Abstract
Several RNA-targeted therapeutics, including antisense oligonucleotides (ONs), small interfering RNAs, and miRNAs, constitute immunostimulatory CpG motifs as an integral part of their design. The limited success with free antisense ONs in hematologic malignancies in recent clinical trials has been attributed to the CpG motif–mediated, TLR-induced prosurvival effects and inefficient target modulation in desired cells. In an attempt to diminish their off-target prosurvival and proinflammatory effects and specific delivery, as a proof of principle, in the present study, we developed an Ab-targeted liposomal delivery strategy using a clinically relevant CD20 Ab (rituximab)–conjugated lipopolyplex nanoparticle (RIT-INP)– and Bcl-2–targeted antisense G3139 as archetypical antisense therapeutics. The adverse immunostimulatory responses were abrogated by selective B cell–targeted delivery and early endosomal compartmentalization of G3139-encapsulated RIT-INPs, resulting in reduced NF-κB activation, robust Bcl-2 down-regulation, and enhanced sensitivity to fludarabine-induced cytotoxicity. Furthermore, significant in vivo therapeutic efficacy was noted after RIT-INP–G3139 administration in a disseminated xenograft leukemia model. The results of the present study demonstrate that CD20-targeted delivery overcomes the immunostimulatory properties of CpG-containing ON therapeutics and improves efficient gene silencing and in vivo therapeutic efficacy for B-cell malignancies. The broader implications of similar approaches in overcoming immunostimulatory properties of RNA-directed therapeutics in hematologic malignancies are also discussed.
Key Points
Toll-like receptor–mediated immune stimulation poses major hurdle for antisense oligonucleotides and RNA-based therapies.
A novel targeted delivery strategy that overcomes these immunostimulatory effects while potentiating gene silencing in B-cell malignancies.
Introduction
Therapeutic oligonucleotides (ONs), including antisense oligodeoxynucleotides (ODNs), small interfering RNAs (siRNAs), and the more recently discovered miRNAs designed for targeted inhibition of specific mRNA sequences that code for cell survival proteins, are of emerging interest in hematologic malignancies.1-4 Despite their promising roles, clinical trials using ONs in hematologic malignancies have shown limited success. Probably the most studied has been the antisense targeting Bcl-2 G3139. Bcl-2 is a well-characterized member of the Bcl-2 family with multiple antiapoptotic functions that prevent cell death from multiple mechanisms.5,6 Overexpression of Bcl-2 can dramatically increase resistance to therapeutics that promote mitochondrial and endoplasmic reticulum–mediated death in a variety of cancer types. The Bcl-2 protein is dramatically overexpressed in chronic lymphocytic leukemia (CLL) compared with normal B cells and has been shown to promote resistance to fludarabine.7-9 Preclinical studies examining either knock-down (antisense and siRNA) or inhibition of Bcl-2 protein function by small molecules promotes apoptosis in CLL cells, thereby prompting the initiation of clinical trials of G3139 in CLL. Surprisingly, the first phase 1 study of G3139 in CLL identified a lower tolerated dose than in other diseases because of cytokine release syndrome and other immune-activating symptoms unique to CLL.10 Whereas detailed pharmacodynamics validating target down-modulation of Bcl-2 was not performed in CLL patients,11 studies done on AML blasts in vivo suggested that the doses were inadequate to effectively inhibit this protein.12 Despite this lack of pharmacodynamic data, development of G3139 went forth to full phase 3 testing, where it was added to fludarabine and cyclophosphamide and compared with chemotherapy alone.10,13,14 Modest enhancement of clinical activity was observed but was insufficient for regulatory approval. Other attempts to target Bcl-2 family member proteins with BH3 mimetic small molecules such as ABT263 have demonstrated clinical success in trials with objective response rates.15 Unfortunately, these agents are not selective to one Bcl-2 family member and therefore have unanticipated target effects such as severe thrombocytopenia and cellular immune suppression because of antagonizing Bcl-XL. These findings suggest that more selective targeting of specific Bcl-2 proteins such as Bcl-2 may diminish untoward off target effects and potentially improve target modulation.
One reason that G3139 has been suggested to have a lower maximally tolerated dose in CLL patients is its immunostimulatory properties associated with adverse cytokine release and confounding effects on target down-modulation in CLL.10,11,16,17 G3139, which contains 2 CpG dinucleotide motifs, has been shown to induce a potent cytokine response because of immune activation via TLR9 in murine models.18 In vivo treatment of CLL cells promoted Bcl-2 down-regulation in CLL cells in some patients, but was also up-regulated in a significant fraction of patients, particularly at low or suboptimal concentrations. Consistent with this, the observed limitations in the clinical activity with G3139 may be attributed to the confounding effects of antisense mechanism and immune activation.10,11,17,19
In the present study, we describe a novel strategy to minimize the adverse stimulatory side effects of G3139 in CLL B cells using anti-CD20 Ab (rituximab)–conjugated immunoliposomal nanoparticles (RIT-INPs). Because of CD20-targeted delivery of G3139 to CLL B cells, we achieved preferential promotion of uptake, enhanced Bcl-2 target down-regulation. and associated sensitivity to fludarabine-induced apoptosis in vitro and a significant in vivo therapeutic effect. The mechanisms underlying the reduced immunostimulation of G3139 by RIT-INPs are also demonstrated.
Methods
Materials
Phosphorothioate-modified oligos G3139 (5′-TCT CCC AGC GTG CGC CAT-3′), G3622 (5′-TAC CGC GTG CGA CCC TCT-3′), and a fluorescein-modified ODN (5′-(6)-FAM-TAC CGC GTG CGA CCC TCT-3′) were custom synthesized by Alpha DNA. Rituximab (Rituxan) and trastuzumab (Herceptin) were from Genentech. Fludarabine phosphate was from Polymed Therapeutics. Egg phosphatidylcholine and methoxy polyethylene glycol (molecular weight, approximately 2000 Da)-distearoyl phosphatidylethanolamine were from Lipoid. 3β-N-(N′,N′-dimethyl amino ethane)-carbamoyl cholesterol and distearoyl phosphatidylethanolamine-methoxy polyethylene glycol-maleimide were from Avanti Polar Lipids. 2-Iminothiolane (Traut reagent) and other chemicals were from Sigma-Aldrich.
Patient samples and cell culture
Peripheral blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the institutional review board of The Ohio State University. CLL B cells and PBMCs were separated from heparinized venous blood of the B-CLL patients by density gradient centrifugation using Ficoll-Paque (Pharmacia LKB Biotechnology). PBMCs and CLL B cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 2mM l-glutamine, and 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37°C in an atmosphere of 5% CO2.
Flow cytometry
Cell-surface markers were analyzed by staining with fluorescent antibodies against CD20, CD40, CD80, and CD86 or isotype control Ab (BD Biosciences). Apoptotic CLL B cells were assessed by flow cytometry using annexin V/propidium iodide staining (BD Biosciences). Mean fluorescence intensity and positive cell percentages were determined. The data were analyzed using WinMDI Volume 2.8 software.
Western blot analysis
Protein lysates were prepared from purified CLL B cells. Western blot was performed using primary antibodies specific for Bcl-2, Mcl-1 (Santa Cruz Biotechnology), anti-TLR9, phospho-NF-κB p65 (Ser536), NF-κB p65, phospho-p38, and p38 (Cell Signaling Technology), respectively, and secondary anti–rabbit IgG-HRP (Santa Cruz Biotechnology), and visualized by enhanced chemiluminescence (Pierce Chemical). Blots were reprobed with an anti-actin Ab, antitubulin, or GAPDH (Santa Cruz Biotechnology) as a loading control. Signal intensity was quantified using ImageJ Volume 1.6 software.
siRNA knock-down of TLR9
To suppress TLR9 expression in CLL B cells, TLR9 siRNA (GenBank accession no. 54106) and nontargeting control siRNA ONs were purchased from Applied Biosystems. CLL cells (8 × 106) were resuspended in Nucleofector solution (Nucleofector kit V) with 2.8 μg of TLR9 siRNA or control siRNA using the Amaxa Nucleofector apparatus and program U-16. Immediately after transfection, cells were transferred into RPMI 1640 medium with 10% FBS and incubated for 48 hours. The silencing effect of TLR9 on CLL B cells was detected by Western blotting using anti-TLR9 Ab.
Laser-scanning confocal microscopy
CLL B cells were fixed, permeabilized, stained with antibodies specific for EEA-1 or LAMP-1 and TLR9 and further labeled with the Alexa Fluor–conjugated rabbit IgG (H+L) secondary antibodies (Cell Signaling Technology). For compartment localization study of G3139, CLL B cells were pretreated with free FAM-G3139 or RIT-INP carrying FAM-G3139 for 2 hours. After fixing and permeabilization, cells were further stained by EEA-1 or LAMP-1 Ab. Nuclei were stained with DRAQ5 (Biostatus Limited). The cells were mounted on a poly-D-lysine–coated cover glass slide (Sigma-Aldrich) and examined with an Olympus FV1000 confocal microscope.
Cytotoxicity analysis
Cytotoxicity by fludarabine, either alone or in combination with ODN-containing INPs, was evaluated in CLL B cells with the MTT assay.
Preparation of rituximab-conjugated liposomal ODN nanoparticles
An ethanol dilution method was used to prepare the ODN nanoparticles as described previously.20 Further details are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A postinsertion method was adopted to incorporate RIT into nanoparticles carrying ODNs. RIT was reacted with 10 × Traut reagent for 2 hours at room temperature to yield RIT-SH. RIT-SH was then reacted to form micelles of distearoyl phosphatidylethanolamine-methoxy polyethylene glycol-maleimide at a molar ratio of 1:10 and then incubated with ODN nanoparticles for 1 hour at 37°C. INPs with a RIT to a total lipid ratio of 1:4000 (0.025 mol %) were thus prepared. Herceptin (HER) was also synthesized as controls using the same method. The INPs were further purified on a Sepharose CL-4B size-exclusion column to remove free Ab.
EMSA
The induction of NF-κB–binding activity by various indicated conditions on CLL B cells was detected by EMSA using consensus NF-κB–binding activity. Nuclear extracts were prepared and incubated with the [32P]-labeled probe. The NF-κB–binding site ODN derived from the B3 site of the TNF promoter (5′-GATCAAACAGGGGGCTTTCCCTCCTC-3′) was labeled with CTP using Klenow polymerase (Promega). Samples were fractionated on a 7% polyacrylamide gel and visualized by exposing dried gel to film.
Cytokine assays
Purified CLL B cells (2 × 106 cells/mL) were treated with free G3139 or various G3139-formulated INPs at the indicated concentrations and cultured in medium. After 48 hours, supernatants were collected and assessed for levels of human IL-6, IFN-γ, and TNF-α by ELISA (R&D Systems).
In vivo study in Raji xenograft model
Raji (2 × 106 cells/mouse) cells were IV injected into NOD-SCID mice. For the initial survival study, free G3139 was given through IP injections at a dose of 5 mg/kg on alternative days for 2 weeks 3 days after inoculation. Untreated mice (negative controls) showed signs of hind-limb paralysis between 14 and 21 days after engraftment. Weight was measured every day and early removal criteria were observed daily before day 30. After day 30, the mice were weighed twice a week and observed for signs of illness. Hind-limb paralysis was considered as the primary end point. Cured mice in the treated group were kept for 6 months after engraftment. For the in vivo Bcl-2 down-regulation study, free G3139 or RIT-INP–G3139 was injected at a dose of 5 mg/kg for 3 doses (days 10, 12, and 14) and were killed on day 15. The cells isolated from BM were assessed for the level of Bcl-2 protein expression and percentage of human CD20+ cells by flow cytometry, whereas mouse plasma was collected to determine the IFN-γ and IL-6 levels by ELISA using commercial kits (R&D Systems). All animal experiments were done in accordance with an institutional animal care and use committee–approved protocol.
Histopathologic and immunohistochemical analyses
For pathologic analysis, tumor samples were fixed in 10% phosphate-buffered formalin solution. The tissue sections were stained with H&E. For immunohistochemical analysis, tumor samples were stained with rat mAbs against CD45, CD20, or human Bcl-2, followed by staining with HRP-conjugated rabbit anti–rat IgG.
B-cell specificity analysis of the RIT-INP
For surface staining, PBMCs were incubated with APC-labeled anti-CD19 (a B-cell maker) or APC-labeled anti-CD3 (a T-cell marker) on ice for 30 minutes. The cells were washed twice with cold PBS (pH 7.4) and analyzed by flow cytometry. To study B-cell selectivity, PBMCs were incubated with 0.5μM free FAM-ODN or FAM-ODN–encapsulated RIT-INPs for 60 minutes at 37°C. The cells were then centrifuged and rinsed twice with cold PBS (pH 7.4). The treated cells were further stained with APC-labeled anti-CD19 or anti-CD3 antibodies to identify the B- and T-cell populations, respectively. The cells were then washed with PBS and analyzed by flow cytometry for CD19+ and/or CD3+ FAM-ODN+ cells.
Whole-blood analysis of CLL patients
Whole peripheral blood samples from CLL patients were treated with free G3139 and various G3139-formulated INPs on rotary device at 37°C. After 24 hours of incubation, blood was stained directly with CD19, CD40, and CD86 (BD Biosciences) and assessed by flow cytometry. Plasma was collected for ELISA analysis.
Statistical analysis
All statistical analyses were performed in the Center for Biostatistics at The Ohio State University. For experiments using samples from the same patients, linear mixed-effect models were used to take into consideration of the dependency of these observations. For experiments with multiple independent groups involved, general linear models were used. Contrasts in models that answer primary questions directly were tested. For the mouse survival study, the log-rank test was used to test the difference between the 2 survival curves. The Holm method was applied to adjust for multiplicity. The overall family-wise type I error rate was controlled at α = 0.05. SAS Version 9.2 software was used for all statistical analyses.
Results
Free G3139 induces TLR9-driven immunostimulation associated with minimal modulation of Bcl-2 expression
Overexpression of the antiapoptotic Bcl-2 and Mcl-1 proteins in CLL B cells contributes to resistance to spontaneous and drug-induced apoptosis.7-9 The effects of G3139, a Bcl-2–directed antisense ODN, were tested against primary CLL B cells (Figure 1). No marked differences in Bcl-2 protein levels were observed in free G3139-treated cells compared with untreated control cells (Figure 1A). Indeed, Bcl-2 protein levels were moderately up-regulated by G3139. In addition, the expression of Mcl-1, another important member of the antiapoptotic Bcl-2 family of proteins, was also significantly up-regulated at all concentrations tested (Figure 1A). Accordingly, free G3139 failed to mediate cell death, even at high concentrations (Figure 1B).
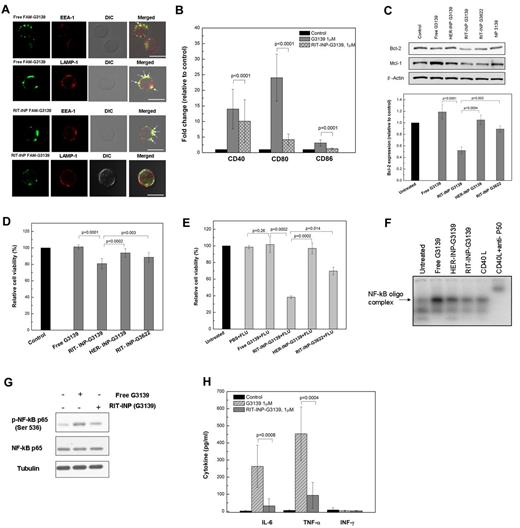
TLR9-driven immunostimulation of free G3139 is associated with limited modulation of Bcl-2 expression. (A) Effect of free G3139 on Bcl-2 and Mcl-1 protein levels. Top panel shows 2 representative Western blot results of n = 10 B-CLL patient cells. Primary CLL B cells were incubated with 1, 2, and 5μM G3139 for 48 hours and then collected and lysed for Western blot analysis. Bottom panel shows average Western blot band intensities determined by densitometry. Data are presented as relative percentages compared with untreated cell controls (for Bcl-2, n = 10, mean ± SEM; for Mcl-1, n = 4, mean ± SEM). (B) Relative CLL B-cell viability normalized to medium control, determined by annexin V/propidium iodide staining (n = 10). Primary CLL B cells were incubated with 1, 2, and 5μM G3139 for 48 hours. Relative cell viability was defined as the percentage of annexin V− and propidium iodide− cells relative to the untreated control group. (C) Fold changes of surface markers relative to medium controls in CLL B cells after G3139 treatment. Primary CLL B cells were incubated in the presence of 1, 2, and 5μM G3139. After 48 hours, expressions of CD40, CD80, and CD86 were measured by flow cytometry. The data are presented according to MFI (n = 10, mean ± SEM). (D) Effect of knock-down of TLR9 on the immunostimulatory properties of G3139. Top panel is the fold changes of surface markers relative to medium controls after TLR9 down-regulation by siRNA. Primary CLL B cells were transfected by TLR 9 siRNA using electroporation and incubated for 48 hours, followed by further treatment with 1μM G3139. After 24 hours, the expressions of CD40, CD80, and CD86 were measured by flow cytometry. The data are presented as MFI (n = 4, mean ± SEM). Variable cell death (5%-20%) after transfections was observed. Bottom panel is a representative Western blot showing down-regulation of TLR9 expression by siRNA. (E) Subcellular distribution analysis of TLR9 in CLL B cells. Purified CLL B cells were fixed and stained intracellularly with anti-TLR9, anti–EEA-1, or anti–LAMP-1 antibodies as described. Images were acquired using an Olympus FV1000 confocal microscope. Scale bar indicates 10 μm. The white arrows indicate colocalization (yellow dots).
TLR9-driven immunostimulation of free G3139 is associated with limited modulation of Bcl-2 expression. (A) Effect of free G3139 on Bcl-2 and Mcl-1 protein levels. Top panel shows 2 representative Western blot results of n = 10 B-CLL patient cells. Primary CLL B cells were incubated with 1, 2, and 5μM G3139 for 48 hours and then collected and lysed for Western blot analysis. Bottom panel shows average Western blot band intensities determined by densitometry. Data are presented as relative percentages compared with untreated cell controls (for Bcl-2, n = 10, mean ± SEM; for Mcl-1, n = 4, mean ± SEM). (B) Relative CLL B-cell viability normalized to medium control, determined by annexin V/propidium iodide staining (n = 10). Primary CLL B cells were incubated with 1, 2, and 5μM G3139 for 48 hours. Relative cell viability was defined as the percentage of annexin V− and propidium iodide− cells relative to the untreated control group. (C) Fold changes of surface markers relative to medium controls in CLL B cells after G3139 treatment. Primary CLL B cells were incubated in the presence of 1, 2, and 5μM G3139. After 48 hours, expressions of CD40, CD80, and CD86 were measured by flow cytometry. The data are presented according to MFI (n = 10, mean ± SEM). (D) Effect of knock-down of TLR9 on the immunostimulatory properties of G3139. Top panel is the fold changes of surface markers relative to medium controls after TLR9 down-regulation by siRNA. Primary CLL B cells were transfected by TLR 9 siRNA using electroporation and incubated for 48 hours, followed by further treatment with 1μM G3139. After 24 hours, the expressions of CD40, CD80, and CD86 were measured by flow cytometry. The data are presented as MFI (n = 4, mean ± SEM). Variable cell death (5%-20%) after transfections was observed. Bottom panel is a representative Western blot showing down-regulation of TLR9 expression by siRNA. (E) Subcellular distribution analysis of TLR9 in CLL B cells. Purified CLL B cells were fixed and stained intracellularly with anti-TLR9, anti–EEA-1, or anti–LAMP-1 antibodies as described. Images were acquired using an Olympus FV1000 confocal microscope. Scale bar indicates 10 μm. The white arrows indicate colocalization (yellow dots).
Given that G3139 contains immunostimulatory unmethylated CpG dinucleotides, we evaluated the induction of activation markers such as CD40, CD80, and CD86 in free G3139-treated CLL B cells (Figure 1C). Free G3139 induced significant up-regulation of CD40, CD80, and CD86 compared with controls. Free G3139 also induced proliferation in CLL B cells, which was assessed by DNA synthesis (supplemental Figure 1). The immunostimulatory effects of G3139 is dependent on TLR9 expression, because siRNA-mediated knock-down of the TLR9 protein almost completely abrogated G3139-induced CD40, CD80, and CD86 (Figure 1D and supplemental Figure 2). Subcellular distribution analysis of TLR9 in B-CLL cells by confocal microscopy revealed the presence of TLR9 in small intracellular vesicles that mainly localized in late endosome/lysosome marked by LAMP-1 (Figure 1E). Our findings demonstrated that G3139 resembles CpG-B ODN that are reported to be potent TLR9-stimulatory agonists compared with CpG-A ODNs in normal and CLL B cells.21,22 The increase in the prosurvival protein expression level may be attributed to the dominance of antisense G3139 in cell activation over the preferred Bcl-2 down-regulation effect.
Design, synthesis, and characterization of RIT-INPs
Given that the TLR9 primarily remains in late endosome/lysosome, we hypothesized that the immunostimulatory effects of G3139 may be altered by subverting its uptake via receptor-mediated endocytosis mechanism, thus prolonging its retention in early endosomal compartment. To achieve this goal, G3139-encapsulated RIT-INPs were designed and synthesized (Figure 2A). The characterization of RIT-INP is shown in Table 1 and Figure 2B. Atomic Force Microscopy imaging was used to determine the morphology of the INPs. Both G3139-NPs and G3139-RIT-INPs are spherical nanostructures (Figure 2B). The colloidal stability of G3139-loaded INPs was evaluated by monitoring changes in the mean diameter. No significant changes in particle size were observed for several weeks at 4°C (data not shown).
Design, characterization, and optimization of RIT-INPs. (A) Design of G3139-loaded RIT-INPs for CLL B cell–specific targeting to overcome immunostimulatory effects. (B) Atomic Force Microscopy images of NPs and RIT-INPs. Shown are G3139 encapsulated in NPs (1) and G3139 encapsulated in RIT-INPs (2). Particle suspensions were dried on a mica substrate. All measurements were recorded in both height and amplitude modes. Height images are presented here. (C) Confocal micrographs of uptake of cy3-G3139 (1μM) loaded in RIT-INPs under various molar ratios of RIT/total lipids (approximately 1/500-1/12 000) in Raji cells after 4 hours of incubation. DIC indicates differential interference contrast (bright-field) images. Scale bar indicates 10 μm (D) Flow cytometry analysis of binding/uptake of RIT-INP carrying FAM-G3139 in Raji cells compared with free FAM-G3139 and nontargeted NP carrying FAM-G3139. Raji cells were treated with FAM-G3139–loaded RIT-INPs with various molar ratios of RIT/total lipids. Data are presented according to mean fluorescence intensity (MFI) changes (n = 3, mean ± SD). (E) Binding of free FAM–labeled G3139 and various NP-formulated FAM-ODN to Raji (CD20+) and Jurkat (CD20−) cells. (F) Binding of free FAM-G3139 and various NP-formulated FAM-G3139 to CLL B cells. The CLL B cells were incubated with free FAM-G3139 or FAM-G3139 in HER-INP or RIT-INP with the concentration of 1μM at 37°C for 1 hour and washed twice with cold PBS. (G) Inhibition of RIT-INP binding to Raji cells by excess RIT or alemtuzumab (anti-CD52). Untreated cells (bold line), cells treated with anti-CD20 ILP (thin solid line), and cells blocked with rituximab or alemtuzumab (broken line) were assessed by flow cytometry.
Design, characterization, and optimization of RIT-INPs. (A) Design of G3139-loaded RIT-INPs for CLL B cell–specific targeting to overcome immunostimulatory effects. (B) Atomic Force Microscopy images of NPs and RIT-INPs. Shown are G3139 encapsulated in NPs (1) and G3139 encapsulated in RIT-INPs (2). Particle suspensions were dried on a mica substrate. All measurements were recorded in both height and amplitude modes. Height images are presented here. (C) Confocal micrographs of uptake of cy3-G3139 (1μM) loaded in RIT-INPs under various molar ratios of RIT/total lipids (approximately 1/500-1/12 000) in Raji cells after 4 hours of incubation. DIC indicates differential interference contrast (bright-field) images. Scale bar indicates 10 μm (D) Flow cytometry analysis of binding/uptake of RIT-INP carrying FAM-G3139 in Raji cells compared with free FAM-G3139 and nontargeted NP carrying FAM-G3139. Raji cells were treated with FAM-G3139–loaded RIT-INPs with various molar ratios of RIT/total lipids. Data are presented according to mean fluorescence intensity (MFI) changes (n = 3, mean ± SD). (E) Binding of free FAM–labeled G3139 and various NP-formulated FAM-ODN to Raji (CD20+) and Jurkat (CD20−) cells. (F) Binding of free FAM-G3139 and various NP-formulated FAM-G3139 to CLL B cells. The CLL B cells were incubated with free FAM-G3139 or FAM-G3139 in HER-INP or RIT-INP with the concentration of 1μM at 37°C for 1 hour and washed twice with cold PBS. (G) Inhibition of RIT-INP binding to Raji cells by excess RIT or alemtuzumab (anti-CD52). Untreated cells (bold line), cells treated with anti-CD20 ILP (thin solid line), and cells blocked with rituximab or alemtuzumab (broken line) were assessed by flow cytometry.
CD20 was chosen because of its cell-specific expression in CLL B cells and because of the safety of rituximab, which has been used successfully with minimal adverse effects in CLL patients. Variability in the expression of CD20 was commonly observed in primary CLL B cells. We chose primary B cells and Raji B-cell line, which express consistent levels of CD20 (supplemental Figure 3) for our in vitro and in vivo evaluation. Although rituximab is known as a noninternalizing Ab in B cells, we obtained the balance between internalization and early endosome retention of RIT-INPs through the optimization of the coated RIT/lipid molar ratio (Figure 2C). The ratio of RIT/lipids of 1/4000 on RIT-INPs gave the best targeting efficiency (Figure 2D). RIT-INPs demonstrated the CD20 antigen-specific targeting on CD20+ Raji and CLL B cells, but not CD20− Jurkat Tcells (Figure 2E-F). Blocking of CD20 by excess rituximab but not excess alemtuzumab (CD52 Ab) prevented the RIT-INP–targeted delivery (Figure 2G). Similar binding to CD20+ B cells but not CD20− T cells was observed in PBMCs from healthy donors (data not shown). In addition, an efficient cytotoxic efficacy was observed with anti-CD20–conjugated liposomes (CD20-ILP) compared with the free anti-CD20 with anti-Fc cross-linker, presumably because of efficient cross-linking of surface CD20 with anti–CD20-ILP, as has been reported previously for other therapeutic antibodies (data not shown and previous results23-25 ).
RIT-INPs mediate early endosomal compartmentalization of G3139, resulting in the inhibition of TLR9-driven immunostimulatory effects and enhanced Bcl-2 down-regulation
Colocalization studies by confocal microscopy revealed differential subcellular trafficking of free G3139 and RIT-INP–G3139. In contrast to free G3139, which was primarily localized in the late endosomal/lysosomal compartment stained by LAMP-1, RIT-INP–G3139 was preferentially detected in early endosomes marked by EEA-1 staining and less so in late endosomes/lysosomes (Figure 3A). After TLR9 staining, the dominant colocalization of LAMP-1/TLR9/free G3139 and EEA-1/RIT-INP–G3139 in B-CLL cells was observed (supplemental Figure 4), indicating relatively increased localization of RIT-INP–encapsulated G3139 in the early compared with the late endosomal compartment. Compared with free G3139, G3139 in RIT-INPs showed significantly reduced induction of activation markers such as CD40, CD80, and CD86 (Figure 3B). Reduction in the immunostimulatory effects of CpG-B–containing ODNs delivered with RIT-INP in B-CLL cells was also independently confirmed with additional CpG-B ODN (ODN 2006)26 (supplemental Figure 5). Because of the reduced activation and enhanced delivery efficiency, > 50% down-regulation of Bcl-2 in RIT-INP–G3139–treated CLL B cells compared with HER-INP–G3139 or free G3139 treatment was observed (Figure 3C). We also observed a lack of induction of Mcl-1 with RIT-INP–G3139 that was observed with free G3139. RIT-INPs loaded with control G3622 (the reversed G3139 sequence) had a slight reduction in Bcl-2 expression compared with free G3139 or that observed with HER-INP–G3139. However, RIT-INP–G3139 induced significant reduction compared with free G3139 (P = .0001), HER-INP G3139 (P = .0004), or RIT-INPG3622 (P = .003;Figure 3C). Consistent with the efficient down-modulation of Bcl-2, a significant reduction in spontaneous cell survival was observed in CLL B cells treated with RIT-INP–G3139 compared with HER-INP–G3139 or free G3139 (Figure 3D). Our study indicates that there is no direct effect of rituximab, rituximab plus anti-Fc, or the empty RIT-INP on Bcl-2 expression in B-CLL cells (supplemental Figure 6). Therefore, the observed modest Bcl-2 down-regulation by RIT-INP–G3622 is presumably from the off-target effect of G3622. Cell apoptosis of RIT-INP–G3622 may be mainly induced by the CD20 cross-linking effect of RIT-INP, because the empty RIT-INPs exhibited similar modest apoptosis of CLL B cells (supplemental Figure 7).
Differential compartmentalization, immunostimulatory effects, target down-modulation, and cytotoxicity by G3139 and RIT-INP–G3139 in CLL B cells. (A) RIT-INPs mediate the early endosomal compartmentalization of G3139. Purified CLL B cells were incubated with free FAM-G3139 (2μM) or RIT-INP–FAM-G3139 (2μM) for 1 hour. Cells were washed, fixed, and stained with anti–EEA-1 or anti–LAMP-1 antibodies for confocal observation. The white arrows indicate the colocalization (yellow dots). Scale bar indicates 10 μm. (B) Fold changes of costimulatory molecules relative to medium control in CLL B cells. Primary CLL B cells were incubated in the presence of free G3139 (1μM), and RIT-INP–G3139 (1μM). The data are based on MFI. Results are shown as means of n = 10 independent experiments. (C) Effect of RIT-INP–G3139 on Bcl-2 protein level. Top panel is a comparison of relative Bcl-2 protein level (n = 5, mean ± SEM). Primary CLL B cells were incubated with free G3139 or HER-INP- or RIT-INP–formulated G3139 and G3622 at 2μM for 48 hours. Average Western blot band intensities were determined by densitometry and data are presented as relative percentages compared with untreated cell controls. Bottom panel is a presentative Western blot analysis of Bcl-2 protein in CLL B cells. (D) Relative percentage of CLL B-cell viability normalized to medium controls. CLL B cells were treated with various conditions at 37°C for 48 hours. The percentage of viable cells was determined by annexin V/propidium iodide staining and was analyzed by flow cytometry (n = 5, mean ± SEM). (E) Improved cytotoxicity of fludarabine after treatment by G3139-loaded RIT-INPs. Relative cell viability was determined by propidium iodide staining of free G3139 and various G3139-containing INPs at 1μM for 24 hours, followed by fludarabine (1μM) for another 48 hours. Results are presented as means of n = 4 independent experiments. (F) Induction of NF-κB–binding activity detected by the EMSA assay. CLL B cells were incubated with free G3139 and INP-loaded G3139 at 1μM for 4 hours. The CLL cells were stimulated with 500 ng/mL of CD40L for 1 hour as a positive control. (G) Western blot analysis of NF-κB (p65) phosphoration. CLL B cells were incubated with free G3139 and INP-loaded G3139 at 1μM for 3 hours, followed by lysis for Western blot analysis. (H) Differential cytokine inductions of IL-6, TNF-α, and IFN-γ on CLL B cells. Primary CLL B cells were treated under the indicated conditions for 48 hours. Supernatants were collected for ELISA analysis.
Differential compartmentalization, immunostimulatory effects, target down-modulation, and cytotoxicity by G3139 and RIT-INP–G3139 in CLL B cells. (A) RIT-INPs mediate the early endosomal compartmentalization of G3139. Purified CLL B cells were incubated with free FAM-G3139 (2μM) or RIT-INP–FAM-G3139 (2μM) for 1 hour. Cells were washed, fixed, and stained with anti–EEA-1 or anti–LAMP-1 antibodies for confocal observation. The white arrows indicate the colocalization (yellow dots). Scale bar indicates 10 μm. (B) Fold changes of costimulatory molecules relative to medium control in CLL B cells. Primary CLL B cells were incubated in the presence of free G3139 (1μM), and RIT-INP–G3139 (1μM). The data are based on MFI. Results are shown as means of n = 10 independent experiments. (C) Effect of RIT-INP–G3139 on Bcl-2 protein level. Top panel is a comparison of relative Bcl-2 protein level (n = 5, mean ± SEM). Primary CLL B cells were incubated with free G3139 or HER-INP- or RIT-INP–formulated G3139 and G3622 at 2μM for 48 hours. Average Western blot band intensities were determined by densitometry and data are presented as relative percentages compared with untreated cell controls. Bottom panel is a presentative Western blot analysis of Bcl-2 protein in CLL B cells. (D) Relative percentage of CLL B-cell viability normalized to medium controls. CLL B cells were treated with various conditions at 37°C for 48 hours. The percentage of viable cells was determined by annexin V/propidium iodide staining and was analyzed by flow cytometry (n = 5, mean ± SEM). (E) Improved cytotoxicity of fludarabine after treatment by G3139-loaded RIT-INPs. Relative cell viability was determined by propidium iodide staining of free G3139 and various G3139-containing INPs at 1μM for 24 hours, followed by fludarabine (1μM) for another 48 hours. Results are presented as means of n = 4 independent experiments. (F) Induction of NF-κB–binding activity detected by the EMSA assay. CLL B cells were incubated with free G3139 and INP-loaded G3139 at 1μM for 4 hours. The CLL cells were stimulated with 500 ng/mL of CD40L for 1 hour as a positive control. (G) Western blot analysis of NF-κB (p65) phosphoration. CLL B cells were incubated with free G3139 and INP-loaded G3139 at 1μM for 3 hours, followed by lysis for Western blot analysis. (H) Differential cytokine inductions of IL-6, TNF-α, and IFN-γ on CLL B cells. Primary CLL B cells were treated under the indicated conditions for 48 hours. Supernatants were collected for ELISA analysis.
Overexpression of Bcl-2 has been attributed to the increased resistance to spontaneous and Ab- or chemotherapy-induced apoptosis. CD19+ CLL B cells treated with RIT-INP–G3139 for 24 hours followed by 48 hours of fludarabine had a significant increase in death compared with PBS and HER-INP–G3139 treatments (Figure 3E). Furthermore, the enhanced apoptosis observed with RIT-INP–G3139 plus fludarabine was significantly higher compared with that observed with RIT-INP–G3622 plus fludarabine. The free G3139 failed to enhance the fludarabine-induced cytotoxicity in CLL B cells compared with the cells treated by fludarabine alone. Interestingly, CLL B cells treated with free G3139 at approximately 1-5μM showed reduced death from fludarabine (supplemental Figure 8). The up-regulation of Bcl-2 and Mcl-1 promoted by free G3139 and associated TLR9 activation (Figure 1) may contribute to this enhanced resistance to fludarabine-mediated death.
Triggering TLR9 by CpG ODN is known to activate the NF-κB pathway.17,27-29 Consistent with this, marked NF-κB activation was observed with free G3139. However, the RIT-INP–encapsulated G3139 showed reduced activity as detected by the EMSA using the consensus NF-κB–binding ONs (Figure 3F). Western blot analysis of phospho-p65 (Ser536) further confirmed that RIT-INP–mediated delivery of G3139 resulted in reduced NF-κB activation compared with free G3139, as evidenced by reduced phospho-p65 (Figure 3G). Consistent with the observed effects on NF-κB activation, significantly lower levels of NF-κB–regulated IL-6 and TNF-α were observed in supernatants obtained from B-CLL cells treated with RIT-INP–G3139 compared with free G3139 (Figure 3H). In addition, the activation of NF-κB by free G3139 also explains the observed up-regulation of Bcl-2 and Mcl-1, downstream targets of NF-κB.30,31
Rituximab signaling is not compromised in RIT-INP formulations
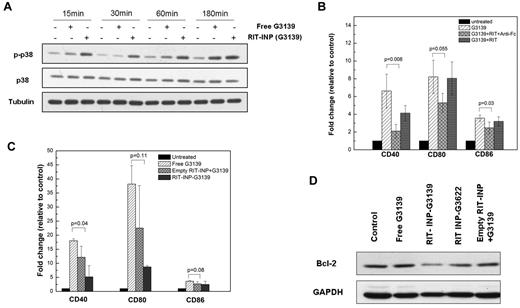
Rituximab is a therapeutic signaling Ab that has been demonstrated to induce phosphorylation of p38 in CLL B cells when cross-linked.32 Given the requirement for cross-linking in rituximab-mediated p38 MAPK activation and cytotoxicity, we tested the effect of free and RIT-INP–G3139 on p38 MAPK phosphorylation. In contrast to free G3139, RIT-INP–G3139 demonstrated potent phosphorylation of p38 MAPK (Figure 4A), which was further confirmed by intracellular staining of p-p38 detected by flow cytometry (supplemental Figure 9A). Empty RIT-INPs, but not empty HER-INPs, also showed similar phosphorylation of p38 (supplemental Figure 9B), indicating that the RIT-INP formulation preserved rituximab-induced signaling, presumably through the liposomal nanoparticle–facilitated cross-linking effect. Consistent with this, although rituximab alone did not alter G3139-induced CD40, CD80, and CD86 expression, rituximab together with anti-Fc cross-linker significantly inhibited free G3139–mediated activation (Figure 4B). Further, the empty RIT-INPs also demonstrated inhibition of free G3139–induced activation (Figure 4C). As shown in Figure 4D, G3139 encapsulated in RIT-INPs achieved the best Bcl-2 down-regulation.
Rituximab signaling is not compromised in RIT-INP formulation. (A) Time-dependent changes of p38 in CLL B cells activated by free G3139 and G3139-containing RIT-INPs. The p38 MAPK activation in CLL B cells treated by free G3139 (1μM) and RIT-INP–encapsulated G3139 (1μM) was monitored by Western blotting. (B) Partial inhibition of costimulation of free G3139 by cross-linking of rituximab with anti-Fc. Fold changes of costimulatory molecules on CLL B cells with treatment of G3139 (1μM), RIT (10 μg/mL), and anti-Fc (50 μg/mL) after 48 hours were measured by flow cytometry. Results are shown as means of n = 3 independent experiments. (C) Partial inhibition of costimulation of free G3139 with cross-linking of empty INP. Similarly, CLL B cells were treated by free G3139 plus empty RIT-INP for 48 hours and then the expressions of costimulatory molecules were measured by flow cytometry (n = 3, mean ± SEM). (D) The effect of CLL B cells cotreated by free G319 and empty RIT-INP on Bcl-2 down-regulation. Comparison of protein levels of CLL B cells treated by free G3139 (1μM) and G3139 (1μM) plus empty RIT-INP and RIT-INP–G3139 (1μM) were measured by Western blotting after 48 hours.
Rituximab signaling is not compromised in RIT-INP formulation. (A) Time-dependent changes of p38 in CLL B cells activated by free G3139 and G3139-containing RIT-INPs. The p38 MAPK activation in CLL B cells treated by free G3139 (1μM) and RIT-INP–encapsulated G3139 (1μM) was monitored by Western blotting. (B) Partial inhibition of costimulation of free G3139 by cross-linking of rituximab with anti-Fc. Fold changes of costimulatory molecules on CLL B cells with treatment of G3139 (1μM), RIT (10 μg/mL), and anti-Fc (50 μg/mL) after 48 hours were measured by flow cytometry. Results are shown as means of n = 3 independent experiments. (C) Partial inhibition of costimulation of free G3139 with cross-linking of empty INP. Similarly, CLL B cells were treated by free G3139 plus empty RIT-INP for 48 hours and then the expressions of costimulatory molecules were measured by flow cytometry (n = 3, mean ± SEM). (D) The effect of CLL B cells cotreated by free G319 and empty RIT-INP on Bcl-2 down-regulation. Comparison of protein levels of CLL B cells treated by free G3139 (1μM) and G3139 (1μM) plus empty RIT-INP and RIT-INP–G3139 (1μM) were measured by Western blotting after 48 hours.
In vivo evaluation of RIT-INP–G3139 in a preclinical model
We chose to evaluate the in vivo therapeutic efficacy of RIT-INP–G3139 in an aggressive Raji cell–inoculated disseminated xenograft mouse model because primary CLL B cells failed to engraft in immunodeficient mice with recapitulation of the disease. Histologic analysis of tissue sections from NOD-SCID mice engrafted with Raji cells revealed extensive infiltration of donor human B cells in the lymph nodes, thymus, and BM 14-18 days after inoculation (supplemental Figure 10). Multifocal neoplastic cell infiltration was also observed in the CNS and brain.33
In vitro, free G3139 neither down-regulated Bcl-2 protein (supplemental Figure 11A) nor caused cell death (supplemental Figure 11B) in the Raji cell line, which is similar to primary CLL B cells. In contrast to CLL B cells, no noticeable CpG-mediated activation was observed with free G3139 in the Raji cell line (supplemental Figure 11C). The RIT-INPs carrying G3139 were much more effective in Bcl-2 down-regulation than free G3139 (Figure 5A). Accordingly, induction of apoptosis by RIT-INP–G3139 was more potent than free G3139 and HER-INP–G3139 (Figure 5B).
Evaluation of in vivo therapeutic efficacy of RIT-INP–G3139. (A) Enhanced Bcl-2 down-regulation of RIT-INP–G3139 in Raji cells. Raji cells were treated with free G3139 (2μM) or various formulated G3139 (2μM) and G3622 (2μM) for 48 hours. Cells were collected and lysed for Western blot analysis. (B) Relative percentage of Raji cell viability of RIT-INP–G3139 normalized to medium control. The percentage of viable cells was determined by annexin V/propidium iodide staining and was analyzed by flow cytometry. Results are presented as means of n = 3 independent experiments. (C) Survival curve for NOD-SCID Raji mice treated with various formulations of G3139 at 5 mg/kg. NOD-SCID mice engrafted with 2 × 106 Raji cells on day 0. The 10-day treatment was initiated via IP injection on day 4. For another in vivo study (D-G), mice were engrafted by Raji cells and treated with free G3139 (5 mg/kg), RIT-INP–G3139 (5 mg/kg), or HER-INP–G3139 (5 mg/kg) on day 10 after inoculation and total 3 treatments were given for each group. On day 15, mice were killed for analysis. (D) Percentage of CD20+ cells from BM assessed by flow cytometry. (E) Immunohistochemical staining of Bcl-2 on BM from the Raji xenograft model. The arrows indicate the intracellular Bcl-2 stained Raji cells (brown color) among the control and treated groups. (F) Fold changes of costimulatory molecules (CD40 and CD86) in cells from BM. Data were normalized to the PBS-treated group (n = 4, mean ± SEM). (G) Cytokine production of IL-6 (n = 4) and IFN-γ (n = 4) in serum from mice treated with PBS, free G3139 (5 mg/kg), and RIT-INP–G3139 (5 mg/kg) or HER-INP–G3139 (5 mg/kg). Cytokine release was determined by ELISA.
Evaluation of in vivo therapeutic efficacy of RIT-INP–G3139. (A) Enhanced Bcl-2 down-regulation of RIT-INP–G3139 in Raji cells. Raji cells were treated with free G3139 (2μM) or various formulated G3139 (2μM) and G3622 (2μM) for 48 hours. Cells were collected and lysed for Western blot analysis. (B) Relative percentage of Raji cell viability of RIT-INP–G3139 normalized to medium control. The percentage of viable cells was determined by annexin V/propidium iodide staining and was analyzed by flow cytometry. Results are presented as means of n = 3 independent experiments. (C) Survival curve for NOD-SCID Raji mice treated with various formulations of G3139 at 5 mg/kg. NOD-SCID mice engrafted with 2 × 106 Raji cells on day 0. The 10-day treatment was initiated via IP injection on day 4. For another in vivo study (D-G), mice were engrafted by Raji cells and treated with free G3139 (5 mg/kg), RIT-INP–G3139 (5 mg/kg), or HER-INP–G3139 (5 mg/kg) on day 10 after inoculation and total 3 treatments were given for each group. On day 15, mice were killed for analysis. (D) Percentage of CD20+ cells from BM assessed by flow cytometry. (E) Immunohistochemical staining of Bcl-2 on BM from the Raji xenograft model. The arrows indicate the intracellular Bcl-2 stained Raji cells (brown color) among the control and treated groups. (F) Fold changes of costimulatory molecules (CD40 and CD86) in cells from BM. Data were normalized to the PBS-treated group (n = 4, mean ± SEM). (G) Cytokine production of IL-6 (n = 4) and IFN-γ (n = 4) in serum from mice treated with PBS, free G3139 (5 mg/kg), and RIT-INP–G3139 (5 mg/kg) or HER-INP–G3139 (5 mg/kg). Cytokine release was determined by ELISA.
Consistent with the in vitro observations, a significant in vivo therapeutic effect of RIT-INPs loaded with G3139 was demonstrated in a NOD-SCID mouse xenograft Raji model (Figure 5C). The median survival time for placebo controls was 15-18 days without liposomal G3139 treatment. CD20-targeting RIT-INP–G3139 showed very significant therapeutic efficacy with > 80% survival rate (n = 14) on day 120 of the study as a single agent compared with the mice treated with the control HER-INP–G3139, all of which showed hind-limb paralysis before day 40.
To further illustrate the underlying mechanism of the potent therapeutic effect by RIT-INP–G3139, we carried out another in vivo study in Raji xenograft NOD-SCID model by starting treatment on day 10 after inoculation with a total 3 treatments for each group. On day 15, mice were killed and cells derived from the BM were analyzed. The depletion of transferred tumor cells in the BM was confirmed by flow cytometry using antibodies directed against human CD20 antigen (Figure 5D). Our ability to detect CD20+ cells in the BM could be attributed to incomplete engagement of all CD20 molecules at the time point tested and/or to internalization of the engaged CD20 with RIT-INP–G3139 that did not mask the remaining CD20 on the surface, which allowed the PE-labeled anti-CD20 Ab to detect the CD20+ cells. Immunohistochemical staining of BM demonstrated down-regulation of Bcl-2 protein expression in the RIT-INP–G3139–treated group (Figure 5E). This indicates that RIT-INP–G3139 can more efficiently down-regulate the Bcl-2 protein expression in the Raji-engrafted model. Cells derived from the BM were further evaluated by intracellular Bcl-2 staining determined by flow cytometry. RIT-INP–G3139–treated NOD-SCID mice expressed reduced Bcl-2 levels compared with the free G3139–treated group (supplemental Figure 12). Furthermore, the up-regulation of CD40 and CD86 molecules was not observed in tumor cells from the BM (Figure 5F), which is in agreement with our in vitro data (supplemental Figure 11C).
G3139 is able to stimulate immune responses in mouse models.18,34,35 Therefore, we examined cytokine production in Raji-engrafted NOD-SCID mice. Although only modest levels of serum IL-6 and IFN-γ were detected in the mice treated with free G3139, the levels of IL-6 and IFN-γ were found to be consistently reduced in the RIT-INP–G3139–treated group (Figure 5G). In addition to the elevated production of IL-6 and IFN-γ, free G3139 or RIT-INP–G3139 treatment also resulted in the enlargement of spleens compared with the saline-treated mice (supplemental Figure 13) on day 16. Splenomegaly and elevated plasma levels of IL-6 and IFN-γ suggest that G3139 can be immunostimulatory even in NOD-SCID mice.35 Because NOD-SCID mice lack functional B and T cells and deficit natural killer cells, the observed pro-inflammatory cytokine response is less likely attributed to natural killer cells.
B cell–specific delivery of the payload and lack of immunostimulation by RIT-INP in whole blood from CLL patients
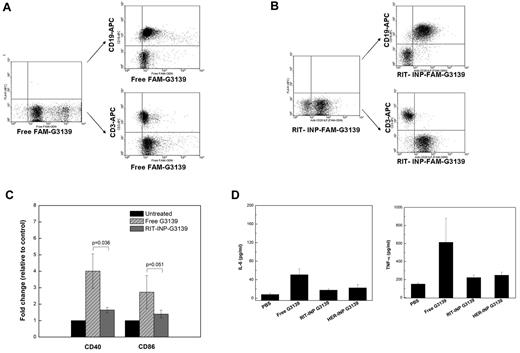
Considering the limitations of an animal model of CLL, further studies are warranted to determine whether inhibiting the immunostimulatory effects of CpG motifs in G3139 by RIT-INPs can minimize the side effects of Bcl-2 antisense treatment in the clinical setting. For in vivo application, it is important to achieve specific targeting and enhanced intracellular delivery and down-regulation of the Bcl-2 target in CD20+ CLL B cells. The selectivity of CD20-targeted RIT-INP was also confirmed in PBMCs, in which FAM-ODN encapsulated in RIT-INPs was selectively taken up by CD20+ B cells but not CD20− T cells identified by CD19 and CD3 staining (Figure 6A). In contrast, free FAM-ODNs (nonformulated) showed nonspecific uptake by both CD19+ B cells and CD3+ T cells (Figure 6B).
B cell–specific delivery of the payload and lack of immunostimulation by RIT-INP in whole blood from CLL patients. (A) Nonselectivity of free FAM-ODN (1μM) in PBMCs. (B) Selectivity of RIT-INP–encapsulated FAM-ODN (1μM) in PBMCs. (C) Fold changes of costimulatory molecules (CD40 and CD86) in whole blood from CLL patients. Expression was determined by flow cytometry. Data were normalized to PBS-treated blood. (D) Cytokine production of IL-6 (n = 4) and TNF-α (n = 4) in serum from patient whole blood treated with PBS, free G3139 (25 μg/mL), and RIT-INP–G3139 (25 μg/mL) or HER-INP–G3139 (25 μg/mL). Cytokine release was determined by ELISA.
B cell–specific delivery of the payload and lack of immunostimulation by RIT-INP in whole blood from CLL patients. (A) Nonselectivity of free FAM-ODN (1μM) in PBMCs. (B) Selectivity of RIT-INP–encapsulated FAM-ODN (1μM) in PBMCs. (C) Fold changes of costimulatory molecules (CD40 and CD86) in whole blood from CLL patients. Expression was determined by flow cytometry. Data were normalized to PBS-treated blood. (D) Cytokine production of IL-6 (n = 4) and TNF-α (n = 4) in serum from patient whole blood treated with PBS, free G3139 (25 μg/mL), and RIT-INP–G3139 (25 μg/mL) or HER-INP–G3139 (25 μg/mL). Cytokine release was determined by ELISA.
The diminished immunostimulatory effects of G3139 by RIT-INPs were further confirmed in the whole blood from CLL patients. Similar to purified B-CLL cells (Figure 2B), immunostimulation of free G3139 was almost completely abrogated in B cells in whole blood from CLL patients after the encapsulation in RIT-INPs (Figure 6C). Consistent with this, reduced levels of IL-6 and TNF-α were also observed in supernatants obtained from the whole blood treated with RIT-INP–G3139 compared with free G3139 (Figure 6D). Similar to the observation in CLL B cells, G3139 induced activation of B cells but not T cells from normal healthy donors that was abrogated by RIT-INP–G3139 (data not shown).
Discussion
Aberrant Bcl-2 expression and the associated poor response to chemotherapy and decreased overall survival in hematologic malignancies have led to the development of novel Bcl-2–directed therapeutic agents. Although small molecules targeting many BH3 domain proteins (ABT263 and AT101) have shown promise, an off-target effect against mature platelets has been observed. More specific targeting of Bcl-2 with antisense ODNs has been hindered by both insufficient delivery and indirect CpG effects of the free antisense ODNs. G3139 has been studied clinically alone or in combination with chemotherapy in CLL. A randomized phase 3 study in patients with relapsed or refractory CLL showed that the addition of G3139 to fludarabine and cyclophosphamide was superior to fludarabine and cyclophosphamide alone in terms of major response (defined as complete response or nodular partial response). Nonetheless, these benefits were very modest, likely reflecting the potential activation of the cells by G3139-mediated CpG effects through TLRs and/or the low plasma (and perhaps intracellular) levels of G3139 obtained in vivo. Further dose escalation was also precluded because of prohibitive toxicity due to cytokine-release syndrome, presumably caused by undesirable cell activation by free G3139. Therefore, the alternative methodologies described herein to overcome the activation properties and targeted delivery of G3139 to B cells offer a new opportunity for this promising therapeutic agent in CLL and other B-cell malignancies.
Our findings confirmed that the sequence-specific antisense mechanism of G3139 targeting to the Bcl-2 protein in CLL is confounded by its immunostimulatory effects associated with up-regulation of antiapoptotic proteins such as Bcl-2 and Mcl-1 via TLR9 (Figure 1). In melanoma cells, very low concentrations of G3139 (eg,100nM) have been shown to induce apoptosis independently of Bcl-2 down-regulation, and G3139 and other phosphorothioate ODNs can block the mitochondrial VDAC channel.36,37 Although free G3139 failed to induce apoptosis in either the Raji B-cell line or CLL patient cells even at concentrations as high as 5μM (Figure 1 and supplemental Figure 8), it caused strong immunostimulation and cytokine release in CLL B cells and CLL patient–derived whole blood samples (Figures 3 and 6), suggesting that the observed poor efficacy of G3139 in recent clinical trials were caused by poor intracellular delivery, suboptimal target down-modulation, and CpG motif–mediated immune stimulation of CLL B cells.
To overcome these limitations, in the present study, we used a novel strategy to minimize the adverse stimulatory effects of G3139 in B-CLL using RIT-INPs. CD20-targeted delivery of G3139 is shown to mediate B-cell preferential uptake, enhanced Bcl-2 target down-regulation, and the associated sensitivity to fludarabine-induced apoptosis in vitro and a significant in vivo therapeutic effect. Selective delivery to CD20+ CLL B cells in PBMCs (Figure 6A) may minimize its nonspecific uptake by other immune cells in vivo. Differential trafficking and cross-linked rituximab-triggered signaling attribute to the differential activation by free G3139 and G3139-encapsulated RIT-INPs in CLL B cells. The RIT-INP–mediated delivery of G3139 prolonged its retention in early endosomal compartments, while free G3139 was predominantly localized in late endosomes or lysosomes (Figure 6A). The weaker immunostimulation of liposomal nanoparticles carrying G3139 is similar to that of CpG-A ODN described previously in CLL.21,28 This phenomenon was not restricted to G3139, because reduced activation of CLL B cells was observed when ODN 2006, a well-known CpG-B ODN, was encapsulated in RIT-INPs (supplemental Figure 3).
The G3139 used in these studies contained the 5′-TCT CCC AGC GTG CGC CAT-3′ sequence. The mismatched control analog G4126 (5′-TCT CCC AGC ATG TGC CAT-3′) and the reverse sequence control G3622 (5′-TAC CGC GTG CGA CCC TCT-3′) are often used as the negative controls in relevant G3139 studies. However, each of these negative controls, including G4126, G3622, and the other tested therapeutic ODNs GTI2040 (targets R2 gene, 5′-GGCTAAATCGCTCCACCAAG-3′) and the widely used ODN2006, (sequence 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′), all retained various levels of B-cell activation properties (data not shown). Our attempts to identify an ideal negative control for G3139 were unsuccessful, so we used reverse sequence control G3622 ON as the “negative” control in our studies. RIT-INP–G3622 exhibited a slight reduction in Bcl-2 expression compared with free G3139 or that observed with HER-INP–G3139. However, RIT-INP–G3139 induced a significant reduction compared with free G3139 (P = .0001), HER-INP–G3139 (P = .0004), or RIT-INP–G3622 (P = .003; Figure 3C). Because free RIT, cross-linked RIT, or empty RIT-INP (supplemental Figure 6) failed to modulate the Bcl-2 levels in the CLL B cells, we attribute the observed effects of RIT-INP–G3622 on Bcl-2 levels to a nonspecific off-target effect.
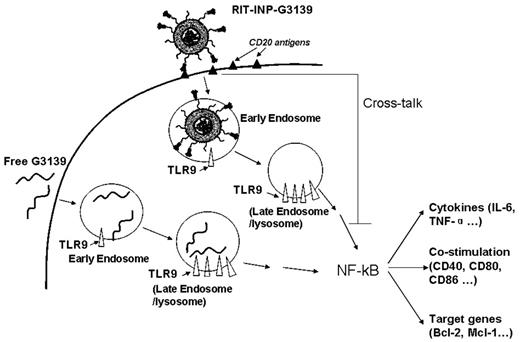
Differential endosomal compartmentalization of CpG ODNs has been shown to result in differential responses such as costimulation and cytokine release in plasmacytoid dendritic cells.38,39 Our studies are the first to show that the immune-targeted liposomal nanoparticles can alter the immunostimulatory effects of CpG ODN by altering its compartment localization in B-CLL cells. Furthermore, the cross-linking effect of rituximab provides additional signaling that inhibits the immunostimulation of G3139 (Figure 4). The cross-linking effect of RIT-INP also explains the observed minimal apoptosis of RIT-INP carrying G3622. Similar findings have been reported recently by others.24 In short, both the intracellular compartment effect of liposomal nanoparticles and the cross-linking effect of rituximab lead to the reduced activation of G3139 in RIT-INPs, which is elucidated schematically in Figure 7.
Proposed mechanisms of the reduced activation of G3139 in RIT-INPs. The recognition of G3139 by TLR9 mainly in late endosomes activates NF-κB pathway due to the CpG motifs in the G3139 sequence, thereby up-regulating its downstream target antiapoptotic proteins and inducing cytokine release. We present a new strategy of using INPs to control endosomal compartmentalization of ONs using the preserved signaling from antibodies on INPs. The early endosomal retention of liposomal nanoparticles and the cross-linking effect of rituximab lead to the reduced activation of G3139 in RIT-INPs, thus enhancing their gene-silencing effects in leukemic cells in vitro and in vivo.
Proposed mechanisms of the reduced activation of G3139 in RIT-INPs. The recognition of G3139 by TLR9 mainly in late endosomes activates NF-κB pathway due to the CpG motifs in the G3139 sequence, thereby up-regulating its downstream target antiapoptotic proteins and inducing cytokine release. We present a new strategy of using INPs to control endosomal compartmentalization of ONs using the preserved signaling from antibodies on INPs. The early endosomal retention of liposomal nanoparticles and the cross-linking effect of rituximab lead to the reduced activation of G3139 in RIT-INPs, thus enhancing their gene-silencing effects in leukemic cells in vitro and in vivo.
Similar to G3139, unsuccessful clinical studies with other phosphorothioate antisense ODN may indicate the dominance of off-target mechanisms such as TLR9-driven action over the desired antisense mechanism.17,27 To avoid TLR-mediated immunostimulation, the development of PS ODNs without CpG motifs has been attempted. However, the modified PS ODNs still retained significant immunostimulatory responses in B cells and plasmacytoid dendritic cells possibly mediated through TLR activation.38,40,41 Similar off-target effects were also observed in siRNA therapeutics, which can be recognized by TLR3 or TLR7/8.3,42 The antisense ODN delivery by INPs described herein could be of great benefit because this approach eliminates adverse immunologic effects.
The in vivo therapeutic studies provide evidence that the antitumor effect of RIT-INP–G3139 in this model is due to a Bcl-2 antisense effect that is independent of CpG immune stimulation. Based on these exciting preclinical data, further clinical development of RIT-INP–G3139 is warranted to resurrect the use of G3139 in CLL and other B-cell malignancies in which Bcl-2 is implicated in pathogenesis and survival. RIT-INP–G3139 could be used alone or in combination with fludarabine. Several factors, such as insufficient dosage and duration of Bcl-2 down-regulation, insufficient or variable surface CD20 expression, and the reduced CD20 internalization property, may contribute to limited therapeutic efficacy with RIT-INP–G3139. Detailed pharmacokinetic and pharmacodynamic evaluation of the RIT-INP–G3139 formulations is warranted to achieve a reproducible therapeutic outcome. Our recent report suggests that the treatment of CLL B cells with lenalidomide can enhance the CD20-mediated internalization and delivery of the CD20-directed immunoliposomal particles.43 It is therefore feasible to combine these with lenalidomide to enhance CD20-targeted delivery of RIT-INP–G3139 in CLL B cells.
In conclusion, the results of the present study have established preclinical evidence for the feasibility of using RIT-INP–G3139 therapy in the treatment of CLL. A similar strategy using relevant antibodies to tumor-specific antigens might also extend this approach to other malignancies. The application of this approach in selective targeting and to circumvent the immunostimulatory properties of novel ON-based therapeutics, including antisense, siRNA, and miRNA therapies, should have broader implications in targeted therapy in lymphoid and other malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Science Foundation Nanoscale Science and Engineering Center (grant EEC-0425626), the National Institutes of Health (R01 CA135332, R01 CA135243, and P50-CA140158), the Leukemia & Lymphoma Society, and the D. Warren Brown Foundation.
National Institutes of Health
Authorship
Contribution: B.Y. designed and performed the research, analyzed the data, and wrote the manuscript; Y.M. assisted in the animal studies and reviewed the manuscript; L.-Y.B., S.E.M.H., A.R., Y.J., X.W., and C.C. assisted with the research and reviewed the manuscript; X.M., K.K.C., D.J., and G.M. analyzed the data and reviewed the manuscript; R.J.L. and J.C.B. designed the research, reviewed the manuscript drafts, and approved the final version of the manuscript; L.J.L. and N.M. designed and supervised the research, analyzed the data, reviewed the manuscript drafts, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natarajan Muthusamy, DVM, PhD, Associate Professor of Medicine, Division of Hematology, 455E, OSUCCC, 410 West 12th Ave, Columbus, OH 43210; e-mail: raj.muthusamy@osumc.edu; or L. James Lee, PhD, Professor of Chemical and Biomolecular Engineering, Nanoscale Science and Engineering Center, 1012 Smith Laboratory, 174 W 18th Ave, Columbus, OH 43210; e-mail: lee.31@osu.edu.
References
Author notes
R.J.L., J.C.B., L.J.L., and N.M. are co–senior authors and contributed equally to this work.