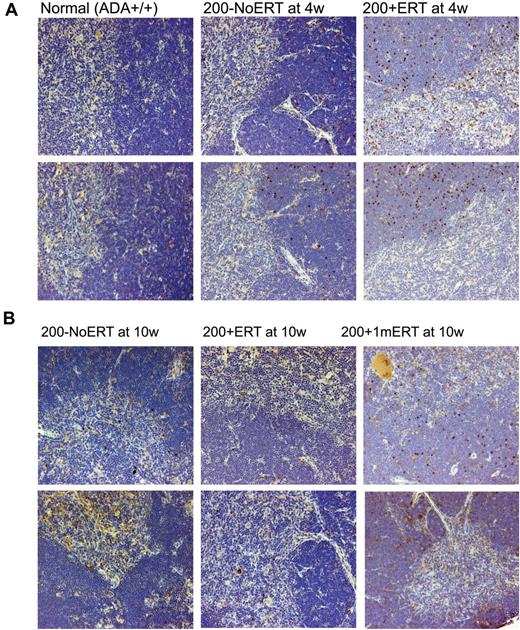
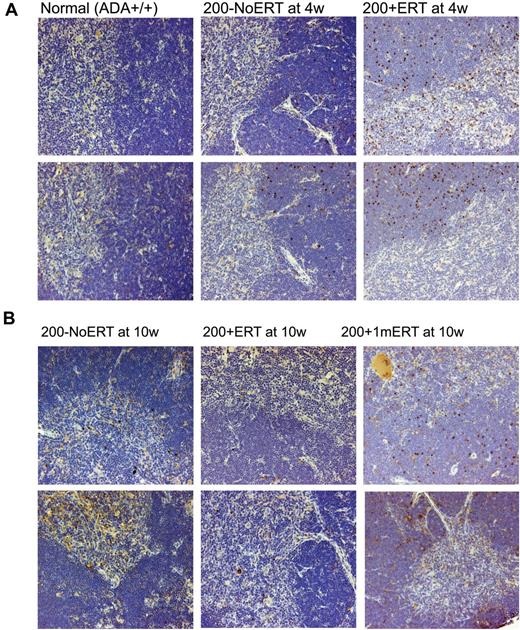
VCN was similar across all time points in all tissues analyzed, independent of ERT treatment (Figure 3B). Compared with mice not receiving ERT, the absolute numbers of gene-marked thymocytes from mice receiving ERT was significantly greater only at the earliest time point (2 weeks) immediately after GT but not at subsequent time-points (P < .05), probably because of the loss of uncorrected cells without ERT (supplemental Figure 2A-D). Immunohistologic staining of thymi with an antibody specific for human ADA demonstrated the presence of stained (brown) lymphocytes distributed throughout the thymic cortex and medulla, indicative of human ADA expression at 4 and 10 weeks, regardless of ERT treatment (Figure 4A-B). Thus, ERT after GT with mild cytoreduction (200 cGy) did not affect the survival of Ada gene–corrected thymocytes.
Immunohistochemical analysis of thymus from ADA-deficient mice after GT with long- and short-term ERT. Perfused thymi were fixed, sectioned, and stained with antihuman ADA antibody (N-19; Santa Cruz Biotechnology) that was specific to human ADA and did not cross-react with murine ADA. Cells containing immune-reactive protein are stained brown. Micrographs of stained sections were made with an Olympus BX40 Microscope (Olympus America Inc) and an Olympus DP11 Camera (Olympus America Inc) All micrographs were made at room temperature, without the use of imaging medium, fluorochromes, or acquisition software. Magnification: (optical 10) total magnification ×200. (A) Thymi from 2 different normal control (Ada+/+) mice, ×200 + 1mERT, mice and 200−NoERT mice at 4 weeks. (B) Thymi from 2 different 200 + ERT mice, 200−NoERT mice, and 200 + 1mERT mice at 10 weeks.
Immunohistochemical analysis of thymus from ADA-deficient mice after GT with long- and short-term ERT. Perfused thymi were fixed, sectioned, and stained with antihuman ADA antibody (N-19; Santa Cruz Biotechnology) that was specific to human ADA and did not cross-react with murine ADA. Cells containing immune-reactive protein are stained brown. Micrographs of stained sections were made with an Olympus BX40 Microscope (Olympus America Inc) and an Olympus DP11 Camera (Olympus America Inc) All micrographs were made at room temperature, without the use of imaging medium, fluorochromes, or acquisition software. Magnification: (optical 10) total magnification ×200. (A) Thymi from 2 different normal control (Ada+/+) mice, ×200 + 1mERT, mice and 200−NoERT mice at 4 weeks. (B) Thymi from 2 different 200 + ERT mice, 200−NoERT mice, and 200 + 1mERT mice at 10 weeks.
ADA enzyme activity and substrate concentration.
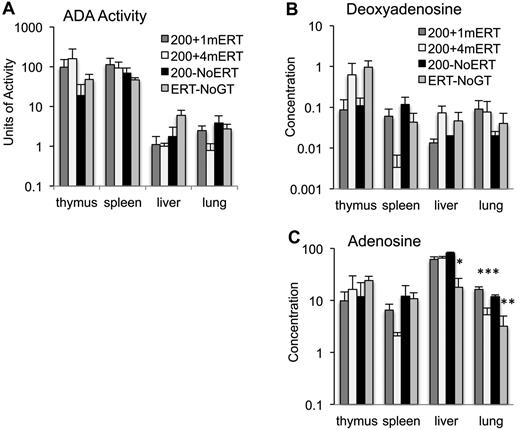
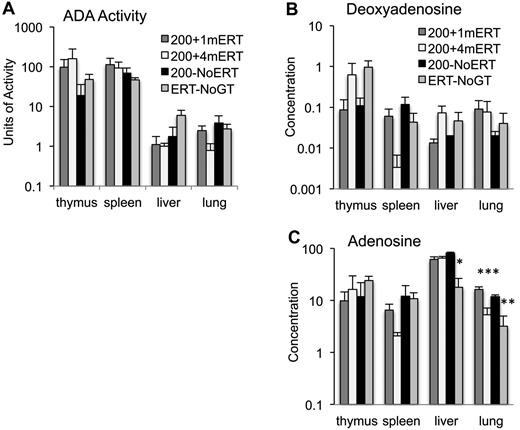
ADA enzyme activity and deoxyadenosine concentrations measured in tissues at 10 weeks were not significantly different in mice maintained on ERT or not (Figure 5A-B). Lung adenosine concentrations were lower in mice maintained on ERT (200 + 4mERT) compared with mice not continuously maintained on ERT (200 + 1mERT and 200−NoERT; P < .05). Ada−/− mice are particularly sensitive to the accumulation of adenosine in lung tissue, and perhaps extracellular ADA (ERT) is more efficacious than intracellular (GT) because extracellular adenosine–mediated signaling through G-coupled adenosine receptors has been implicated in the pulmonary phenotype.39
Effect of ERT on ADA enzyme activity and adenine substrate concentrations. (A) ADA enzyme activity (nmoles/mg/min) was measured in tissues 4 months after GT with 1 month, 4 months, or 0 months ERT (mean ± SEM). (B) Deoxyadenosine concentration (nmoles/mg of protein) in tissues 4 months after GT with 1 month, 4 months, or 0 months ERT (mean ± SEM). (C) Adenosine concentration (nmoles/mg protein) in tissues 4 months after GT with 1 months, 4 months, or 0 months ERT (mean ± SEM). *Significantly lower adenosine levels in ERT-NoGT mice compared with mice in other conditions (P < .05). **Significantly lower adenosine levels in ERT-NoGT compared 200 + 1mERT and 200 + 4mERT (P < .05). ***Significantly lower in 200 + 4mERT compared with 200 + 1mERT.
Effect of ERT on ADA enzyme activity and adenine substrate concentrations. (A) ADA enzyme activity (nmoles/mg/min) was measured in tissues 4 months after GT with 1 month, 4 months, or 0 months ERT (mean ± SEM). (B) Deoxyadenosine concentration (nmoles/mg of protein) in tissues 4 months after GT with 1 month, 4 months, or 0 months ERT (mean ± SEM). (C) Adenosine concentration (nmoles/mg protein) in tissues 4 months after GT with 1 months, 4 months, or 0 months ERT (mean ± SEM). *Significantly lower adenosine levels in ERT-NoGT mice compared with mice in other conditions (P < .05). **Significantly lower adenosine levels in ERT-NoGT compared 200 + 1mERT and 200 + 4mERT (P < .05). ***Significantly lower in 200 + 4mERT compared with 200 + 1mERT.
Study 3: cytoreduction, ERT, and target cell
In studies 1 and 2, whole BM was transduced and infused into recipients. However, in the clinical setting, patients are infused with transduced autologous CD34+ marrow cells, a population enriched ∼ 50-fold for human HSC. The following study was designed to simulate conditions closer to approaches used in current clinical trials for ADA-SCID. Although the CD34 marker is not useful for enrichment of murine HSC, lineage-depleted murine BM cell preparations are similarly enriched ∼ 50-fold for murine HSC. Ada−/− mice were conditioned with 0 cGy (0) or 200 cGy (200) TBI and transplanted with either transduced wm or transduced lin− and maintained on ERT for 4 months (+4mERT), 1 month (+1mERT), or ERT was stopped after GT (−NoERT).
Survival.
Unlike ADA-deficient patients, survival can be used as a measure of efficacy (engraftment of gene-marked HSC) in the Ada−/− mice because ADA deficiency is a lethal condition unrelated to the SCID phenotype. Overall, mice conditioned with 200 cGy of TBI had greater survival than unconditioned mice, regardless of transduced cell type (wm or lin−) or ERT course of treatment (P < .01; Figure 6A). When unconditioned mice were not maintained on ERT, survival was significantly reduced compared with unconditioned mice that were maintained on a short course of ERT (P < .01; Figure 6B). In the condition closest to current clinical GT protocols for ADA-SCID (nonmyeloablative conditioning and no ERT), survival was also significantly reduced in mice conditioned with 200 cGy and transplanted with lin− and not maintained on ERT compared with mice maintained on ERT for 1 or 4 months (P < .01). As seen previously, for mice conditioned with 200 cGy and transplanted with wm, there were no differences in survival with or without ERT.
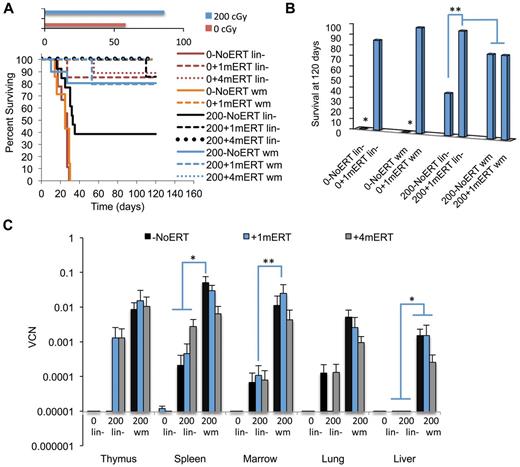
Survival and VCN 4 months after GT with transduced lin− cells or transduced wm cells. The recipients were conditioned with either 0 cGy or 200 cGy and either remained on ERT long term (4 months), short term (1 months), or not at all. (A) Survival. Solid lines are as follows: −NoERT: 0−NoERT lin− (n = 9), 0−NoERTwm (n = 7), 200−NoERT lin− (n = 13), 200−NoERTwm (n = 8). Dashed lines are as follows: +1mERT: 0 + 1mERTlin− (n = 7), 0 + 1mERTwm (n = 4), 200 + 1mERTlin− (n = 7), 200 + 1mERTwm (n = 4). Dotted lines are +4mERT: 0 + 4mERTlin− (n = 7), 200 + 4mERTlin− (n = 11), 200 + 4mERTwm (n = 10). Survival was decreased with no conditioning compared with 200 cGy TBI (P < .0001). (B) Percent survival at day 120. *Survival with no conditioning and no ERT (0−NoERT) after infusion of transduced lin− or transduced wm cells was significantly decreased compared with all others (P < .01). **Survival in 200−NoERTlin− mice was significantly less than 200 + 1mERTlin− or 200 + 4mERTlin− mice (P < .05). (C) VCNs in cell suspension of tissues analyzed 4 months after GT: 200−NoERTlin− (n = 5), 200 + 1mERTlin− (n = 6), 200 + 4mERTlin− (n = 11), 200−NoERTwm (n = 8), 200 + 1mERTwm (n = 4), or 200 + 4mERTwm (n = 10; means ± SEM). *Spleen VCN is significantly greater in 200−NoERTwm compared with 200−NoERTlin−, 200 + 1mERTlin−, 200 + 4mERTlin−, and 200 + 4mERTwm (P < .05). **Marrow VCN is significantly greater in 200 + 1mERTwm compared with 200 + 1mERTlin− (P < .05).
Survival and VCN 4 months after GT with transduced lin− cells or transduced wm cells. The recipients were conditioned with either 0 cGy or 200 cGy and either remained on ERT long term (4 months), short term (1 months), or not at all. (A) Survival. Solid lines are as follows: −NoERT: 0−NoERT lin− (n = 9), 0−NoERTwm (n = 7), 200−NoERT lin− (n = 13), 200−NoERTwm (n = 8). Dashed lines are as follows: +1mERT: 0 + 1mERTlin− (n = 7), 0 + 1mERTwm (n = 4), 200 + 1mERTlin− (n = 7), 200 + 1mERTwm (n = 4). Dotted lines are +4mERT: 0 + 4mERTlin− (n = 7), 200 + 4mERTlin− (n = 11), 200 + 4mERTwm (n = 10). Survival was decreased with no conditioning compared with 200 cGy TBI (P < .0001). (B) Percent survival at day 120. *Survival with no conditioning and no ERT (0−NoERT) after infusion of transduced lin− or transduced wm cells was significantly decreased compared with all others (P < .01). **Survival in 200−NoERTlin− mice was significantly less than 200 + 1mERTlin− or 200 + 4mERTlin− mice (P < .05). (C) VCNs in cell suspension of tissues analyzed 4 months after GT: 200−NoERTlin− (n = 5), 200 + 1mERTlin− (n = 6), 200 + 4mERTlin− (n = 11), 200−NoERTwm (n = 8), 200 + 1mERTwm (n = 4), or 200 + 4mERTwm (n = 10; means ± SEM). *Spleen VCN is significantly greater in 200−NoERTwm compared with 200−NoERTlin−, 200 + 1mERTlin−, 200 + 4mERTlin−, and 200 + 4mERTwm (P < .05). **Marrow VCN is significantly greater in 200 + 1mERTwm compared with 200 + 1mERTlin− (P < .05).
Marking.
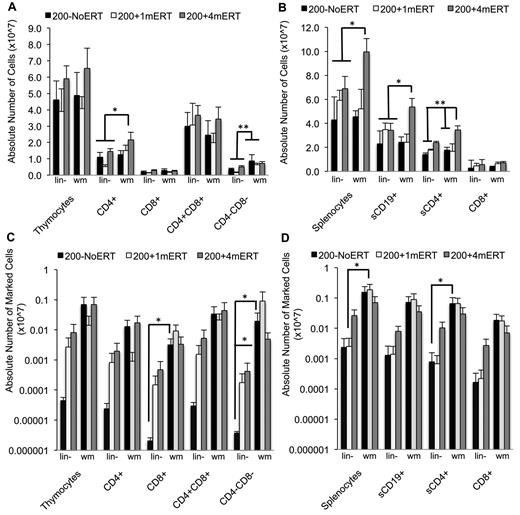
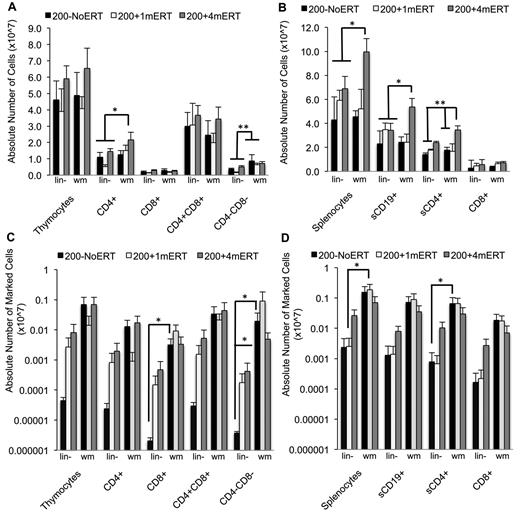
Overall, mice receiving transduced wm had 10-100× greater BM VCN compared with mice receiving lin− regardless of ERT course (P < .05; Figure 6C). For most tissues analyzed, ERT did not affect VCN or numbers of gene-marked cells when mice received transduced wm. Mice transplanted with transduced wm had greater gene-marked thymocytes (CD8+ and CD4−CD8−) compared with mice transplanted with lin− (P < .05). Although spleen VCN was significantly greater in mice not maintained on ERT (200−NoERTwm) compared with mice maintained on ERT (200 + 4mERTwm), there were no differences in the number of gene-marked cells (P < .05), probably because the absolute numbers of splenocytes were significantly increased with ERT, indicating uncorrected and corrected splenocyte survival was supported with ERT. Mice transplanted with transduced wm with no ERT had greater numbers of gene-marked splenic lymphocytes (total, sCD4+, and sCD19+) compared with mice transplanted with transduced lin− with no ERT (P < .05), suggesting that the target cell population is an important factor in the engraftment of transduced cells in the absence of ERT.
In mice transplanted with lin− without conditioning (0 + 1mERTlin− or 0 + 4mERTlin−) there were few gene-marked lymphocytes present (Figure 7A-D). Furthermore, there was a complete lack of detectable vector sequences in the thymi of mice receiving nonmyeloablative conditioning and no ERT (200−NoERTlin− mice), the condition most similar to current clinical GT protocol. However, if the mice were maintained on ERT for 1 or 4 months (200 + 1mERTlin− or 200 + 4mERTlin−), vector marking was detected. These results demonstrate that cytoreduction is very important in the engraftment of transduced cells and that a short course of ERT may be beneficial, especially when transplanting a transduced, enriched HSC population.
Absolute numbers of lymphocytes and gene-marked lymphocytes and their function 4 months after GT with transduced lin− cells or transduced wm cells. (A) Absolute numbers of thymocytes (mean ± SEM). *Significantly greater in 200 + 4mERTwm mice compared with 200−NoERTwm, 200−NoERTlin−, and 200 + 1mERTlin− (P < .05). **Significantly greater in all mice receiving transduced wm compared with transduced lineage-depleted marrow (P < .05). (B) Absolute numbers of splenocytes (mean ± SEM). *Significantly greater in 200 + 4mERT wm mice compared with all other conditions (P < .05). **Significantly greater in 200 + 4mERTwm mice compared with all other conditions except 200 + 4mERTlin− (P < .05). (C) Absolute numbers of gene-marked thymocytes (mean ± SEM). *Significantly greater than 200−NoERTlin− (P < .05). (D) Absolute numbers of gene-marked splenocytes (mean ± SEM). *Significantly greater than 200−NoERTlin− (P < .05).
Absolute numbers of lymphocytes and gene-marked lymphocytes and their function 4 months after GT with transduced lin− cells or transduced wm cells. (A) Absolute numbers of thymocytes (mean ± SEM). *Significantly greater in 200 + 4mERTwm mice compared with 200−NoERTwm, 200−NoERTlin−, and 200 + 1mERTlin− (P < .05). **Significantly greater in all mice receiving transduced wm compared with transduced lineage-depleted marrow (P < .05). (B) Absolute numbers of splenocytes (mean ± SEM). *Significantly greater in 200 + 4mERT wm mice compared with all other conditions (P < .05). **Significantly greater in 200 + 4mERTwm mice compared with all other conditions except 200 + 4mERTlin− (P < .05). (C) Absolute numbers of gene-marked thymocytes (mean ± SEM). *Significantly greater than 200−NoERTlin− (P < .05). (D) Absolute numbers of gene-marked splenocytes (mean ± SEM). *Significantly greater than 200−NoERTlin− (P < .05).
Discussion
Initial GT trials for ADA-deficient SCID patients used enriched populations of BM CD34+ cells, patients were not given cytoreductive conditioning, and they remained on ERT.10-13 These studies did not demonstrate clinical efficacy because the frequency of gene-corrected cells was low. It was hypothesized that omission of cytoreductive marrow conditioning limited HSC engraftment and that the continuous administration of ERT blunted any selective survival advantage the corrected lymphocytes may have had over the uncorrected cells. Thus, in subsequent clinical trials, patients have been treated with nonmyeloablative doses of busulfan or melphalan and ERT was discontinued before reinfusion of the autologous, transduced CD34+ cells.14,19-22
The premise for these protocol changes was 2-fold: cytoreductive conditioning would “make space” in the marrow compartment and ERT cessation would allow the transduced, corrected lymphocytes to realize their survival advantage. This approach has resulted in a high frequency of gene-marked cells, especially in the T-cell compartment, and has had a clear clinical benefit for the majority of patients.19,22 However, despite the clinical benefits achieved, it was not possible to determine the individual roles of cytoreduction and ERT because both changes were implemented together. Although both factors may add to efficacy, they also add to potential risks, from chemotherapy-related toxicities and from an increased risk of infection because of lymphopenia that occurs after ERT withdrawal. In one study, ADA-deficient SCID patients receiving HSC GT with ERT cessation but without conditioning had delayed immune reconstitution,38 supporting the role for cytoreduction. As GT becomes the standard of care for ADA-deficient SCID, it will be important to understand the roles of conditioning with enhanced engraftment of transduced HSC and the cessation of ERT, with increased selective advantage for gene-corrected cells for achieving best clinical outcomes. We explored these 2 issues in gene transfer/BMT studies in ADA gene knockout mice.
Cytoreductive conditioning
ADA-deficient mice die postnatal d20-21 unless treated with ERT, HSCT, or GT. If treatment is discontinued (ERT; < 14 days) or ineffective (HSCT/GT), the adult mice will eventually die of pulmonary insufficiency (pathogen-free housing); thus, survival can be a measure of efficacy of a treatment. In our studies, greater intensity conditioning consistently led to greater levels of gene-marked cells in lymphoid and nonlymphoid organs. Mice receiving 900 cGy always had greater VCN (10- to 1000-fold) in the thymus, spleen, marrow, lung, and liver than mice receiving 200 cGy, regardless of whether ERT was continued. Also, the absolute numbers of gene-marked cells in the thymus and spleen were 100- to 1000-fold greater in the 900 cGy mice compared with the 200 cGy mice. Furthermore, mice not conditioned (0 cGy) had poor survival without short (1-month)– or long (4-month)–term ERT and VCN was dramatically reduced.
These results indicate that the TBI dose of cytoreductive conditioning is critical for determining the amount of engraftment and expansion of transduced HSC and progenitors, as has been shown in other murine models of GT.39 Similarly, in clinical trials of GT for ADA-deficient SCID, patients with the longest period of neutropenia after conditioning with busulfan had the greatest frequency of gene-marked cells19 and when 2 protocols were used, one with and without busulfan, marking is greatly improved with busulfan. It is important to note that radiation is not equivalent to busulfan, and that the possible effects of radiation should be considered when interpreting these results. Mice receiving 900 cGy did have lower total numbers of immature and mature lymphocytes than those receiving 200 cGy, suggesting that the greater dose of radiation may damage the lymphoid organ stroma and impair immune reconstitution.40
ERT
In the early clinical trials, ERT was reduced in a few patients at various times after GT, which resulted in an increase in the measured frequency of gene-marked cells in peripheral blood T lymphocytes.13,14 In one ADA-deficient patient who experienced a spontaneous reversion of a mutant Ada allele in a progenitor cell, ADA-replete T cells that arose were obscured when the patient resumed ERT.41 From these results, it was hypothesized that continued ERT may blunt the selective advantage of the gene-corrected cells, resulting in a lower number of corrected cells and vector sequence detection.42 However, when only the average VCN is measured (eg, qPCR for vector sequence in tissue DNA), it would be difficult to distinguish between a decrease in vector detection from reduced selective pressure from a decrease in VCN from dilution of vector sequence by the increased amount of uncorrected cell DNA. Therefore, to understand whether there is a true diminution of the absolute numbers of gene-corrected cells, we calculated the absolute number of gene-marked cells in tissues when ADA-deficient mice remained on ERT or not. It is of interest that we found no difference in the absolute numbers of gene-marked cells in any analyzed tissue when mice received 900 cGy before receiving transduced whole marrow cells, with or without ERT. Likewise, we found no difference in the absolute numbers of gene-marked lymphocytes from mice receiving cytoreduction with 200 cGy, with or without ERT, despite increases in the absolute numbers of lymphocytes with ERT and apparent dilution of vector sequences in gene-corrected cell DNA by uncorrected cell DNA. Likewise, a few subjects in ADA GT clinical trials have been restarted on ERT after GT and PBMC DNA VCN did not decrease.19-22 Thus, it could be argued that the frequency/survival of gene-corrected cells actually increased on ERT because VCN remained the same despite the putative dilution of vector sequence with continued ERT.
We cannot say for sure how ERT improves survival of gene-corrected cells, but we hypothesize ERT may augment the ADA supplied by the gene-corrected thymocytes by providing a good “soil” for thymopoiesis to further expand the T-cell population because it has been shown that CD4−CD8− thymocyte differentiation is disrupted and apoptosis is initiated when ADA substrates accumulate in ADA-deficient fetal thymic organ culture.43 Study 3 also supports this hypothesis. When mice received lineage-depleted marrow with 200 cGy and no ERT (the closest condition to current clinical trial approaches), vector sequences were only detected in the thymus when mice remained on ERT for 1 or 4 months.
Whole marrow versus lineage-depleted marrow
Mice transplanted with transduced whole marrow (5.0 × 106 total cells) had engraftment and detectable gene-marking in thymus, spleen, marrow, lung, and liver regardless of ERT course. However, in mice transplanted with transduced lineage-depleted marrow (5.0 × 10−5 total cells), and a theoretically greater HSC dose, there was no detectable vector in thymus and lower numbers of gene-marked lymphocytes detected without ERT compared with mice transplanted with whole marrow without ERT. It is not clear what mechanism(s) is responsible for the enhanced engraftment of transduced HSC and/or development of gene-corrected lymphocytes when whole marrow is transplanted instead of lineage-depleted marrow, or how ERT after GT enhances engraftment with lineage-depleted marrow. Transplanting transduced whole marrow may provide one or more of the following: (1) a more effective HSC dose, (2) cells that enhance the BM niche and support hematopoiesis, and (3) transient ADA expression from transduced mature marrow cells, creating a detoxified microenvironment and/or improving the function of other cells residing in the niche.
Transduced whole marrow may represent a more effective HSC dose because there is virtually no processing/enrichment of HSC and their stromal support remains intact. In matched sibling HSCT, ADA-deficient patients receive unmanipulated, whole marrow where 100% of the cells are ADA replete, with 2 functional copies of the ADA gene per cell. Even nonengrafting cells may provide enough ADA early in the engraftment period to detoxify the microenvironment for engraftment and for other facilitating cells residing in the BM niche. Indeed, Sauer et al found that within the BM niche, ADA-deficient mice have decreased osteoblast function in situ, suggesting an altered BM microenvironment.44 Furthermore, they found decreased colony formation when Ada−/− lineage-depleted cells were cocultured with Ada−/− stromal feeder layers compared with ADA+/+ stromal feeder layers. These parameters were all reversed with ERT, HSCT, and GT in Ada−/− mice.
Likewise, transduced whole marrow will also contain many types of transduced cells expressing ADA, albeit not all are transduced with 2 copies/cell as in HSCT. We hypothesize that many transduced cells will not achieve long-term engraftment but could provide ADA for detoxification of the niche, as well as transduced stromal cells that may be important for HSC localization within the BM niche and other tissues. Unexpectedly, we found VCN was greater in liver and lung from recipients of transduced whole marrow compared with lineage-depleted marrow cells, regardless of ERT treatment. Both lung and liver exhibit significant pathology in the ADA-deficient mouse, and transduced whole marrow may have other hematopoietic and mesenchymal cells that facilitate “liver/lung engraftment.”45 Although it is not clear how relevant this observation is to the human experience, a recent study showed that many ADA-deficient patients, unlike X-linked SCID patients, presented with lung pathology not associated with an infectious agent and with histopathology similar to that observed in ADA-deficient mice.45
Although the mechanism for improved engraftment is not fully elucidated, these studies suggest early ADA expression from transduced whole marrow from exogenously supplied ERT combined with transduced lineage-depleted marrow cells may act to enhance the marrow and thymic microenvironments to allow for the engraftment and development of gene-corrected cells. These results, combined with the clinical experience in HSCT and GT in ADA-deficient patients, strongly suggest a positive role for ERT during the early engraftment period after GT, especially with transplant of HSC-enriched transduced cells and nonmyeloablative conditioning.
Future clinical protocol development
The data presented here indicate that cytoreduction conditioning probably plays a larger role in the success of GT in current clinical trials than the cessation of ERT. Post-GT ERT did not diminish the absolute numbers of gene-marked cells and may even have improved engraftment and development of gene-corrected cells. These findings raise important questions about the clinical benefit of ERT cessation before GT for ADA-SCID. There may be a role for short-term ERT in the early engraftment period after cytoreductive conditioning and infusion of gene-corrected cells, with the understanding that the goal of GT is to be curative and not to have patients remain on ERT. At a minimum, there appears to be no detriment to the development and survival of gene-corrected cells from providing ERT for 1 month after GT. ADA-deficient patients may have a quicker emergence of gene-corrected T cells and may experience fewer infections if a short course of ERT is continued after GT.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Renee Traub Workman for the excellent animal care.
This work was supported by grants from the National Institutes of Health to D.B.K. (HL073104 and AI074043), including the Cores for Animal Care, Research Vectors, and Cell & Tissue Analysis. Enzon Pharmaceuticals Inc and Sigma-Tau Pharmaceuticals Inc provided Adagen for these studies, facilitated by Lynn Gordon of Enzon and Kristine David of Sigma-Tau.
This work is in memory of X.-J.Y., a contributing author on this paper.
National Institutes of Health
Authorship
Contribution: D.A.C., X.J., X-J.Y., M.L.K., Y.Z., and M.R.B. performed experiments; X.W. prepared the immunohistologic specimens; N.R. performed immunohistologic/pathologic analysis; X.W. and D.G. performed statistical analysis; and D.A.C. and D.B.K. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denise Ann Carbonaro Sarracino, PhD, Department of Microbiology, Immunology and Molecular Genetics, UCLA, 3159 TLSB, 610 Charles E. Young Dr South, Los Angeles, CA 90095; e-mail: dsarracino@ucla.edu.