Abstract
Therapy of relapsed pediatric acute lymphoblastic leukemia (ALL) is hampered by low remission rates and high toxicity, especially in second and subsequent relapses. Our phase 1 study, T2005-003, showed that the combination of bortezomib with vincristine, dexamethasone, pegylated asparaginase, and doxorubicin had acceptable toxicity. We report the phase 2 expansion of this combination in patients with relapsed ALL who failed 2-3 previous regimens. Twenty-two patients with relapsed ALL were treated with bortezomib combined with this regimen; their ages ranged from 1 to 22 years, and they had either B-precursor ALL (n = 20) or T-cell ALL (n = 2). Grade 3 peripheral neuropathy developed in 2 (9%) patients. After 3 patients died from bacterial infections, treatment with vancomycin, levofloxacin, and voriconazole prophylaxis resulted in no further infectious mortality in the last 6 patients. Fourteen patients achieved complete remission (CR), and 2 achieved CR without platelet recovery, for an overall 73% response rate, meeting predefined criteria allowing for early closure. B-precursor patients faired best, with 16 of 20 (80%) CR + CR without platelet recovery, whereas the 2 patients with T-cell ALL did not respond. Thus, this combination of bortezomib with chemotherapy is active in B-precursor ALL, and prophylactic antibiotics may be useful in reducing mortality. Bortezomib merits further evaluation in combination therapy in pediatric B-precursor ALL. This study is registered at http://www.clinicaltrials.gov as NCT00440726.
Introduction
Outcomes after bone marrow (BM) relapse remain poor in pediatric patients with acute lymphoblastic leukemia (ALL), especially after the second or subsequent relapse. Even marrow remission may prove elusive. Gaynon reviewed a variety of phase 2 studies and found that remission rates clustered around 40% for combination chemotherapy in the second and subsequent relapse.1 Investigators from the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) consortium retrospective study found remission rates of 44%, 27%, and 12% with 3rd, 4th, and subsequent therapeutic attempts, respectively.2
Bortezomib, the first proteasome inhibitor approved by the Food and Drug Administration for multiple myeloma and relapsed non-Hodgkin lymphoma, showed preclinical activity against ALL. However, little single-agent activity was observed in relapsed leukemia in adults3 or children.4 Preclinical studies demonstrated that bortezomib was synergistic with dexamethasone in vitro and additive with asparaginase, vincristine, doxorubicin, and cytarabine.5 Anecdotally, a child with ALL who experienced multiple relapses previously had transient clinical response with the administration of bortezomib and dexamethasone.6
In 2010, we reported the phase 1 results of our TACL study, T2005-003 and showed that a bortezomib 1.3 mg/m2/dose on days 1, 4, 8, and 11 might be safely combined with vincristine, dexamethasone, pegylated asparaginase, and doxorubicin (VXLD).7 We now report the planned phase 2 expansion and our findings that this regimen is indeed tolerable and active in B-precursor ALL.
Methods
TACL study T2005-003 was approved by the institutional review boards of all participating TACL centers. An independent Data Safety Monitoring Committee monitored study progress and patient safety. Individual and/or parental informed consent was obtained from all subjects as per local and federal requirements. Eligible patients had relapsed B-precursor or T-cell ALL with ≥ 25% BM blasts by morphology and were < 21 years of age at original diagnosis and > 1 year of age at study entry. CNS involvement was allowed. For the phase 2 expansion reported here, patients were eligible only after they failed 2 or 3 previous treatment regimens. Patients were eligible after allogeneic stem cell transplantation (ie, BM transplatation) as long as they were not being treated actively for graft-versus-host disease. A Karnofsky (patients > 10 years of age) or Lansky (≤ 10 years of age) score ≥ 50 was required at study entry. Patients with allergies to Escherichia coli asparaginase product, boron, or mannitol, with lifetime exposure to > 350 mg/m2 of anthracyclines (in doxorubicin equivalent dosing), or with left ventricular fractional shortening < 30% were excluded from participation. Other exclusion criteria were as follows: serum creatinine greater than the upper limit of normal (ULN) for age; direct bilirubin > 1.5 times the institutional ULN; alanine transaminase > 4 times ULN; a history of pancreatitis; serum amylase or lipase > 2 times ULN; or active uncontrolled infection. Because neuropathy has been described for both bortezomib and vincristine, patients with preexisting ≥ grade 2 motor or sensory neuropathy also were excluded.
Treatment
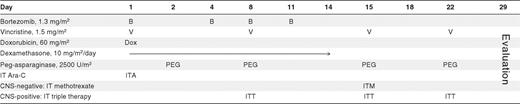
The treatment regimen is shown in Table 1. Bortezomib 1.3 mg/m2/dose was administered intravenously by push on days 1, 4, 8, and 11. Dexamethasone 10 mg/m2/day divided into 2 doses was given orally for 14 consecutive days. Doxorubicin 60 mg/m2 was given intravenously over 15-30 minutes on day 1. Vincristine 1.5 mg/m2/dose (2 mg maximum dose) was administered intravenously by push day 1, 8, 15, and 22. Pegylated asparaginase 2500 units/m2/dose was given intramuscularly as 4 weekly doses. All patients received cytarabine intrathecal (IT) on day 1. Patients who had no CNS involvement with ALL (≤ CNS 2) were treated with 1 methotrexate IT dose on day 15. Patients with CNS involvement (CNS 3) were given methotrexate, hydrocortisone, and cytarabine IT (IT triple therapy) on days 8, 15, and 22.
Toxicity evaluation
Toxicity was graded via use of the Common Terminology Criteria for Adverse Events (CTCAE) Criteria Version 3.0.8 Bortezomib was immediately discontinued for significant reactions as previously described.7 Patients could continue with the platform therapy excluding bortezomib. Pegylated asparaginase was discontinued for anaphylaxis, symptomatic pancreatitis, significant bleeding, or significant thrombosis. Neither pegylated asparaginase nor dexamethasone doses were modified for hyperglycemia. The decision to hold or continue vincristine after peripheral neuropathy developed was left to the local investigator.
After 3 patients died from bacterial infections, significant supportive care modifications were made for the remaining 6 patients enrolled. We required prophylactic vancomycin and levofloxacin, as well as inpatient hospitalization stay from day 8 until neutrophil recovery to > 500/μL. We also required prophylaxis with either voriconazole or posaconazole from day 1 to day 36.
Response evaluation
BM aspirate/biopsy and complete blood count (CBC) to assess response were performed on day 29. A BM procedure was not required if the patient had an absolute blast count ≥ 2500/μL in the peripheral blood on day 29. If the marrow was hypoplastic, a repeat marrow aspiration and CBC were requested weekly until recovery or progression. If the patient had < 5% lymphoblasts in the BM (M1 marrow) but did not achieve an absolute neutrophil count (ANC) > 750/μL and a platelet count > 75 000/μL, CBC and BM evaluation were requested weekly until count recovery or relapse.
Standard definitions of ALL response were used in this study and are as follows: complete remission (CR) was defined as M1 (< 5% blasts) BM with no evidence of circulating blasts or extramedullary disease and with recovery of peripheral counts (ANC > 750/μL and platelet count > 75 000/μL); CR without platelet recovery (CRp) was defined as M1 BM with no circulating blasts or extramedullary disease and recovery of ANC (> 750/μL) but insufficient recovery of platelets (< 75 000/μL). Partial remission (PR) was defined as the complete disappearance of circulating blasts and achievement of M2 (5%-25% blasts) marrow status, without new sites of extramedullary disease, and with recovery of ANC (> 750/μL). Stable disease (SD) was defined as not satisfying the criterion for progressive disease (PD), or has a recovery of ANC (> 750/μL) but failing to qualify for CR, CRp, or PR. PD was defined as an increase of at least 25% in the absolute number of circulating leukemia cells, development of new sites of extramedullary disease, or other laboratory or clinical evidence of PD, with or without recovery of ANC or platelets. Minimal residual disease was not evaluated as a part of this study.
Statistical methods
Achievement of CR at the completion of 1 course of therapy was the primary end point for this phase 2 expansion. Patients who did not complete reinduction therapy because of toxicity or complications of disease were considered evaluable for response and classified as nonresponders. A 3-stage group sequential design was used, with interim analyses after 6, 14, and 28 patients. In stage 1, ≥ 2 of 6 CRs were required to proceed to stage 2, and ≥ 7 of 14 CRs were then required to proceed to the final stage, with ≥ 14 of 28 patients in CR at the end of the study indicating sufficient efficacy. The study could be terminated also as soon as 14 patients with CR were confirmed. This design has 10% 1-sided type I error at a CR rate of 0.38 and 20% type I error at a CR rate of 0.42, 80% power to detect a CR rate of 0.60, and at least 90% power to detect a CR rate of 0.65. The design benchmarks were selected with reference to an historical response rate of 0.39 ± 0.031 in patients who have experienced multiple relapses, which was later confirmed in a TACL retrospective study,2 and our desire to identify candidate regimens with CR rate at least 20% points greater than this historical reference. The maximum likelihood estimates of response rate in the context of the multistage design differed negligibly from the simple binomial estimate, so the latter are presented.
Secondary end points included the CTCAE 3.0 toxicities and grade during the course of therapy and Kaplan-Meier estimate of overall survival (OS). The OS was calculated from time of enrollment and also from the end of induction. The log-rank test was used to assess any differences in OS from the end of induction by response type (CR/CRp vs nonresponse). Permutation test9 (10 000 rearrangements) was used to confirm the results of the log-rank test.
Results
Twenty-two patients were included in the phase 2 expansion. Demographic, clinical data, and treatment-related information are displayed in Table 2. Twenty (91%) had B-precursor ALL, and 2 (9%) had T-cell ALL. Seventeen patients (77%) were enrolled on the study after failing 2 previous regimens and 5 patients (23%) after failing 3 previous regimens. Four had relapsed after BM transplantation. All patients had > 25% marrow blasts at study entry.
Efficacy
The CR rate was 14 of 22, or 64% (95% exact confidence interval 41%-83%), and CRp rate was 2/22 (9%) for overall response rate of 73% (95% exact confidence interval 50%-89%; Table 3). The study reached its predefined early stopping rule for efficacy when 14 CRs were observed among the first 22 patients enrolled.
Nineteen patients completed the course, and 3 patients died from bacterial sepsis. BM response was not available for the patients who died. One patient achieved an M1 marrow status (< 5% blasts) but then received additional chemotherapy before peripheral blood count recovery, contrary to protocol, and is counted as a nonresponder.
Outcome
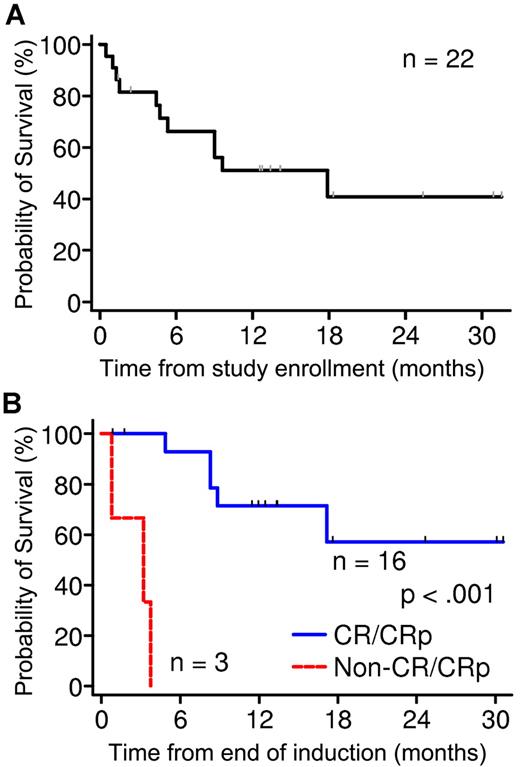
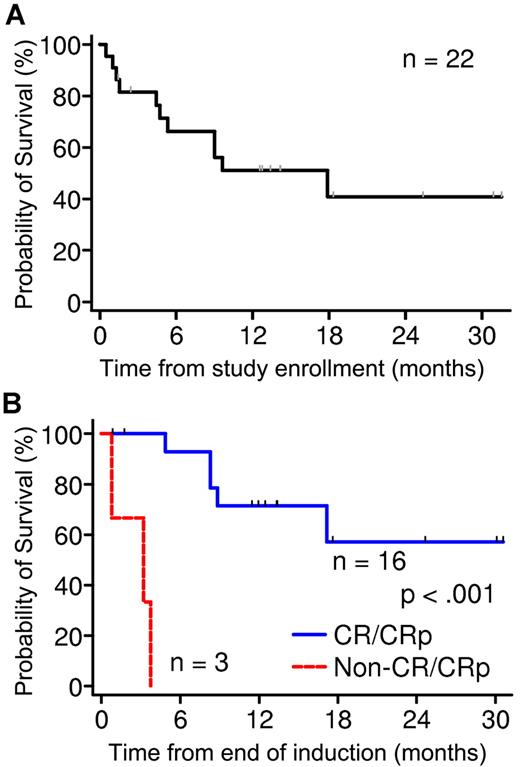
Overall, 3 patients died, and 19 completed induction (Table 2). Eight died after induction, and 11 were alive at 1.4-31.4 months at last contact (8 in remission and 3 with disease). Overall survival at 24 months is estimated to be 41% ± 13%, with 4 patients surviving > 18 months (Figure 1A). Among patients who completed reinduction, postinduction survival was significantly better in those who responded (CR or CRp) compared with those who did not (P < .001; Figure 1B). Nine patients underwent hematopoietic stem cell transplantation, and 8 are alive (7 are still in remission).
Survival analysis of phase 2 patients. (A) OS of all 22 patients in phase 2 at 24 months is estimated to be 41% ± 13%. (B) Comparison of survival of patients who completed reinduction. Survival was significantly better in those who responded (CR or CRp) compared with those who did not (P < .001).
Survival analysis of phase 2 patients. (A) OS of all 22 patients in phase 2 at 24 months is estimated to be 41% ± 13%. (B) Comparison of survival of patients who completed reinduction. Survival was significantly better in those who responded (CR or CRp) compared with those who did not (P < .001).
Toxicity
All 22 patients completed 4 planned doses of bortezomib (Table 2). Details of the grades 3-5 regimen-related toxicity are shown in Table 4. In total, 10 of 22 patients had infection ≥ grade 3. There were 3 septic deaths in this phase 2 part, with multiorgan failure (culture-positive: Stenotrophomonas maltophilia, E coli with Staphylococcus Hominis, E coli) on days 15, 31, and 40 of protocol therapy. One of these patients experienced cerebral ischemia as part of the multiorgan failure. One patient had grade 4 hypoxia and grade 4 pleural effusion secondary to fatal bacterial sepsis. Another patient developed grade 4 hypoxia secondary to bacterial sepsis that resolved completely. One patient experienced invasive mucor infection of the sinuses, skin, and lung on day 15 and required sinus debridement and orbital exploration and treatment with liposomal amphotericin, caspofungin, posaconazole, and GM-CSF. This patient was removed from protocol therapy and achieved CRp but died after 5 months. After the 3 septic deaths, in consultation with the Data Safety Monitoring Committee, mandatory prophylactic therapy with vancomycin intravenously, levofloxacin, and voriconazole (or posaconazole) and inpatient management was required. Subsequently, no further cases of bacteremia or fungal infections were reported in the last 6 patients enrolled.
Two patients developed grade 3 peripheral neuropathy on day 15. Both patients had received all 4 doses of bortezomib before the onset of peripheral neuropathy, and 1 dose of vincristine was omitted for 1 of them. One of them had previous vincristine neuropathy, which had resolved before study entry but then reflared. Both patients have improved to grade 2 but are left with residual neuropathy at 2.5 and 30 months of follow-up.
No patient developed BM aplasia, and responding patients achieved platelets > 75 000/μL at median day 35 (range, 27-41) and ANC > 750/μL at median day 38 (range, 27-48; Table 2).
Low fibrinogen and thrombosis were uncommon (but also were expected in a regimen that includes asparaginase). Coagulopathy and lower gastrointestinal hemorrhage followed E coli sepsis in 1 patient.
Three patients experienced grade 3 asymptomatic hypophosphatemia. Hyponatremia, hypocalcemia, and hypokalemia were seen and have been described with bortezomib10 but did not result in drug discontinuation (Table 4). Other metabolic toxicities (hyperglycemia, hypoalbuminemia, elevated lipase and amylase, and liver enzyme abnormalities) were rare and expected with platform therapy that includes dexamethasone and pegylated asparaginase. One patient had encephalopathy with signs of adrenal insufficiency immediately after completing dexamethasone and improved with hydrocortisone replacement.
Discussion
This study confirms our earlier report that bortezomib in combination with VXLD is tolerable at 1.3 mg/m2 intravenously on days 1, 4, 8, and 11.7 Lethal bacterial sepsis was the principal toxicity encountered in 3 of the first 16 patients. Using a regimen of vincristine, dexamethasone, idarubicin, and pegylated asparaginase without bortezomib in patients with ALL who replasped, the Children's Oncology Group (COG) found an unacceptably high infectious mortality, resulting in the replacement of dexamethasone by prednisone and replacement of idarubicin by doxorubicin.11 We opted for a different approach, namely, starting prophylaxis with vancomycin and levofloxacin from day 8 of therapy.12-15 On the basis of the fungal infections observed in the phase 17 and in 1 of the patients treated on the phase 2, voriconazole or posaconazole were used from day 1. We found no further bacterial or fungal morbidity or mortality in the last 6 patients. Routine antibiotic and antifungal prophylaxis may be warranted in such a vulnerable, heavily pretreated population.
Severe lung injury is described in adults treated with bortezomib.16-19 In 31 patients on this TACL T2005-003 protocol (phase 1 and phase 2), pleural effusions, hypoxia, and/or pneumonitis were associated with bacterial infection. Thus, severe lung injury was not found in this population, possibly because of the protective effect of dexamethasone that is given in parallel to bortezomib, or because of the young age of the subjects.
Peripheral neuropathy is common in adults treated with bortezomib, mostly after repeated courses,20 but was not seen in the pediatric single-agent trial.4 Our regimen combined bortezomib with vincristine, which is also a neurotoxin. During phase 1, 2 of the 9 patients had transient grade 1-2 peripheral neuropathy.7 In phase 2, 2 patients experienced grade 3 motor and sensory peripheral neuropathy that have yet to resolve. Of note, 1 of these patients had previous vincristine neuropathy that flared after exposure to bortezomib and VXLD. Certainly, future studies in which investigators combine vincristine with bortezomib, and the addition of voriconazole to these drugs, should be closely monitored for peripheral neuropathy.
In the phase 1 study, no BM aplasia was seen. Here we confirm that this regimen does not lead to BM aplasia. However, recovery may be moderately prolonged. Median platelet recovery time was day 35 but can be delayed to day 41. Similarly, although median ANC recovery time was day 38, it can be delayed to day 48. Such delay in BM recovery is not surprising for these heavily pretreated patients.
Historically, patients in second or subsequent relapse (failing 2 or more previous regimens) achieved a remission rate of approximately 40% with a variety of multiagent regimens.1,2 In this phase 2 study, all patients failed 2-3 previous regimens. The response rate of 73% (80% for B-precursor ALL) was significantly better than previous trials. This response rate allowed the early termination of this trial before the planned 28 patients were accrued. To our knowledge, this is the first and only pediatric ALL phase 2 study that has been stopped early because of superior response. Although bortezomib lacks single-agent activity in ALL,3,4 our results may reflect the synergistic and additive effects of bortezomib with chemotherapy demonstrated in vitro5 and in a previous patient.6
Although numbers are small, a remarkably high response rate in B-precursor ALL was observed with 80% response rate and 85% BM response rate at the end of 1 course of therapy. Combining the phase 1 and 2 data, the 24 B-precursor ALL patients who were treated with bortezomib at 1.3 mg/m2 had similar remission rate of 83% (75% CR and 8% CRp). As expected, achieving a remission resulted in survival benefit supporting previous observation that induction remission is a prerequisite for survival in multiple relapsed patients.10,21 Four patients survived longer than 18 months, which is remarkable for patients who were in second or subsequent relapse.
Both patients with T-cell ALL did not respond to this regimen. In T-cell ALL, a regimen of bortezomib, dexamethasone, and liposomal doxorubicin induced remission in a patient in first relapse but only reduced the blast count in another patient in second relapse.22 A reduction of NF-κB binding activity was also demonstrated. The COG is treating a larger group of T-cell ALL patients in first relapse on a similar phase 2 protocol, and the results are not yet known.
The molecular basis for the apparent synergy of bortezomib with VXLD is not clear. Proteasome inhibition induces apoptosis in tumor cells via the intrinsic mitochondrial pathway, the extrinsic death-receptor pathway and the endoplasmic reticulum stress response pathway.23-25 This activity may be a consequence of the multiple effects of proteasome inhibition: deregulation of the turnover of cyclins and disruption of cyclin-dependent kinase activity; c-Jun N-terminal kinase (JNK) stabilization and FAS (tumor necrosis factor receptor superfamily member 6; TNFRSF6) up-regulation; stabilization of p53, a tumor suppressor; activation of caspase-8; shifting of the proapoptotic and antiapoptotic balance in the Bcl-2 family of proteins; and reactive oxygen species generation.26,27 NF-κB is constitutively active in ALL and in most in vitro studies bortezomib was thought to inhibit NF-κB. However, recent reports indicate that bortezomib in fact induces NF-κB, instead of inhibiting it,28,29 suggesting that bortezomib is unlikely to mediate its anticancer effects through suppression of NF-κB.30 Endoplasmic reticulum stress via the unfolded protein response seems to be a critical mediator of chemotherapy resistance in B-precursor ALL and is a target of not only bortezomib but other new agents showing intriguing activity against B-precursor ALL.31-33 Evaluating the unfolded protein response in patients treated with this regimen may be an important biologic correlate for future studies.
The authors recognize that a limitation of this study is the small number of patients who were treated. The results of the ongoing COG phase 2 study for ALL in first relapse, AALL07P1, which has a similar but not identical platform with prednisone in place of dexamethasone, may support our findings in B-precursor ALL and further clarify the activity in T-cell ALL.
In conclusion, the regimen of bortezomib combined with the 4-drug induction—vincristine dexamethasone, pegylated asparaginase, and doxorubicin—is highly effective and tolerable for children with relapsed B-precursor ALL. This regimen merits evaluation in randomized studies on childhood B-precursor ALL protocols. For highly vulnerable patients with ALL who have relapsed multiple time, preliminary evidence suggests that vancomycin, levofloxacin, and voriconazole may be an effective prophylaxis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Rally for Kids with Cancer Foundation, the University of Southern California–Children's Hospital Los Angeles Institute for Pediatric Cancer Research, and the Children's Hospital Los Angeles Children's Center for Cancer and Blood Diseases.
Authorship
Contribution: Y.H.M. was the principal investigator and takes primary responsibility for the paper; Y.H.M., P.S.G., R.S., and B.C.B. designed the study; R.S. and J.M. provided statistical analysis; J.v.d.G. and E.E. recruited the patients, collected the data, and helped with data analysis; and Y.H.M., P.S.G., R.S., J.M., and B.C.B. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: Y.H.M. declares serving 1 time on the advisory board of Sanofi Aventis (Genzyme). P.S.G. declares serving on the advisory boards of EUSA, Sanofi Aventis (Genzyme), and Seattle; Genetics; chairs a DMC for Bristol Meyers Squibb; and gave a talk for Sigma Tau. The remaining authors declare no competing financial interests.
The TACL group investigators are listed in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Yoav H. Messinger, MD, Pediatric Hematology and Oncology, Children's Hospitals and Clinics of Minnesota, 2525 Chicago Ave S, Minneapolis, MN 55404; e-mail: yoav.messinger@childrensmn.org.