Abstract
Sickle cell disease (SCD) is associated with a complex vascular pathophysiology that includes activation of coagulation and inflammation. However, the crosstalk between these 2 systems in SCD has not been investigated. Here, we examined the role of tissue factor (TF) in the activation of coagulation and inflammation in 2 different mouse models of SCD (BERK and Townes). Leukocytes isolated from BERK mice expressed TF protein and had increased TF activity compared with control mice. We found that an inhibitory anti-TF antibody abrogated the activation of coagulation but had no effect on hemolysis or anemia. Importantly, inhibition of TF also attenuated inflammation and endothelial cell injury as demonstrated by reduced plasma levels of IL-6, serum amyloid P, and soluble vascular cell adhesion molecule-1. In addition, we found decreased levels of the chemokines MCP-1 and KC, as well as myeloperoxidase in the lungs of sickle cell mice treated with the anti-TF antibody. Finally, we found that endothelial cell-specific deletion of TF had no effect on coagulation but selectively attenuated plasma levels of IL-6. Our data indicate that different cellular sources of TF contribute to activation of coagulation, vascular inflammation, and endothelial cell injury. Furthermore, it appears that TF contributes to these processes without affecting intravascular hemolysis.
Introduction
Sickle cell disease (SCD) is caused by a single nucleotide mutation that substitutes glutamic acid with valine at the sixth position of the β-globin protein.1-3 Under hypoxic conditions, polymerization of mutant hemoglobin tetramers results in the formation of sickled red blood cells that are less flexible, prone to hemolysis, and adhere to the endothelium. This primary event results in the obstruction of the microvasculature and intravascular hemolysis.1-3 However, it is thought that multiple, highly interconnected biologic processes contribute to the pathophysiology of SCD.2
SCD is also associated with chronic vascular inflammation.4 Vaso-occlusive episodes within postcapillary venules result in tissue ischemia and inflammation. Subsequent reperfusion of the ischemic tissue leads to oxidative stress, vascular injury, increased expression of adhesion molecules on the endothelium, and further enhancement of inflammation.1,2,4 Patients with SCD have increased numbers of circulating leukocytes and platelets, as well as elevated plasma levels of various cytokines, soluble adhesion molecules, and C-reactive protein (CRP).4,5 Similarly, transgenic sickle mice, such as the BERK model, have leukocytosis, increased plasma levels of IL-6, and serum amyloid P (SAP), which is the mouse homolog of human CRP.6
Another prominent feature of SCD is the activation of coagulation.7 Increased plasma levels of tissue factor (TF)–positive microparticles (MPs), thrombin antithrombin complexes (TAT), prothrombin fragment F1.2, and D-dimers have been reported in humans with SCD.7 Furthermore, TF-positive monocytes as well as plasma levels of TAT and D-dimer correlate with measures of hemolysis and anemia (lactate dehydrogenase [LDH], indirect bilirubin, and hemoglobin) and levels of soluble vascular cell adhesion molecule-1 (sVCAM-1), a marker of endothelial cell activation.8 In mouse models of SCD, increased TF expression has been observed in the endothelium of the pulmonary microvasculature and in circulating monocytes.9 Endothelial cell TF expression required activation of NF-κB in mononuclear cells and was reduced by endothelial nitric oxide synthase or lovastatin.9-11 Furthermore, it has been reported that a genetic deficiency of TF in nonhematopoietic cells reduces vascular congestion in the livers of sickle cell mice.12
In animal models of endotoxemia, sepsis, and ischemia-reperfusion injury, TF-dependent activation of coagulation enhances inflammation.13-16 This observation indicates that there is a crosstalk between coagulation and inflammation in a variety of pathologic states. A recent study demonstrated that inhibition of TF or thrombin, as well as neutrophil depletion, attenuates enhanced thrombosis in the cerebral microvessels of mice expressing the sickle form of hemoglobin, suggesting a possible link between inflammation and thrombosis in this disease state.17 However, a detailed analysis of the contribution of TF to the pathophysiology of SCD has not been performed.
In this study, we determined the effects of TF inhibition and a genetic deficiency of TF in endothelial cells on activation of coagulation, endothelial cell activation, and vascular inflammation in 2 different mouse models of SCD.
Methods
Mice
We used BERK mice on a mixed genetic background (FVB/N, 129, DBA/2, C57BL/6, and Black Swiss).18 BERK mice have a transgene containing normal human α-, γ-, δ-globins and sickle β-globin and targeted deletions of murine α- and β-globins (α−/−, β−/−,Tg). We generated these mice by intercrossing α−/−, β−/−,Tg males with α−/−, β+/−,Tg females. As a control, we used wild-type (WT) mice on the similar mixed genetic background that have no human transgenes (α+/+, β+/+). Mice 4 to 6 months old were used. In addition, we used Townes mice that have both human α- and β- (βA and βS form) globin genes knocked into the mouse locus, allowing the generation of littermate AA and SS mice (here referred to as TWAA and TWSS) after intercrossing TWAS mice.19 The generation of TFflox/flox,Tie-2 Cre+ mice has been previously described.20 All mouse experiments were approved by the University of North Carolina Animal Care and Use Committees and complied with National Institutes of Health guidelines.
Bone marrow transplantation
C57BL/6 recipient mice (8 weeks old) were lethally irradiated with 14 Gy (2 doses of 7 Gy 4 hours apart) to ablate bone marrow cells using a Cesium-137 irradiator (MarkI Irradiator; J. L. Shepherd & Associates). Recipient mice were then injected via the retro-orbital sinus with 2 × 106 nucleated bone marrow cells from BERK mice or WT controls. These mice will be referred to as BERKBM or WTBM. Mice were used for experiments 4 to 5 months after bone marrow transplantation. Reconstitution of bone marrow was determined by cellulose acetate electrophoretic analysis of hemoglobin type (Helena Laboratories).
Administration of anti-TF antibody and sample collection
Mice were treated with an intraperitoneal injection of rat anti–mouse TF (1H1) or control rat IgG antibodies (20 mg/kg) on days 0, 3, and 6 and were killed at day 7. Blood was drawn from the inferior vena cava into sodium citrate–anticoagulated syringes (final ratio = 9:1) and centrifuged at 4000g for 15 minutes at 4°C. Plasma was collected and immediately stored at −80°C for further analysis. In clotting assays, the inhibitory activity of 1H1 was observed only when samples and antibody were allowed to preincubate before addition of plasma, indicating that 1H1 binds to TF and competes for binding with factor VII.21
Generation of sickle cell mice with an endothelial cell-specific deletion of the TF gene
Nonsickle and sickle cell mice with either normal expression of TF or endothelial cell-specific deletion of the TF gene were generated by transplanting TFflox/flox or TFflox/flox,Tie-2 Cre+ mice (8 weeks old) with bone marrow from WT or BERK mice, respectively. Mice were used for experiments 5 months after bone marrow transplantation. Reconstitution of bone marrow was determined by cellulose acetate electrophoretic analysis of hemoglobin type (Helena Laboratories).
Determination of hematologic profile and blood cell number
Blood cell count and hematologic profile were determined using automatic system HEMAVET (Drew Scientific).
Analysis of LDH
Plasma levels of LDH were analyzed using the QuantiChrom Lactate Dehydrogenase kit (BioAssay Systems).
Multiplex cytokine assay
Plasma cytokine and chemokine levels (GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, CCL2, CXCL-1, MIP-2, TNF-α, and VEGF) were determined using the mouse Fluorokine MAP Multiplex (R&D Systems). The fluorescent signal was measured with a Luminex200 reader (Luminex).
Analysis of TAT complexes, SAP, IL-6, IL-18, and adhesion molecules
Plasma levels of TAT (ELISA kit, Enzygnost TAT micro; Siemens Healthcare Diagnostics), IL-6, IL-18, sVCAM-1, soluble intercellular adhesion molecule-1 (sICAM-1), soluble E-selectin (Quantikines; R&D Systems) and SAP (ELISA kit; Kamiya Biomedical Company) were analyzed using commercial ELISA assays following the manufacturers' instructions.
Analysis of MPO, MCP-1, and KC levels in the tissue
Lung, liver, and kidney tissues were homogenized in ice-cold lysis buffer (1% SDS, 10% glycerol, 10mM Tris, pH 7.6) supplemented with 1% protease-inhibitor cocktail (Sigma-Aldrich). The lysates were incubated on ice for 30 minutes and centrifuged at 10 000g for 20 minutes at 4°C. Supernatants were used to measure levels of myeloperoxidase (MPO; Hycult Biotech) and MCP-1 and KC (R&D Systems). Data were normalized to the protein concentration determined by the Bio-Rad Dc Protein Assay (Bio-Rad).
Real-time PCR analysis of TF mRNA
Total RNA was isolated from different tissue using TRIzol (Invitrogen). cDNA was synthesized from 1 μg of RNA using a RETROscript Kit (Applied Biosystems/Ambion) on a MyCycler themal cycler (Bio-Rad). Real-time PCR was then performed using SYBR Green RealMasterMix (Eppendorf AG) on a Mastercycler ep realplex machine (Eppendorf AG). Mouse TF mRNA was detected using forward primer spanning exon 4 and 5 (5′-TCAAGCACGGGAAAGAAAAC-3′) and reverse primer located within exon 5 (5′-CTGCTTCCTGGGCTATTTTG-3′), which generated a 137-base pair product. TF expression was normalized to the expression of hypoxanthine-guanine phosphoribosyltransferase (forward, 5′-GTGGTGAAAAGGACCTCTCG-3′; and reverse, 5′-TGAAGTACTCATTATAGTCAAGGGGA-3′), and relative expression levels were calculated using the comparative threshold cycle method.
Clotting and TF activity assay
The procoagulant activity of tissue lysates was measured using a 1-stage clotting assay with a STart 4 Clotting Machine (Diagnostica Stago), as described.20 The procoagulant activity of the sample was calculated by reference to a standard curve generated using recombinant human relipidated TF Innovin (Dade Behring). The procoagulant activity of each sample was normalized to total protein concentration determined using a Bio-Rad DC protein assay (Bio-Rad). TF-dependent procoagulant activity was determined by incubating the samples in the presence of anti-TF antibody (1H1) or control IgG. TF activity of MPs isolated from 50 μL of plasma was measured as previously described.22 White blood cells were isolated from 50 μL of blood by centrifugation at 400g for 5 minutes after removing the red cells with lysis buffer (4.14 g NH4CL, 0.5 g NaHCO3, 0.0185 g EDTA in 500 mL of sterile H2O). Cell pallets were resuspended in HBSA buffer, and TF activity was determined as above.
Immunocytochemistry
Paraffin sections (4-μm thick) were obtained from lungs, livers, and kidneys of WT and BERK mice. Before staining, sections were subjected to antigen retrieval solution (10mM citrate buffer, pH 6.0) for 25 minutes at 95°C. Endogenous peroxidase and endogenous biotin were blocked using hydrogen peroxide and avidin/biotin blocking kit (Vector Laboratories), respectively. Neutrophil staining was performed using a rat anti–mouse neutrophil monoclonal antibody (1:1000, NIMP-R14; Abcam). For TF staining, slides with blood smear from WT and BERK mice were fixed with ice-cold acetone for 1 minute and washed in PBS/0.05% Tween 20. Slides were than incubated with rat anti–mouse TF monoclonal antibody (20 μg/mL, 1H1) at room temperature for 1 hour. Subsequently, sections were incubated with a biotinylated anti–rat secondary antibody (Vector Laboratories), followed by Vectastain ABC kit reagents (Vector Laboratories). Slides were developed using ImmPACT DAB peroxidase substrate (Vector Laboratories) and counterstained with hematoxylin (Dako). Images were captured with the Olympus DP70 digital camera (Olympus America) attached to the Olympus DX51WI microscope using the 40 × Olympus UPlanFln aperture that resulted in a final magnification of 400×. Images were acquired using DP controller Version 02.03 software (Olympus).
Immunofluorescence staining
Rat anti–mouse TF monoclonal 1H1 antibody was prelabeled with AlexaFlour-488 using the Monoclonal Antibody Labeling kit (Invitrogen). Slides with blood smear from WT and BERK mice were fixed with ice-cold acetone for 1 minute and washed with ice-cold PBS. Slides were blocked with 1% BSA in PBS for 30 minutes and incubated with AlexaFlour-488–labeled rat anti–mouse TF monoclonal antibody (20 μg/mL). After washing, slides were mounted with a Vectashield Mounting Medium with DAPI (Vector Laboratories).
FACS analysis
Citrated blood samples from TWAA and TWSS mice treated with either 1H1 or control IgG antibodies were incubated with PE-labeled rat anti–mouse Ter-119 antibody (clone Ter-119, BD Biosciences) and FITC-labeled rat anti–mouse CD71 antibody (clone C2, BD Biosciences). The expression of Ter-119 and CD71 on red blood cells was determined using an LSRII (BD Biosciences) flow cytometer and analyzed by FlowJo Version 7.6.5 software (TreeStar).
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 5 software. Data are presented as mean plus or minus SEM. For 2-group comparison of parametric data, a t test was performed. For multiple-group comparison, 2-way ANOVA tests were performed, followed by Bonferroni posttest analysis. The criterion for significance was P less than .05.
Results
Hematologic profile of WTBM and BERKBM mice
We used a bone marrow transplantation strategy to generate sufficient numbers of WTBM and BERKBM mice for most of the experiments in our study. Reconstitution of bone marrow was confirmed by electrophoretic analysis of the different forms of hemoglobin. BERKBM mice that demonstrated the presence of normal mouse hemoglobin 4 to 5 months after bone marrow transplantation were excluded from experiments (data not shown). Interestingly, in WTBM mice treated with IgG, we observed increased total numbers of white blood cells, lymphocytes, and monocytes, reduced numbers of red blood cells, lower hemoglobin, and reduced hematocrit compared with WT donor mice (Table 1). The hematologic profile of BERKBM mice treated with IgG was similar to that observed in donor BERK mice (Table 1).
TF expression in sickle cell mice
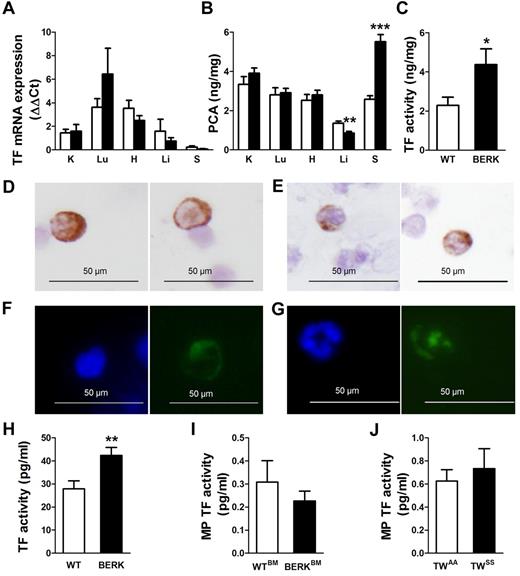
To study the role of TF in the activation of coagulation in SCD, we first analyzed TF expression in various tissues of WT and BERK mice. We did not see any significant differences in the TF mRNA expression between the organs from WT and BERK mice (Figure 1A). Interestingly, the spleen was the only organ demonstrating increased procoagulant activity in BERK mice, whereas livers showed decreased procoagulant activity (Figure 1B). The increased procoagulant activity observed in the spleen was TF dependent (Figure 1C). Next, we analyzed the TF expression by immunohistochemistry in leukocytes isolated from WT and BERK mice. We observed small numbers of TF+ leukocytes isolated from the blood of BERK mice but not WT mice. TF+ cells had a nuclear morphology resembling that of both monocytes (Figure 1D,F) and neutrophils (Figure 1E,G). To determine whether TF expressed by leukocytes may contribute to activation of coagulation, we analyzed the TF activity of the leukocytes isolated from the same volume of blood. We observed a statistically significant increase in TF activity of leukocytes isolated from BERK mice compared with the WT mice (Figure 1H).
TF expression in sickle cell mice. (A) TF mRNA expression and (B) procoagulant activity (PCA) in organs of WT (open bar; n = 9) and BERK mice (filled bar; n = 8). K indicates kidney; Lu, lung; H, heart; Li, liver; and S, spleen. Asterisks directly above the bars indicate statistically significant difference compared with WT controls: **P < .01 and ***P < .001. (C) TF-dependent PCA activity in the spleens of WT (open bar) and BERK mice (filled bar). *P < .05. Immunocytochemistry (D-E) or immunofluorescence (F-G) staining demonstrates TF-positive leukocytes with nuclear morphology resembling monocytes (D,F) or neutrophils (E,G). TF is shown as brown staining (top panels) or green staining (bottom panels). Nuclei are stained blue. (H) TF activity of the leukocytes isolated from the same volume of blood obtained from WT (n = 13) or BERK (n = 10) mice. **P < .01. (I) TF activity of the microparticles (MP TF) isolated from the plasma of WT (open bar; n = 6) and BERK (filled bar; n = 15) mice. (J) MP TF activity in TWAA (open bar; n = 13) and TWSS (filled bar; n = 17) mice.
TF expression in sickle cell mice. (A) TF mRNA expression and (B) procoagulant activity (PCA) in organs of WT (open bar; n = 9) and BERK mice (filled bar; n = 8). K indicates kidney; Lu, lung; H, heart; Li, liver; and S, spleen. Asterisks directly above the bars indicate statistically significant difference compared with WT controls: **P < .01 and ***P < .001. (C) TF-dependent PCA activity in the spleens of WT (open bar) and BERK mice (filled bar). *P < .05. Immunocytochemistry (D-E) or immunofluorescence (F-G) staining demonstrates TF-positive leukocytes with nuclear morphology resembling monocytes (D,F) or neutrophils (E,G). TF is shown as brown staining (top panels) or green staining (bottom panels). Nuclei are stained blue. (H) TF activity of the leukocytes isolated from the same volume of blood obtained from WT (n = 13) or BERK (n = 10) mice. **P < .01. (I) TF activity of the microparticles (MP TF) isolated from the plasma of WT (open bar; n = 6) and BERK (filled bar; n = 15) mice. (J) MP TF activity in TWAA (open bar; n = 13) and TWSS (filled bar; n = 17) mice.
Finally, we did not find a difference between TF activity of MPs isolated from the plasma of BERK and WT mice (Figure 1I) or between TF activity of MP from TWSS and TWAA mice (Figure 1J). These data indicate that TF expressed by white blood cells may contribute to the activation of coagulation in sickle cell mice.
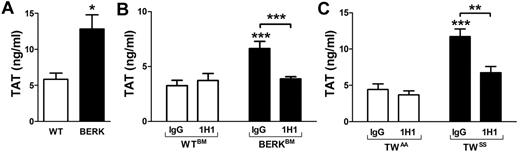
Activation of coagulation in sickle cell mice is TF dependent
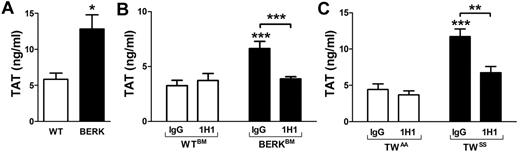
Activation of coagulation was assessed by measuring plasma levels of TAT complexes. TAT levels were significantly elevated in BERK, BERKBM, and TWSS mice compared with control mice (Figure 2A-C). To determine whether TF contributes to the activation of coagulation, BERKBM and TWSS mice were treated with either the anti–mouse TF antibody 1H1 or control IgG. Importantly, inhibition of TF significantly reduced plasma TAT levels, indicating that activation of coagulation in both mouse models of SCD was TF dependent (Figure 2B-C).
Activation of coagulation in sickle cell mice. (A) Plasma TAT levels in WT (open bar; n = 6) and BERK (filled bar; n = 16) mice (both on a similar mixed genetic background). *P < .05. (B) Plasma TAT levels in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti–mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). (C) Plasma TAT levels in TWAA (open bar) or TWSS (filled bar) mice injected with either a rat anti–mouse TF (n = 6 for TWAA; n = 7 for TWSS) or control rat IgG antibodies (n = 7 for TWAA; n = 11 for TWSS). Asterisks directly above the bars indicate statistically significant difference compared with WTBM or TWAA controls within the same treatment: **P < .01; and ***P < .001.
Activation of coagulation in sickle cell mice. (A) Plasma TAT levels in WT (open bar; n = 6) and BERK (filled bar; n = 16) mice (both on a similar mixed genetic background). *P < .05. (B) Plasma TAT levels in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti–mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). (C) Plasma TAT levels in TWAA (open bar) or TWSS (filled bar) mice injected with either a rat anti–mouse TF (n = 6 for TWAA; n = 7 for TWSS) or control rat IgG antibodies (n = 7 for TWAA; n = 11 for TWSS). Asterisks directly above the bars indicate statistically significant difference compared with WTBM or TWAA controls within the same treatment: **P < .01; and ***P < .001.
Inhibition of TF has no effect on hemolysis or red blood cell maturation and turnover in sickle cell mice
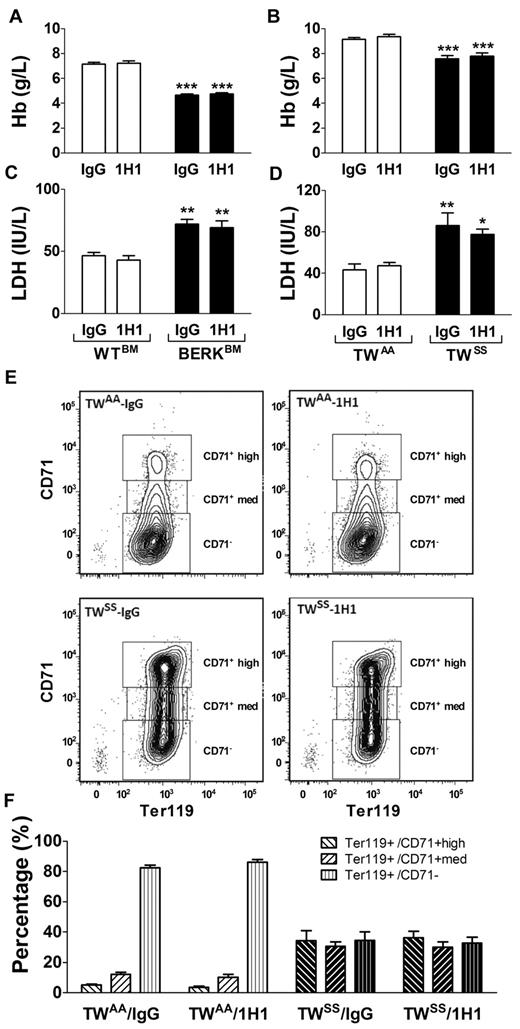
Decreased hemoglobin and elevated plasma levels of LDH are indicators of hemolysis in SCD patients. In this study, we found that both BERKBM and TWSS mice had decreased levels of hemoglobin and elevated plasma levels of LDH compared with control mice, consistent with baseline hemolysis (Figure 3A-D). However, 1H1 treatment did not change the rate of hemolysis or improve the anemic state in either mouse model of SCD (Figure 3A-D).
Hemolysis and red blood cell maturation. (A) Levels of hemoglobin (Hb) in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti –mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). (B) Levels of Hb in TWAA (open bar) or TWSS (filled bar) mice injected with either a rat anti–mouse TF (n = 6 for TWAA; n = 7 for TWSS) or control rat IgG antibodies (n = 7 for TWAA; n = 11 for TWSS). (C-D) Plasma levels of LDH were analyzed in the same group of mice as described for panels A and B. Asterisks directly above the bars indicate statistically significant difference compared with WTBM or TWAA controls within the same treatment: *P < .05; **P < .01; and ***P < .001. (E) Representative density plots demonstrating levels of CD71 expression on Ter119+ cells (red blood cells) from TWAA and TWSS mice treated with 1H1 or IgG. (F) Red blood cell maturation profiles in TWAA or TWSS mice treated with 1H1 or IgG (n = 6-11).
Hemolysis and red blood cell maturation. (A) Levels of hemoglobin (Hb) in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti –mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). (B) Levels of Hb in TWAA (open bar) or TWSS (filled bar) mice injected with either a rat anti–mouse TF (n = 6 for TWAA; n = 7 for TWSS) or control rat IgG antibodies (n = 7 for TWAA; n = 11 for TWSS). (C-D) Plasma levels of LDH were analyzed in the same group of mice as described for panels A and B. Asterisks directly above the bars indicate statistically significant difference compared with WTBM or TWAA controls within the same treatment: *P < .05; **P < .01; and ***P < .001. (E) Representative density plots demonstrating levels of CD71 expression on Ter119+ cells (red blood cells) from TWAA and TWSS mice treated with 1H1 or IgG. (F) Red blood cell maturation profiles in TWAA or TWSS mice treated with 1H1 or IgG (n = 6-11).
Furthermore, we used flow cytometry to analyze circulating levels of immature red cells in TWAA and TWSS mice treated with either IgG or 1H1. TWSS mice had a higher number of immature red blood cells (Ter119+ cells with high or medium expression of CD71) and a lower number of mature red blood cells (Ter119+/CD71−) compared with TWAA mice (Figure 3E-F). Consistent with the absence of an effect on hemoglobin or plasma levels of LDH, 1H1 treatment did not change the red blood cell maturation profiles in either TWAA or TWSS mice (Figure 3E-F). Moreover, inhibition of TF with 1H1 did not change the hematocrit values or numbers of red cells in any group of mice (Tables 1 and 2).
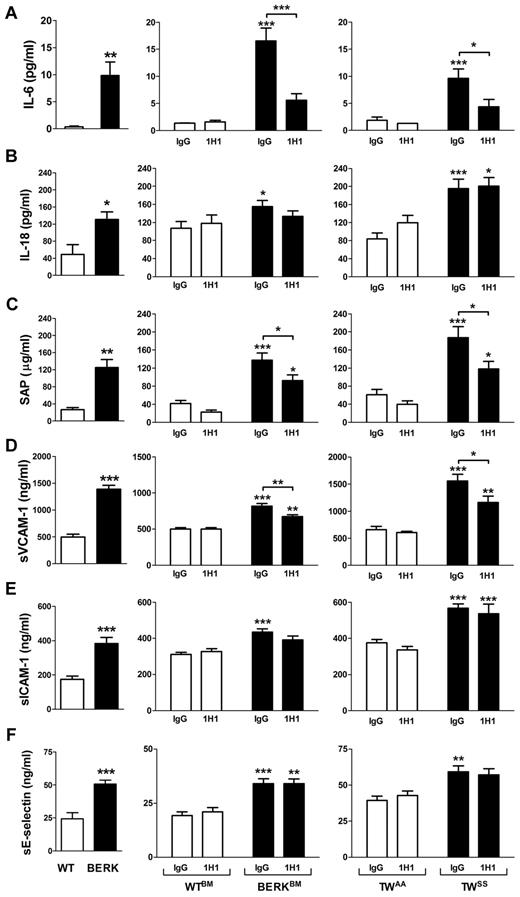
Inhibition of TF reduces inflammation and endothelial cell activation in mouse models of SCD
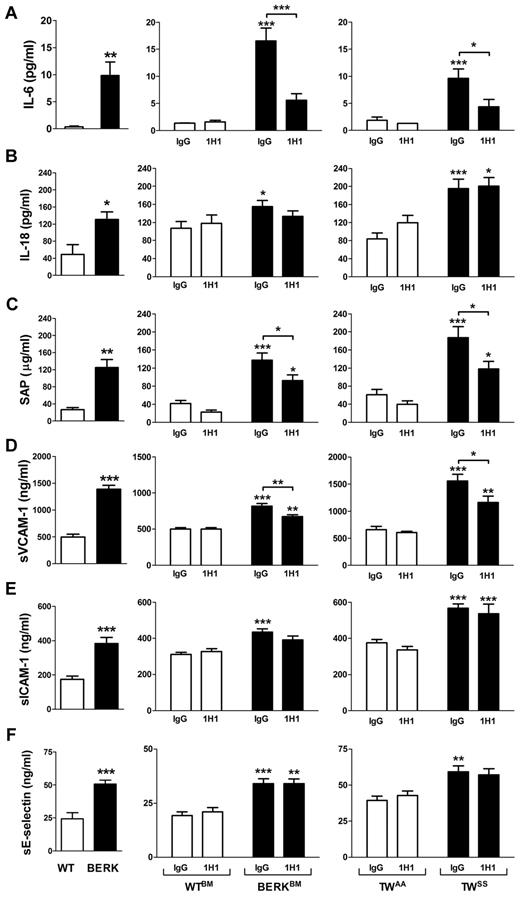
We analyzed plasma levels of various cytokines and chemokines (GM-CSF, IFN-γ, IL-10, IL-12p70, IL-13, IL-17, IL-1β, IL-2, IL-4, IL-5, IL-6, MCP-1, KC, MIP-2, TNF-α, and VEGF) in sickle cell mice using a multiplex cytokine assay. Interestingly, IL-6 was the only cytokine that was significantly increased in both BERK mice or BERKBM compared with controls (data not shown). The elevated level of IL-6 in BERK or BERKBM mice was confirmed by ELISA (Figure 4A). In addition, BERK mice also demonstrated increased plasma levels of IL-18 (Figure 4B). We also showed that BERK mice manifest elevated plasma levels of sVCAM-1, sICAM-1, and sE-selectin, which are markers of endothelial cell injury, as well as SAP, which is a major acute phase protein in mice (Figure 4C-F).
Inflammation and endothelial cell activation in sickle cell mice. Plasma levels of various inflammatory mediators and markers of endothelial cell activation in: WT (open bar; n = 12) and BERK (filled bar; n = 22) mice (left column); WTBM (open bar; n = 5-8) and BERKBM (filled bar; n = 14) mice (middle column) treated with 1H1 or IgG antibodies; TWAA (open bar; n = 6 or 7) and TWSS (filled bar; n = 7-11) mice (right column) treated with 1H1 or IgG antibodies. Asterisks directly above the bars indicate statistically significant difference compared with wild-type controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
Inflammation and endothelial cell activation in sickle cell mice. Plasma levels of various inflammatory mediators and markers of endothelial cell activation in: WT (open bar; n = 12) and BERK (filled bar; n = 22) mice (left column); WTBM (open bar; n = 5-8) and BERKBM (filled bar; n = 14) mice (middle column) treated with 1H1 or IgG antibodies; TWAA (open bar; n = 6 or 7) and TWSS (filled bar; n = 7-11) mice (right column) treated with 1H1 or IgG antibodies. Asterisks directly above the bars indicate statistically significant difference compared with wild-type controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
To determine whether TF contributes to the inflammatory response in SCD, these inflammatory markers were measured in WTBM and BERKBM mice that were treated with either 1H1 or control IgG. Inhibition of TF significantly reduced plasma levels of IL-6, sVCAM-1, and SAP (Figure 4A,C-D). In contrast, inhibition of TF did not attenuate the increased plasma levels of IL-18, sICAM-1, and sE-selectin (Figure 4B,E-F). Furthermore, 1H1 had no effect on the numbers of white blood cells in either WTBM or BERKBM mice (Table 1). Similar effects were observed in Townes mice treated with the anti-TF antibody (Figure 4; Table 2).
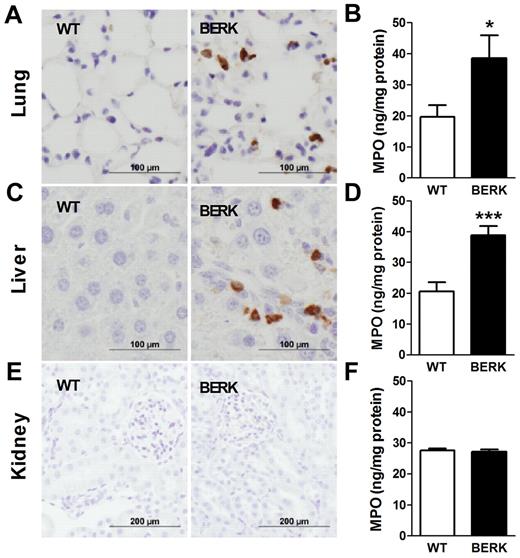
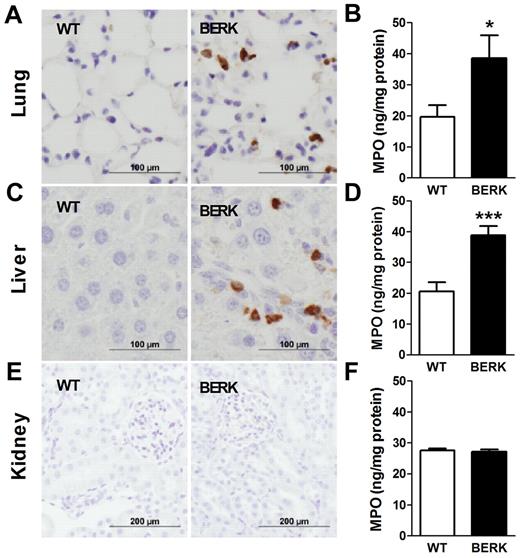
Blocking TF attenuates neutrophil infiltration/activation into the lungs of BERKBM mice
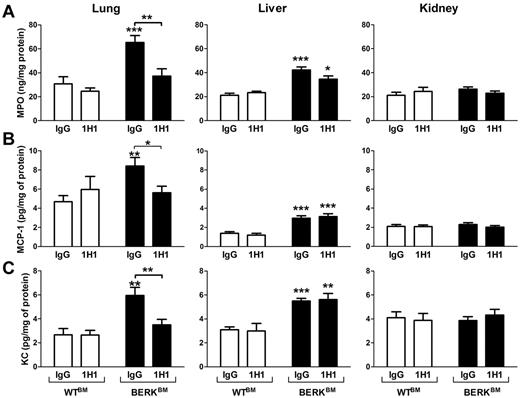
In SCD, chronic vascular inflammation leads to pathologic changes in multiple organs, including the lung, liver, and kidney.18 Recent studies have demonstrated a significant contribution of neutrophil infiltration/activation in this process.23,24 We observed infiltration of neutrophils in the lungs and livers but not kidneys of BERK mice compared with WT controls (Figure 5). Consistent with this observation, levels of MPO, a marker of neutrophil infiltration and activation, were also increased in the lungs and livers of BERK (Figure 5) and BERKBM mice (Figure 6A). Interestingly, 1H1 significantly reduced levels of MPO in the lungs but not livers in BERKBM mice (Figure 6A). Furthermore, increased levels of MPO in lung and liver were associated with increased expression of the chemokines MCP-1 and KC in BERKBM mice compared with WTBM mice (Figure 6B-C). Here again, 1H1 treatment significantly attenuated expression of both chemokines in the lung but not in the liver (Figure 6B-C).
Neutrophil infiltration and activation in various organs of BERK mice. Neutrophils (brown staining) are present in the lungs and livers, but not kidneys, of BERK mice. Tissue levels of MPO were determined in the organs of WT (n = 8 or 9) and BERK (n = 10-12) mice. Asterisks directly above the bars indicate statistically significant difference compared with WT group: *P < .05.
Neutrophil infiltration and activation in various organs of BERK mice. Neutrophils (brown staining) are present in the lungs and livers, but not kidneys, of BERK mice. Tissue levels of MPO were determined in the organs of WT (n = 8 or 9) and BERK (n = 10-12) mice. Asterisks directly above the bars indicate statistically significant difference compared with WT group: *P < .05.
Effect of TF inhibition on MPO and chemokine expressions in BERKBM mice. Tissue levels of MPO (A), MCP-1 (B), and KC (C) were analyzed in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti–mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). Asterisks directly above the bars indicate statistically significant difference compared with WTBM controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
Effect of TF inhibition on MPO and chemokine expressions in BERKBM mice. Tissue levels of MPO (A), MCP-1 (B), and KC (C) were analyzed in WTBM (open bar) or BERKBM (filled bar) mice injected with either a rat anti–mouse TF (n = 5 for WTBM; n = 14 for BERKBM) or control rat IgG antibodies (n = 8 for WTBM; n = 14 for BERKBM). Asterisks directly above the bars indicate statistically significant difference compared with WTBM controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
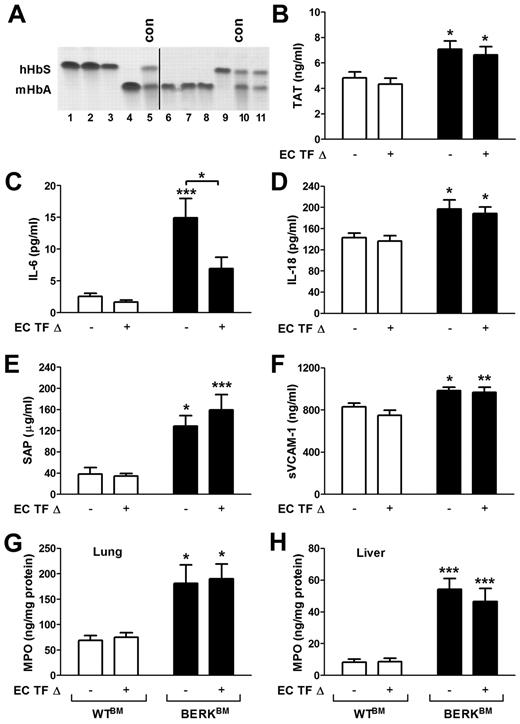
Endothelial cell-specific deletion of TF has no effect on activation of coagulation in sickle cell mice
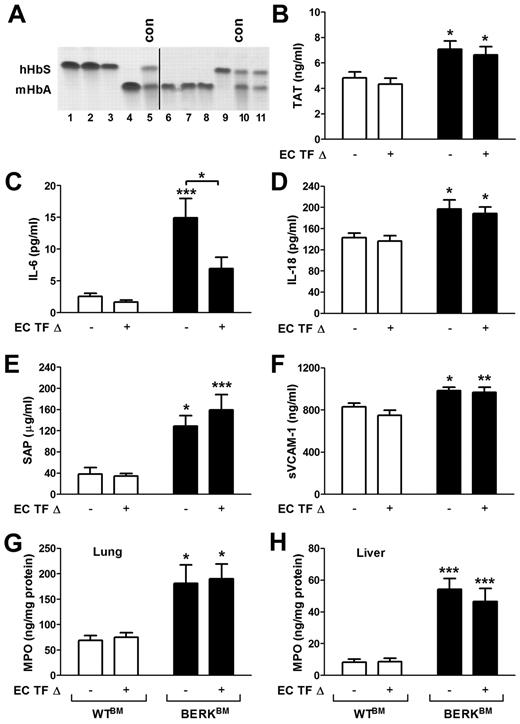
It has been previously demonstrated that TF expression is increased in the pulmonary vein endothelium of BERK mice.24 To investigate the role of TF expressed by endothelial cells in the activation of coagulation in a mouse model of SCD, we generated sickle cell mice with an endothelial cell-specific deletion of the TF gene. We have recently demonstrated that TFflox/flox,Tie-2 Cre+ mice have almost complete deletion of the TF gene in both lung endothelial cells and hematopoietic cells.20 To generate sickle cell and control mice with endothelial cell-specific deletion of TF, we transplanted these mice with bone marrow from BERK or WT mice, which resulted in the restoration of normal TF expression in the hematopoietic cells. To generate sickle and control mice with normal expression of TF, we also transplanted bone marrow from BERK and WT mice into the TFflox/flox mice (expressing normal levels of TF). Reconstitution of bone marrow was confirmed by electrophoretic analysis of the different forms of hemoglobin. TFflox/flox,Tie-2 mice that demonstrated the presence of normal mouse hemoglobin 4 to 5 months after bone marrow transplantation were excluded from experiments (Figure 7A line 11). Interestingly, a similar increase in plasma TAT levels was observed in sickle cell mice with either normal level of TF expression or with the endothelial-specific deletion of TF gene (Figure 7B). These data demonstrated that a deletion of the TF gene in endothelial cells did not reduce the activation of coagulation.
Role of endothelial cell TF in the activation of coagulation, inflammation, and endothelial cell activation in sickle cell mice. (A) Representative gel demonstrating the analysis of different hemoglobin types by cellulose acetate electrophoresis in TFflox/flox,Tie-2 Cre mice transplanted with bone marrow from BERK mice (lines 1-3 and 9) or WT mice (lines 4 and 6-8). Upper and lower bands correspond to human sickle hemoglobin and normal mouse hemoglobin. Lines 5 and 10 contain positive controls (con) for both forms of hemoglobin. Line 11 shows the example of unsuccessful transplantation of bone marrow from BERK into TFflox/flox,Tie-2 Cre mouse. Mice like this were excluded from experiments. A vertical line has been added to indicate 2 separate gels. (B-H) Plasma levels of TAT, IL-6, IL-18, SAP, and sVCAM-1 as well as tissue levels of MPO in lung and liver were analyzed in WT (open bar) and sickle cell mice (filled bar) with (+) or without (−) TF gene deletion in endothelial cells (EC TF Δ; n = 9-14). Asterisks directly above the bars indicate statistically significant difference compared with WTBM controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
Role of endothelial cell TF in the activation of coagulation, inflammation, and endothelial cell activation in sickle cell mice. (A) Representative gel demonstrating the analysis of different hemoglobin types by cellulose acetate electrophoresis in TFflox/flox,Tie-2 Cre mice transplanted with bone marrow from BERK mice (lines 1-3 and 9) or WT mice (lines 4 and 6-8). Upper and lower bands correspond to human sickle hemoglobin and normal mouse hemoglobin. Lines 5 and 10 contain positive controls (con) for both forms of hemoglobin. Line 11 shows the example of unsuccessful transplantation of bone marrow from BERK into TFflox/flox,Tie-2 Cre mouse. Mice like this were excluded from experiments. A vertical line has been added to indicate 2 separate gels. (B-H) Plasma levels of TAT, IL-6, IL-18, SAP, and sVCAM-1 as well as tissue levels of MPO in lung and liver were analyzed in WT (open bar) and sickle cell mice (filled bar) with (+) or without (−) TF gene deletion in endothelial cells (EC TF Δ; n = 9-14). Asterisks directly above the bars indicate statistically significant difference compared with WTBM controls within the same treatment: *P < .05; **P < .01; and ***P < .001.
Deletion of TF gene in endothelial cells reduces plasma levels of IL-6
Finally, we determined whether endothelial cell-specific deletion of TF has any impact on inflammation and endothelial cell activation. We found that sickle cell mice that lack the TF gene in endothelial cells had significantly reduced plasma levels of IL-6 compared with sickle cell mice with unaltered TF expression (Figure 7C). In contrast, increased plasma levels of IL-18, SAP, and markers of EC activation as well as increased levels of MPO in the lung and liver were not affected by endothelial cell-specific deletion of TF in sickle cell mice (Figure 7D-H). These data indicate that endothelial cell TF contributes only to IL-6 expression, whereas other cellular sources of TF contribute to the local tissue inflammation and endothelial cell activation.
Discussion
Multiple processes have been implicated in the pathophysiology of SCD, including red cell sickling, intravascular hemolysis, attenuated NO bioavailability, oxidative stress, vascular stasis, endothelial cell activation, reperfusion injury, inflammation, activation of coagulation, and thrombosis.2,3 Clinical studies demonstrated correlations between many of these pathologies, suggesting that they are interdependent phenomena. Mouse models of SCD provide the opportunity to further study the pathophysiologic mechanisms (and potential causality) underlying these associations. Our study demonstrates that TF not only activates coagulation but also promotes systemic and local inflammation, as well as endothelial cell activation in mouse models of SCD. Our recent clinical study found that coagulation activation in SCD patients is associated with a history of stroke,25 whereas elevated levels of markers of endothelial activation and inflammation are correlated with a history of pulmonary hypertension.26 Taken together, these studies indicate that inhibition of coagulation may have beneficial effects on clinically relevant endpoints of SCD. Importantly, TF inhibition had no effect on intravascular hemolysis, indicating that TF contributes to the manifestations of inflammation and endothelial cell activation downstream from intravascular hemolysis.
Patients with SCD demonstrate elevated levels of whole blood TF procoagulant activity that correlates with increased expression of TF antigen on monocytes.8,27 Consistent with these observations, as well as previously published data in BERK mice,9 we found increased TF expression and activity on leukocytes from BERK mice. These data strongly suggest that TF expressed by hematopoietic cells contributes to the activation of coagulation in sickle cell mice. Recently, it has been shown that depletion of neutrophils abolished enhanced brain microvasculature thrombosis in sickle cell mice.17 However, the authors of this study did not examine the mechanism by which neutrophils contributed to the microvascular thrombosis. Our data demonstrating TF on leukocytes with neutrophil-like morphology suggests that neutrophils probably contribute to thrombosis by expressing TF.
TF antigen has also been demonstrated on circulating endothelial cells isolated from the blood of sickle cell patients as well as on pulmonary vein endothelial cells in mouse models of SCD.9,28 These data place SCD on the very short list of diseases where TF has been demonstrated on endothelial cells in vivo. However, the expression of TF antigen in BERK mice was only demonstrated by fluorescent staining, raising the possibility that this TF staining may be the result, in part, of the binding of TF-bearing MPs derived from leukocytes rather than aberrant expression of TF by endothelial cells themselves. The same explanation has been proposed to explain the presence of TF-positive endothelium in the aorta branches of septic baboons.29 Moreover, we have shown that endothelial cell-specific deletion of TF had no effect on the activation of coagulation in endotoxemic mice.20 Importantly, our data from the sickle cell mice with a targeted deletion of the TF gene from endothelial cells indicate that endothelial cells in BERKBM mice express TF that contributes to IL-6 expression but does not contribute to the activation of coagulation. This result suggests that endothelial cell TF is primarily involved in signaling rather than coagulation.
Surprisingly, MP TF activity was not elevated in either BERK or TWSS mice. This result is in contrast to our previous observation of increased levels of circulating TF-positive MPs in sickle cell patients.30 However, we found that the spleen of BERK mice was the only organ demonstrating increased procoagulant activity without an increase in TF mRNA expression. Intriguingly, splenic pathology of SCD differs between humans and mice. Whereas most adult sickle cell patients are asplenic because of auto-infarction,31,32 adult BERK mice develop splenomegaly.18,19 A recent study demonstrated that the spleen is the primary organ responsible for clearance of MPs from the circulation.33 Therefore, we speculate that, in BERK mice, leukocyte-derived MPs may be trapped in the spleen, resulting in increased procoagulant activity of this organ with an absence of elevated plasma MP TF activity. This hypothesis is also compatible with the increase in thrombotic propensity that occurs after surgical splenectomy in several other hemolytic disorders.34
Currently, we cannot say whether TF promotes inflammation in SCD directly or via generation of downstream coagulation proteases. The reduced plasma levels of IL-6, but not TAT, observed in sickle cell mice lacking the TF gene in endothelial cells strongly suggest that endothelial cell TF contributes to the expression of IL-6 independently from thrombin generation. The most likely mechanism involves factor VIIa– and/or factor Xa–dependent activation of protease-activated receptor-2 (PAR-2).35,36 Several studies have demonstrated that TF/factor VIIa–dependent activation of PAR-2 promotes inflammation.37-39
In SCD, chronic inflammation contributes to pathologic changes in multiple organs. We demonstrated reduced levels of MPO, a marker of neutrophil infiltration/activation, in the lungs of BERKBM mice. Our data indicate that the reduction of MPO levels reflects reduced neutrophil accumulation in the lung because of local attenuation of chemokine production. Deletion of the TF gene in endothelial cells did not affect the levels of MPO in the lung; therefore, 1H1 presumably inhibited other cellular sources of TF (eg, lung epithelial cells), which are known to express TF.40 Epithelial cell TF could contribute to chemokine expression directly or via local thrombin generation. However, we cannot exclude the possibility that 1H1 may also attenuate activation of neutrophils by interfering with TF/factor VIIa–dependent activation of PAR-2 on these cells. It has been previously demonstrated that the PAR-2 pathway plays an important role in neutrophil activation in a mouse model of antiphospholipid syndrome-induced pregnancy loss.37 Interestingly, we observed a modest reduction of MPO in the liver of BERKBM mice treated with 1H1 (P = .061) in the absence of chemokine reduction, which further suggests that neutrophil TF may directly contribute to the activation of these cells. Because TF expression in the liver is lower compared with TF expression in the lung, the TF-dependent contribution to the increased expression of chemokines could also be relatively weaker and may explain the absence of effect of 1H1 on the increased chemokine expression in the livers of BERKBM mice.
TF can also promote inflammation via thrombin-dependent fibrin generation. It has been demonstrated that leukocyte engagement of fibrin via Mac-1 (αMβ2) contributes to local inflammation by activating leukocytes and increasing the expression of proinflammatory cytokines.41,42 Furthermore, the E1 degradation fragment of fibrin has been shown to facilitate neutrophil infiltration into infarcted tissues after ischemia-reperfusion injury.43 However, in contrast to the beneficial effects of blocking TF observed in this study, fibrinogen deficiency was associated with increased mortality in a milder mouse model of SCD (SAD mice).44 Importantly, however, patients with afibrinogenemia have increased levels of circulating thrombin.45 Therefore, the pathologic effects of fibrinogen deficiency observed in sickle cell mice may be the result of an exacerbation of endothelial cell injury caused by increased thrombin signaling. Consistent with this hypothesis, fibrinogen-deficient mice transplanted with bone marrow from BERK mice had increased plasma levels of TAT and sVCAM-1.12
Despite decades of basic research, only 1 agent (hydroxyurea) has been approved for clinical use in SCD.1-3 Although there are many promising therapeutic targets that have been identified using a systems biology approach to this disorder, much work is needed to determine which of these will prove to be the most efficacious and safe.1-3 Several clinical studies have investigated the effect of different anticoagulants, including warfarin, heparin, or acenocoumarol, on acute pain crisis in sickle cell patients.7 These studies have demonstrated modest effects at best. However, most of these studies, performed on a very small number of patients, used pain crisis as the only clinical endpoint and were poorly controlled.7 Notably, the only adequately powered, appropriately designed (ie, double-blind, placebo-controlled) study to examine the effect of anticoagulation (low molecular weight heparin for 7 days) in SCD showed a positive result, with a reduction in the duration of pain crisis and hospital stay.46 In addition, a recent abstract demonstrated that low molecular weight heparin reduces plasma levels of sVCAM-1 in sickle cell mice.47 The reduction of plasma levels of sVCAM-1 by low molecular weight heparin was similar to what we observed after inhibition of TF. However, it is unclear whether the beneficial effects of low molecular weight heparin are mediated by its anticoagulant properties or are the result of interruption of P-selectin-mediated cellular interactions.48
In conclusion, our data demonstrate that TF not only plays an important role in the activation of coagulation but also contributes to inflammation and vascular injury in sickle cell mice. Hemolysis and anemia observed in sickle cell mice were not affected by TF inhibition. Future mice studies will determine the precise mechanism by which TF-dependent activation of coagulation contributes to the pathophysiology of SCD. Moreover, based on our results, we contend that the contribution of the hypercoagulable state to the pathophysiology of SCD should be revisited and investigated in clinical studies using targeted anticoagulant agents, such as novel orally available FXa and thrombin inhibitors rivaroxaban and dabigatran etexilate, respectively. These future studies should focus not only on the frequency of pain crisis but should also include vascular inflammation, vaso-occlusion, and pulmonary hypertension as endpoints.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Tim Townes for providing TWAS mice generated in his laboratory.
This work was supported by the National Institutes of Health (grant HL096679, R.P.; grant HL006350, N.M.).
National Institutes of Health
Authorship
Contribution: P.C. designed and performed the experiments and interpreted the data; N.M. and N.S.K. aided in data interpretation and editing the manuscript; E.S. and J.-G.W. performed experiments; L.V.P and D.K. provided valuable reagents and critically read the manuscript; and R.P. designed the study and the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: L.V.P. is a consultant for Biogen-Idec. The remaining authors declare no competing financial interests.
Correspondence: Rafal Pawlinski, Division of Hematology/Oncology, Department of Medicine, 320A Mary Ellen Jones Bldg, 98 Manning Dr, Chapel Hill, NC 27599; e-mail: rafal_pawlinski@med.unc.edu.