Key Points
We evaluated interference with integrin alpha4–mediated stromal adhesion as a new acute lymphoblastic leukemia treatment.
Integrin alpha4 blockade using natalizumab in combination with chemotherapy sensitizes pre-B acute lymphoblastic leukemia to chemotherapy.
Abstract
Bone marrow (BM) provides chemoprotection for acute lymphoblastic leukemia (ALL) cells, contributing to lack of efficacy of current therapies. Integrin alpha4 (alpha4) mediates stromal adhesion of normal and malignant B-cell precursors, and according to gene expression analyses from 207 children with minimal residual disease, is highly associated with poorest outcome. We tested whether interference with alpha4-mediated stromal adhesion might be a new ALL treatment. Two models of leukemia were used, one genetic (conditional alpha4 ablation of BCR-ABL1 [p210+] leukemia) and one pharmacological (anti-functional alpha4 antibody treatment of primary ALL). Conditional deletion of alpha4 sensitized leukemia cell to nilotinib. Adhesion of primary pre-B ALL cells was alpha4-dependent; alpha4 blockade sensitized primary ALL cells toward chemotherapy. Chemotherapy combined with Natalizumab prolonged survival of NOD/SCID recipients of primary ALL, suggesting adjuvant alpha4 inhibition as a novel strategy for pre-B ALL.
Introduction
Although the overall prognosis of pediatric acute lymphoblastic leukemia (ALL) has improved, relapse, originating from leukemia cells that have evaded chemotherapy, continues to occur. Contact to bone marrow (BM) stromal cells is required for survival of ALL cells in the presence of chemotherapy.1-3 The integrin alpha4 chain associates with the integrin β1 chain to form very late antigen-4 (VLA4),4 which binds to its counter receptors vascular cell adhesion molecule (VCAM)-1, fibronectin, or osteopontin,5,6 and regulates homing, adhesion, and engraftment of hematopoietic progenitors in BM7 and engraftment of ALL cells.8 VLA4 was shown to be a dominant adhesion molecule for acute myeloid leukemia cells,9 indicating that alpha4 expression might be an unfavorable risk factor in acute myeloid leukemia, but alternative observations have also been reported.10 Formal studies of the role of alpha4 as a potential therapeutic target in ALL have not been performed. Using genetic and pharmacological models of alpha4 modulation, we tested whether alpha4 blockade can overcome drug resistance in pre-B ALL.
Study design
Correlation of integrin alpha4 gene expression on leukemic blasts with clinical outcomes of pre-B ALL patients
Patient clinical and outcome data were obtained from the National Cancer Institute TARGET Data Matrix of the Children’s Oncology Group Clinical Trial P9906.11 Analysis is described in the “Supplemental Methods.” All studies have been approved by the institutional review board or Institutional Animal Care and Use Committee of Children's Hospital Los Angeles. Human studies were conducted in accordance with the Declaration of Helsinki.
In vitro and in vivo studies with integrin alpha4fl/fl cells transduced with BCR/ABL1 (p210)
Quantitative reverse transcriptase-polymerase chain reaction (PCR), PCR, and flow cytometry
Information is listed in “Supplemental Methods” and supplemental Tables 1 and 2.13-15
Patient ALL samples and cell lines
Primary pre-B ALL samples were used for in vivo and in vitro as described in “Supplemental Methods.”12
Pharmacological integrin alpha4 blockade
A detailed protocol for in vivo and in vitro assays can be found in “Supplemental Methods.”
Results and discussion
Integrin alpha4 expression on pre-B ALL cells inversely correlates with clinical outcome of patients with ALL
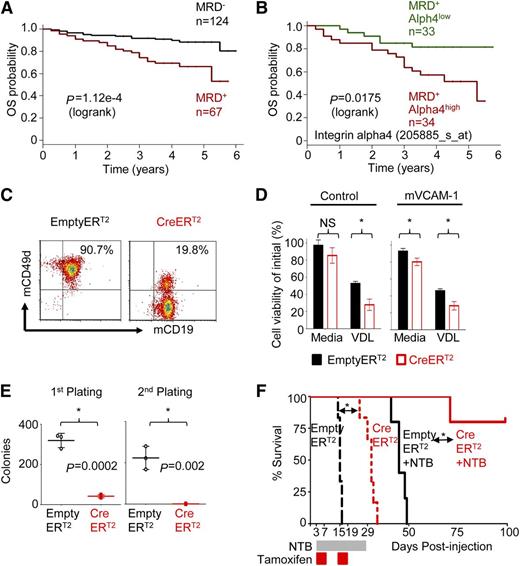
To determine the role of alpha4 in ALL, expression of alpha4 mRNA (ITGA4) in 207 ALL patients uniformly treated with the Children’s Oncology Group P9906 clinical trial11 was correlated with outcome. Overall survival of minimal residual disease–positive patients (MRD+) (n = 67) was analyzed further by alpha4 expression and could be separated into MRD+ alpha4high (ITGA4 expression ≥ mean; n = 34) and MRD+ alpha4low-expressing cases (ITGA4 expression < mean, n = 33) (Figure 1A-B). Alpha4high leukemias were associated with inferior outcome (supplemental Figure 1A-C), indicating the potential use of alpha4 as a therapeutic target because it is expressed especially highly in the prognostically poorest cases.
Integrin alpha4 expression inversely correlates with clinical outcomes of pre-B ALL patients and mediates adhesion-dependent chemoprotection in leukemia cells. (A) Kaplan-Meier estimates of overall survival (OS) for ALL patients negative (black) or positive (red) with MRD at the end of the induction chemotherapy cycle of flow cytometry (day 29).11 (B) Analysis of the OS of MRD-positive patients (MRD+) (n = 67) and alpha4 expression (205885_at) separates MRD+ integrin alpha4high (alpha4 expression ≥ mean; n = 34) and MRD+ alpha4low expressing cases (alpha4 expression < mean, n = 33) (P = .0175, log-rank test). (C) Deletion of alpha4 induced by tamoxifen was confirmed by flow cytometry 6 days after treatment. (D) Alpha4-deleted cells (CreERT2: red) and nondeleted control cells (Empty-ERT2: black) were cultured with mVCAM-1(+) or without mVCAM-1 (control). Cells were then treated with standard chemotherapy VDL (0.005 µM vincristine, 0.05 nM dexamethasone, 0.005 IU/mL L-asparaginase) for 4 days. Cell viability relative to the initial viability on day 0 was assessed by Trypan blue exclusion of dead cells. *P < .05, mean ± standard deviation (SD), unpaired t test, 3 independent experiments performed in triplicate. NS, nonsignificant. (E) Colony-forming ability in primary and secondary platings. *P < .05, mean ± SD, unpaired t test, 3 independent experiments performed in triplicate. (F) Kaplan-Meier survival curve of alpha4-CreERT2 and Empty-ERT2 cells injected C57/BL6 Ly5.1+ recipient mice treated with or without nilotinib (NTB). MST was calculated for each group by log-rank test.
Integrin alpha4 expression inversely correlates with clinical outcomes of pre-B ALL patients and mediates adhesion-dependent chemoprotection in leukemia cells. (A) Kaplan-Meier estimates of overall survival (OS) for ALL patients negative (black) or positive (red) with MRD at the end of the induction chemotherapy cycle of flow cytometry (day 29).11 (B) Analysis of the OS of MRD-positive patients (MRD+) (n = 67) and alpha4 expression (205885_at) separates MRD+ integrin alpha4high (alpha4 expression ≥ mean; n = 34) and MRD+ alpha4low expressing cases (alpha4 expression < mean, n = 33) (P = .0175, log-rank test). (C) Deletion of alpha4 induced by tamoxifen was confirmed by flow cytometry 6 days after treatment. (D) Alpha4-deleted cells (CreERT2: red) and nondeleted control cells (Empty-ERT2: black) were cultured with mVCAM-1(+) or without mVCAM-1 (control). Cells were then treated with standard chemotherapy VDL (0.005 µM vincristine, 0.05 nM dexamethasone, 0.005 IU/mL L-asparaginase) for 4 days. Cell viability relative to the initial viability on day 0 was assessed by Trypan blue exclusion of dead cells. *P < .05, mean ± standard deviation (SD), unpaired t test, 3 independent experiments performed in triplicate. NS, nonsignificant. (E) Colony-forming ability in primary and secondary platings. *P < .05, mean ± SD, unpaired t test, 3 independent experiments performed in triplicate. (F) Kaplan-Meier survival curve of alpha4-CreERT2 and Empty-ERT2 cells injected C57/BL6 Ly5.1+ recipient mice treated with or without nilotinib (NTB). MST was calculated for each group by log-rank test.
Integrin alpha4 deletion sensitizes murine BCR-ABL1 (p210)+ leukemia to chemotherapy
First, we compared the course of alpha4-competent and alpha4-deficient murine leukemia. We used BM cells from alpha4fl/fl mice6,16,17 and retrovirally transduced them in vitro using BCR-ABL1(p210)12,18,19 to generate B220+/CD19+ murine leukemia (supplemental Figure 2A). Subsequently, leukemia cells were transduced with either Empty-ERT2 control or Cre-ERT2 vector to delete alpha4 (supplemental Figures 2B-C and S3A-C). Deletion of alpha4 in transduced cells was efficient, as demonstrated by genetic (supplemental Figure 3D) and flow cytometric (Figure 1C) analyses. Alpha4-deficient murine leukemia cells adhered to mVCAM-1–coated plates with markedly reduced efficiency compared with Empty-ERT2 (alpha4 competent) control cells (P < .05) (supplemental Figure 3E) and alpha4-ablation sensitized murine leukemia cells to chemotherapy (Figure 1D and supplemental Figure 3F and supplemental Table 1). Moreover, alpha4 deletion was associated with loss of colony-forming units (CFU) of BCR/ABL1+ cells in primary and secondary platings (Figure 1E and supplemental Table 1). When these in vitro alpha4 predeleted and undeleted murine leukemia cells were injected into sublethally irradiated mice, nilotinib treatment led to prolonged survival of alpha4-deleted recipients as opposed to recipients of alpha4-competent cells (supplemental Figure 3G-H). To account for potential differences in engraftment of deleted leukemia cells, alpha4 deletion was induced in vivo 3 days after transfer of alpha4-competent alpha4-Empty-ERT2 or Cre-ERT2 cells to sublethally irradiated C57/BL6 Ly5.1+ recipient mice. Both cell types showed similar viability and proliferation rate (data not shown). After engraftment, all animals were treated with tamoxifen to induce alpha4 deletion in Cre-ER leukemia cells in vivo. Kaplan-Meier survival analysis revealed prolonged survival of the group receiving alpha4-Cre-ERT2 in vivo ablated leukemia cells compared with the Empty-ERT2 group (mean survival time [MST] = 31 days vs MST = 15 days; P = .0008; Figure 1F). Flow cytometric analyses confirmed complete in vivo alpha4 deletion in sacrificed animals (supplemental Figure 4A-B). Importantly, animals receiving alpha4-Cre-ERT2 murine leukemia cells plus nilotinib survived until the end of follow-up compared with animals receiving alpha4-Empty-ERT2 (ie, nonablated) murine leukemia cells plus nilotinib (MST = undefined vs MST= 45 days; P = .002). Similar results were obtained with intrafemoral injection of leukemia cells (supplemental Figure 4C-D). We further determined that mice died of leukemia-associated anemia and thrombocytopenia as assessed by blood count analysis (supplemental Figure 4E) and that chemotherapy treatment of alpha4-deficient mice did not result in excessive hematopoietic toxicity against normal cells (supplemental Figure 5).
Pharmacological integrin alpha4 blockade sensitizes resistant human leukemia blasts to ALL chemotherapy in vitro, and its addition to standard ALL chemotherapy prolongs survival of NOD/SCID recipients of human pre-B ALL cells in vivo
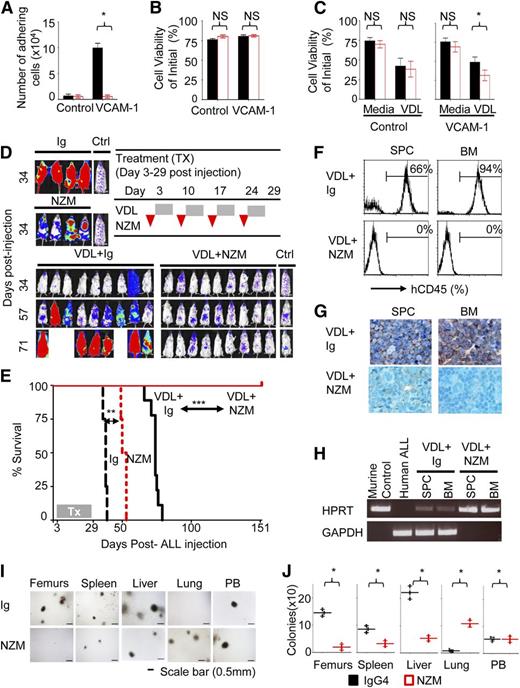
To corroborate our observations in an alternative model, we next tested in a pharmacological model whether alpha4 blockade with antifunctional antibodies can overcome drug resistance of primary human precursor B-ALL cells. The humanized anti-alpha4 monoclonal antibody (mAb) natalizumab (NZM), licensed as a disease-modifying treatment of autoimmune diseases, including multiple sclerosis,20,21 was previously shown to mobilize hematopoietic stem cells into circulation in humans and monkeys.22,23 Chemoresistant LAX7R pre-B ALL cells from a patient with normal karyotype (supplemental Figure 6A-B), who had relapsed despite treatment with chemotherapy, were used for subsequent studies. Anti-alpha4 antibody inhibited adhesion to human VCAM-1 compared with control immunoglobulin (Ig) treatment (Figure 2A and supplemental Figure S7A-B and supplemental Table 2). Marked changes in viability after alpha4-mAb treatment were not observed (Figure 2B). Matched isotypic antibodies served as control. This may not constitute an ideal control antibody, because it does not bind to the ALL cells at a similar density and affinity as the anti-alpha4 antibody, but has been routinely used for similar studies. Alpha4 antibody-dependent cell-mediated cytotoxicity was specifically excluded by demonstrating that natural killer cells (CD56+CD3−) of healthy donors do not impact lysis of primary ALL cells treated with Ig or NZM as determined by a Calcein-AM release assay (supplemental Figure 7C-D). Viability of normal pre-B cells was unaffected by alpha4 mAb treatment (supplemental Figure 8A-B). However, anti-alpha4-mediated de-adhesion significantly sensitized LAX7R cells to chemotherapy (vincristine, dexamethasone, and L-asparaginase, VDL) (Figure 2C and supplemental Table 2). When 6 different primary B-ALL cases were preincubated with function-blocking anti-alpha4 antibodies, we observed that pharmacological alpha4-blockade before in vivo transfer significantly prolonged survival of recipient mice (supplemental Figure 9A-B). We next injected luciferase-labeled LAX7R cells into nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice and subjected them to whole-body in vivo bioluminescent imaging (supplemental Figures 2D and 10A-B). Three days after leukemia cell injection, leukemia cell-bearing mice received 4 weekly injections of function-blocking alpha4 mAb (NZM, a humanized IgG4) or control Ig ± VDL (Figure 2D). Human and murine ALL cells were detected on day 3 after leukemia cell injection by immunohistochemistry (supplemental Figure 11A-B) or by detecting a chromosomal translocation by real-time PCR (supplemental Figure 11C-D), evidence that at least partial BM engraftment had been achieved. Mice treated with NZM survived modestly longer than control antibody-treated mice. Chemotherapy-treated mice relapsed shortly after the end of the 4-week treatment and rapidly succumbed to leukemia, as evidenced by almost complete replacement of normal murine hematopoiesis by human ALL blasts (Figure 2G). In marked contrast, mice treated with chemotherapy plus NZM survived disease-free until day 151 (P < .0001) (Figure 2E), when the animals were euthanized and the absence of human leukemia cells was confirmed using sensitive techniques (Figure 2F-H). These data were confirmed with a repeat experiment with NOD/SCID IL2Rγ−/− hosts (supplemental Figure 12) as well as with 2 additional primary leukemia cases (supplemental Figures S13 and S14). Furthermore, we repeated the experiment starting NZM ± chemotherapy only on day 6 after leukemia cell injection to allow for more time for in vivo expansion of leukemia cells (supplemental Figure 15A-D). Again, NZM ± chemotherapy markedly prolonged survival of leukemia-bearing mice (MST = 75 days vs 147 days). Blood count analysis showed that the death of the animals was due to leukemia-associated anemia and thrombocytopenia (supplemental Figure 15E). We also determined the role of alpha4 in homing and mobilization of primary ALL cells. Anti-alpha4 blockade inhibits homing of primary ALL cells to bone marrow, spleen, and liver; instead, cells were increased in lung compared with control Ig as assessed 18 hours after leukemia injection by CFU counts (Figure 2I-J). Primary ALL cells were mobilized by a 1-time in vivo treatment with NZM out of the bone marrow, spleen, and liver and into the lung and the peripheral blood, whereas proliferation in the bone marrow was unaffected (supplemental Figure 16A-G).
Integrin alpha4 blockade sensitizes primary pre-B ALL cells to chemotherapy. (A) LAX7R cells were plated on bovine serum albumin (BSA) as control or humanVCAM-1 and treated with control IgG4 or anti-alpha4 mAb (NZM). Numbers of viable adhering cells were counted after 48 hours. (B) Cell viability was determined by Trypan blue exclusion of dead cells. NS, nonsignificant (P > .05). (C) LAX7R cells were plated for 3 days on BSA as control or VCAM-1+ and treated with control Ig or anti-alpha4 mAb (NZM) with or without vincristine, dexamethasone, and L-asparaginase (VDL). Depicted is the cell viability by Trypan blue exclusion. *P = .0001 for IgG4+VDL vs NZM+VDL, incubated on VCAM-1–coated plates, mean ± standard deviation (SD), unpaired two-tailed t test, three independent experiments performed in triplicates. (D) Bioluminescent imaging of mice transplanted with LAX7R cells and treated with Ig (n = 4), NZM (n = 4), VDL+Ig (n = 9), or VDL+NZM (n = 9) on day 34, day 57, and day 71 after leukemia cell transfer. A mouse with no leukemia injection treated only with luciferin at time of imaging was included as background control (Ctrl). (E) Kaplan-Meier survival curve was analyzed and MST was calculated for each group: Ig (MST = 38 days), NZM (MST = 52 days), VDL+Ig (MST = 74 days), VDL+natalizumab (euthanized at the end of follow-up, day 151 after leukemia injection). (F) The absence of human LAX7R cells in spleen (SPC) and BM of the VDL+natalizumab group was determined by flow cytometry using an anti-human CD45 Ab. (G) Tissues, including SPC, BM, liver, and lung from two groups were stained with anti-human CD45 antibody by immunohistochemistry (brown). (H) The presence of murine and human DNA in SPC and BM was evaluated using genomic PCR for murine HPRT (hypoxanthine phosphoribosyltransferase) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. (I) Homing of ALL cells to tissues was assessed by CFU assay. (J) Quantified number of colonies. *P < .05, mean ± SD (unpaired two-tailed t test). NS, nonsignificant (P > .05).
Integrin alpha4 blockade sensitizes primary pre-B ALL cells to chemotherapy. (A) LAX7R cells were plated on bovine serum albumin (BSA) as control or humanVCAM-1 and treated with control IgG4 or anti-alpha4 mAb (NZM). Numbers of viable adhering cells were counted after 48 hours. (B) Cell viability was determined by Trypan blue exclusion of dead cells. NS, nonsignificant (P > .05). (C) LAX7R cells were plated for 3 days on BSA as control or VCAM-1+ and treated with control Ig or anti-alpha4 mAb (NZM) with or without vincristine, dexamethasone, and L-asparaginase (VDL). Depicted is the cell viability by Trypan blue exclusion. *P = .0001 for IgG4+VDL vs NZM+VDL, incubated on VCAM-1–coated plates, mean ± standard deviation (SD), unpaired two-tailed t test, three independent experiments performed in triplicates. (D) Bioluminescent imaging of mice transplanted with LAX7R cells and treated with Ig (n = 4), NZM (n = 4), VDL+Ig (n = 9), or VDL+NZM (n = 9) on day 34, day 57, and day 71 after leukemia cell transfer. A mouse with no leukemia injection treated only with luciferin at time of imaging was included as background control (Ctrl). (E) Kaplan-Meier survival curve was analyzed and MST was calculated for each group: Ig (MST = 38 days), NZM (MST = 52 days), VDL+Ig (MST = 74 days), VDL+natalizumab (euthanized at the end of follow-up, day 151 after leukemia injection). (F) The absence of human LAX7R cells in spleen (SPC) and BM of the VDL+natalizumab group was determined by flow cytometry using an anti-human CD45 Ab. (G) Tissues, including SPC, BM, liver, and lung from two groups were stained with anti-human CD45 antibody by immunohistochemistry (brown). (H) The presence of murine and human DNA in SPC and BM was evaluated using genomic PCR for murine HPRT (hypoxanthine phosphoribosyltransferase) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. (I) Homing of ALL cells to tissues was assessed by CFU assay. (J) Quantified number of colonies. *P < .05, mean ± SD (unpaired two-tailed t test). NS, nonsignificant (P > .05).
Taken together, we demonstrated that alpha4 blockade in combination with chemotherapy sensitizes drug- resistant pre-B ALL to chemotherapy, proposing alpha4-blockade as a novel therapy to existing chemotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Donald Kohn (University of California, Los Angeles) for sharing the pCCL-MNDU3-LUC lentiviral vector and Dr Esteban Fernandez of the Imaging Core of the Saban Research Institute for his expert assistance.
This work is supported by grants from the Nautica Triathlon Foundation, Hyundai Hope on Wheels Foundation, and the St. Baldrick’s Foundation Scholar Award (to Y.M.K.). H.B. acknowledges support from LOEWE OSF TP5a and Deutsche Forschungsgemeinschaft BO3553/1-1. M.M. acknowledges support from National Institutes of Health grants (R01CA137060, R01CA139032, and R01CA157644) and Scholar of Leukemia and Lymphoma Society.
National Institutes of Health
Authorship
Contribution: Y.-T.H. and E.J.G. designed and performed research, collected data, performed analysis, and wrote the manuscript; H.G., E.P., S.H., D.C., K.D., P.S., C.S., and H.S. performed research and collected data; M.L., E.-S.K., H.H.K., W.-K.H., J.A., G.M.C., C.L.W., M.M., L.K., S.S., R.P., H.B., N.H., and T.P. contributed vital new reagents, analytical tools, or patient samples and interpreted the data; H.B. designed interpreted data, and wrote the manuscript; and Y.M.K designed, analyzed, interpreted the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Mi Kim, Division of Hematology and Oncology Children’s Hospital Los Angeles, University of Southern California, 4650 Sunset Blvd, Mailstop #57, Los Angeles, CA 90027; e-mail: ymkim@chla.usc.edu.
References
Author notes
Y.-T.H and E.J.G., H.B., and Y.-M.K contributed equally to this study.