Key Points
Mouse hepatocytes express cell surface tissue factor.
Hepatocyte tissue factor activates the coagulation cascade in mice.
Abstract
In this study, we characterized tissue factor (TF) expression in mouse hepatocytes (HPCs) and evaluated its role in mouse models of HPC transplantation and acetaminophen (APAP) overdose. TF expression was significantly reduced in isolated HPCs and liver homogenates from TFflox/flox/albumin-Cre mice (HPCΔTF mice) compared with TFflox/flox mice (control mice). Isolated mouse HPCs expressed low levels of TF that clotted factor VII-deficient human plasma. In addition, HPC TF initiated factor Xa generation without exogenous factor VIIa, and TF activity was increased dramatically after cell lysis. Treatment of HPCs with an inhibitory TF antibody or a cell-impermeable lysine-conjugating reagent prior to lysis substantially reduced TF activity, suggesting that TF was mainly present on the cell surface. Thrombin generation was dramatically reduced in APAP-treated HPCΔTF mice compared with APAP-treated control mice. In addition, thrombin generation was dependent on donor HPC TF expression in a model of HPC transplantation. These results suggest that mouse HPCs constitutively express cell surface TF that mediates activation of coagulation during hepatocellular injury.
Introduction
Tissue factor (TF) is the transmembrane receptor for coagulation factor VII/VIIa (FVII/FVIIa) and the primary activator of the extrinsic blood coagulation cascade.1 Formation of the TF:FVIIa complex triggers activation of a protease cascade culminating in generation of the serine protease thrombin, which initiates fibrin clot formation. Spatial separation of TF from FVII/FVIIa is one mechanism whereby procoagulant activity (PCA) of the TF:FVIIa complex is regulated.2 However, the TF:FVIIa complex can also exist in an encrypted state that has little or no PCA.3 In this molecular state, TF could potentially be expressed on cells in contact with coagulation factors in the blood without triggering pathologic coagulation. Molecular events proposed to increase PCA of the TF:FVIIa complex include interaction of the TF:FVIIa complex with anionic phospholipids on the cell membrane and modification of allosteric disulfides in the TF molecule.3-6 However, the mechanism whereby the TF:FVIIa complex attains full PCA is not completely understood.
TF expression in the liver is very low compared with other organs, such as the heart, brain, and lung.7 In contrast to other vascular endothelial cells, fenestration of endothelial cells lining the liver sinusoids allows for persistent exposure and exchange of plasma components with hepatocytes (HPCs),8 the liver parenchymal cells. HPCs are also the primary cellular source of numerous coagulation factors, including FVII/FVIIa.9,10 Accordingly, TF expression on the surface of HPCs would present a significant regulatory challenge. An increase in the PCA of the TF:FVIIa complex expressed by HPCs could have major pathologic consequences for the liver microcirculation. A previous study found that human HPC membranes had the capacity to generate coagulation factor Xa (FXa).11 Another study suggested that human HPCs expressed both soluble and membrane forms of TF.12 Therefore, the subcellular distribution and regulation of TF:FVIIa PCA in HPCs is not completely understood.
There are numerous diseases in which HPC TF could potentially contribute to pathologic clotting. Disruption of hepatocellular architecture by liver toxicants could potentially trigger TF-dependent activation of the coagulation cascade. Indeed, hepatic injury in mice given a toxic dose of acetaminophen (APAP) occurs concurrently with activation of coagulation, as indicated by increased plasma thrombin-antithrombin (TAT) levels.13 Similarly, patients presenting with APAP overdose have an increase in plasma TAT levels and a decrease in circulating coagulation factors, suggesting that this type of liver injury leads to a consumptive coagulopathy.14,15 We previously have shown that thrombin generation associated with APAP-induced liver injury is significantly reduced in low TF mice (mTF−/−hTF+ mice), which express ∼1% of wild-type TF levels in all tissues compared with APAP-treated control mice expressing 50% of wild-type levels of TF (mTF+/−hTF+ mice).13,16 However, whether HPCs are the primary cellular source of procoagulant TF in this model is not known. Primary isolated HPCs are currently being investigated as an alternative approach to hepatic allograft transplantation. HPC TF could also contribute to the procoagulant response associated with HPC transplantation. Indeed, several studies have reported activation of coagulation in patients undergoing HPC transplantation.17,18 However, the role of donor HPC TF in the procoagulant response in a model of HPC transplantation has not been investigated.
In this study, we characterized TF expression by primary mouse HPCs and evaluated the contribution of TF expressed by HPCs to the generation of thrombin in mouse models of APAP overdose and HPC transplantation.
Materials and methods
Mice
Wild-type C57Bl/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). To selectively delete TF expression in HPCs, TFflox/flox mice backcrossed 6 generations on a C57Bl/6 background19 were crossed with mice expressing Cre recombinase under control of the albumin promoter (Jackson Laboratory, stock no. 003574, congenic C57Bl/6) to generate TFflox/flox/albuminCre mice (HPCΔTF mice) and TFflox/flox mice (control mice). Additionally, low TF mice (mTF−/−hTF+ mice16 ) backcrossed 6 generations onto a C57Bl/6 background between the ages of 8 and 12 wk were used for comparison of whole liver PCA. Mice were housed at an ambient temperature of ∼22°C with alternating 14-/10-h light/dark cycles and provided water and rodent chow ad libitum (Teklad 8604 or 8940; Harlan, Indianapolis, IN) in Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facilities at the University of Kansas Medical Center and Michigan State University. All animal procedures were approved by the University of Kansas Medical Center and/or Michigan State University Institutional Animal Care and Use Committees.
APAP-induced liver injury
Mice fasted overnight were given 300 mg/kg APAP (at 30 µL/g body weight) in sterile saline via an intraperitoneal injection and food was returned. Two hours after APAP administration, mice were anesthetized using isoflurane, and blood was collected from the caudal vena cava into a syringe containing sodium citrate (0.38% final) or an empty syringe for the collection of plasma and serum, respectively.
HPC isolation
Mouse HPCs were isolated by collagenase digestion as previously described.20 HPC viability was evaluated by trypan blue exclusion and a viability of ≥85% was set as the minimum criteria for HPC utilization. Hepatic nonparenchymal cells contaminating HPCs are ∼5% of total cells, as previously described.21 Detailed methods are available in the supplemental Materials.
HPC transplantation
Primary mouse HPCs isolated from control mice or HPCΔTF mice were plated and allowed to adhere for ∼2 h, washed twice with HBSS, then gently scraped into HBSS and collected by centrifugation at 50 × g for 2 min. The cells were then gently resuspended at a density of 2 × 106 cells/mL in Hanks balanced salt solution (HBSS). Recipient mice were anesthetized with isoflurane to achieve deep anesthesia and maintained on a heating pad (SnuggleSafe heat pad [Lentric C21, Littlehampton, UK]) for the duration of the transplant procedure. A laparatomy was performed, the portal vein visualized, and 2 × 105 HPCs (in 100 μL) were injected into the portal vein using a 31-gauge insulin syringe (BD, Franklin Lakes, NJ). The surgical area was covered with sterile gauze soaked in warmed sterile saline post injection. Fifteen minutes after injection of HPCs, blood was collected from the caudal vena cava into sodium citrate [0.38% final] or an empty syringe for the collection of plasma and serum, respectively.
Single-stage clotting assay
Cellular PCA was determined using a single-stage clotting assay as previously described.22 Detailed methods are available in the supplemental Materials.
FXa generation assay
TF-dependent FXa generation by HPCs was determined using an assay based on Khorana et al.23 HPCs isolated from control or HPCΔTF mice were plated on 6- or 24-well plates in FBS containing medium and allowed to adhere for 2 h. The medium was then aspirated to remove unattached cells and then replaced with 375 µL (24-well plate) or 750 µL (6-well plate) of warmed (37°C) sterile HBSA (137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 0.1% bovine serum albumin) immediately followed by the addition of 125 µL (24-well plate) or 250 µL (6-well plate) of 600 nM FX (150 nM final) in HBSA with 20 mM calcium chloride (5 mM final) or vehicle (HBSA with 20 mM calcium chloride without FX) and allowed to incubate at 37°C for 15 min. The reaction was then stopped by the addition of 125 µL of 25 mM EDTA (pH 7.4; 5 mM final). The resulting supernatant was collected and spun at 50 × g for 2 min at 4°C to clear any potential contaminating HPCs. To evaluate the effect of lysis on TF activity, adherent HPCs in 6-well plates were scraped into 750 μL HBSA containing 15 mM N-octyl-β-D-glucopyranoside. The cell suspension was vortexed for 1 min at high speed, incubated for 15 min at 37°C, followed by the addition of 250 µL of 600 nM FX (150 nM final) in HBSA with 20 mM calcium chloride (5 mM final) or vehicle (HBSA with 20 mM calcium chloride without FX) and allowed to incubate at 37°C for 15 min. The reaction was then stopped by the addition of 250 µL of 25 mM EDTA (pH 7.4; 5 mM final). For assessment of FXa generation, 125 µL of sample was added to a 96-well plate in the presence of 115 nM rivaroxaban (FXa inhibitor, MSU Clinical Center Pharmacy) or vehicle (dimethylsulfoxide; J.T. Baker, Phillipsburg, NJ) and 25 µL of 4 mM Pefachrome FXa 8595 (0.667 mM final; Pentapharm, Norwalk, CT), and the change in absorbance at 405 nM was evaluated for 120 min at 37°C using an Infinity M200 plate reader (Tecan, Durham, NC). The average change in absorbance per minute for each sample was compared with a standard curve generated using human FXa (Enzyme Research Laboratories) and FXa generation was expressed as pM/min. Lysed samples were diluted 1:10 prior to determination of FXa generation. In select experiments, adherent intact HPCs were incubated for 15 min at 37°C with HBSA containing either various concentrations of rat anti-mouse TF antibody (clone 1H1) or serum-free medium containing 15 mM sulfo-NHS-SS-biotin. The cells were then washed with HBSA and FXa generation was determined in both intact and lysed HPCs.
RNA isolation, cDNA synthesis, and PCR
RNA was isolated from HPCs using TRI Reagent (MRC Inc., Cincinnati, OH) according to the manufacturer’s protocol. One microgram of total RNA was used for the synthesis of complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) and a C1000 Thermal Cycler (Bio-Rad). Relative levels of mouse full-length TF were determined from 25 ng of cDNA using polymerase chain reaction (PCR) with conditions previously described24 : forward primer 5-GACGAGATCGTGAAGGATGT-3, reverse primer 5-CAGATATGGACAGGAGGATGAT-3 (bases 437–456 and 977–998 of the mouse TF cDNA [NCBI NM010171] in exons 3 and 6, respectively). Levels of HPRT mRNA were assessed as a control for loading (forward primer 5-AAGCCTAAGATGAGCGCAAG-3, reverse primer 5-TTACTAGGCAGATGGCCACA-3) and were resolved and visualized on a 2% agarose gel containing ethidium bromide. An expected, the PCR product for full-length TF was 562 bp (exons 3, 4, 5, and 6) and 104 bp for HPRT.
Clinical chemistry
The serum activity of alanine aminotransferase (ALT) was determined using a commercially available reagent (Thermo Fisher, Waltham, MA). Plasma TAT levels were determined using a commercial enzyme-linked immunosorbent assay kit (Siemens Health Care Diagnostics, Deerfield, IL). For each assay, data were acquired utilizing an Infinite M200 plate reader (Tecan).
Statistics
A comparison of 2 groups was performed using a Student’s t test. Comparison of 3 or more groups was performed using 1- or 2-way analysis of variance, as appropriate, and the Student-Newman-Keuls post hoc test. The criterion for statistical significance was P < .05.
Results
HPC expression of TF represents the major source of PCA in the liver
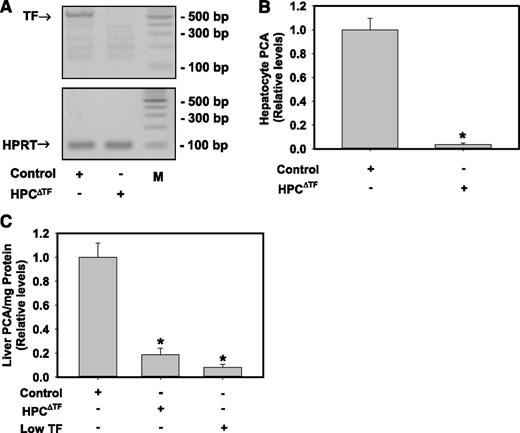
To determine the efficiency of Cre-recombinase deletion of the TF gene in HPCs of HPCΔTF mice, we evaluated levels of TF mRNA and total PCA in HPCs. TF mRNA levels (Figure 1A) were reduced in HPCs isolated from HPCΔTF mice compared with HPCs from TFflox/flox mice (control mice). Assessment of total PCA in lysed HPCs using a single-stage clotting assay revealed a dramatic reduction (>95%) of PCA in HPCs isolated from HPCΔTF mice compared with HPCs from control mice (Figure 1B). To evaluate the contribution of HPC TF to whole liver PCA, a single-stage clotting assay was performed on whole liver homogenates. Compared with control mice, PCA in whole liver homogenates was reduced by ∼80% in HPCΔTF mice and 90% in low TF mice (Figure 1C). These data suggest that HPCs express TF and that the majority of the TF-dependent PCA in whole liver comes from HPCs.
HPCs express TF and contribute the majority of TF-dependent PCA in liver. (A) Representative agarose gel visualization of a TF PCR amplicon from primary HPCs isolated from control mice (TFflox/flox mice) or HPCΔTF mice. HPRT is shown for loading. M: DNA ladder. (B) TF-dependent PCA from lysates of control or HPCΔTF HPCs. Data are expressed as mean ± SEM relative to control HPCs (n = 3 independent experiments). *Significantly different from control HPCs (P < .05). (C) PCA of liver homogenates from control mice, HPCΔTF mice, and low TF mice. Data are expressed as mean +SEM relative to control mice (n = 3-5 mice/group). *Significantly different from control liver homogenates (P < .05).
HPCs express TF and contribute the majority of TF-dependent PCA in liver. (A) Representative agarose gel visualization of a TF PCR amplicon from primary HPCs isolated from control mice (TFflox/flox mice) or HPCΔTF mice. HPRT is shown for loading. M: DNA ladder. (B) TF-dependent PCA from lysates of control or HPCΔTF HPCs. Data are expressed as mean ± SEM relative to control HPCs (n = 3 independent experiments). *Significantly different from control HPCs (P < .05). (C) PCA of liver homogenates from control mice, HPCΔTF mice, and low TF mice. Data are expressed as mean +SEM relative to control mice (n = 3-5 mice/group). *Significantly different from control liver homogenates (P < .05).
TF-dependent FXa generation by intact mouse HPCs occurs independently of exogenous FVIIa
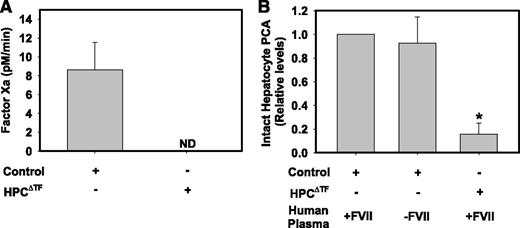
To expand on our studies using the single-stage clotting assay, we used an FXa generation assay to determine HPC TF activity. To our surprise, we found that exogenous human FVIIa, at up to 50 nM, did not impact FXa generation by intact primary mouse HPCs (data not shown). Control HPCs converted exogenous human FX to FXa in the absence of exogenous FVIIa (Figure 2A). Substrate conversion was completely prevented by rivaroxaban, an FXa inhibitor, confirming substrate specificity (data not shown). FXa generation was not detectable in HPCs isolated from HPCΔTF mice (Figure 2A), consistent with deletion of TF mRNA in HPCs and no effect of potential contaminating TF expressing nonparenchymal cells. This result indicates that TF-dependent FXa generation in control HPCs does not require exogenous FVII/FVIIa, suggesting that TF expressed on the surface of primary HPCs is preloaded with FVIIa. To further explore this possibility, we again used a single-stage clotting assay. Human FVII/FVIIa has an ∼6-fold lower affinity for mouse TF, and, by extension, single-stage clotting assays for mouse TF typically employ a small percentage of mouse plasma as a source of murine FVII/VIIa.25-27 Nonetheless, control mouse HPCs had the capacity to clot pooled normal human plasma, and this PCA was markedly reduced in HPCs isolated from HPCΔTF mice (Figure 2B). Moreover, the observed PCA of control HPCs was unaffected by the substitution of FVII-deficient human plasma in the single-stage clotting assay (Figure 2B). Taken together, these results suggest that intact HPC TF is prebound with FVII/FVIIa.
HPC TF PCA does not require exogenous FVIIa. (A) Assessment of FXa generation by intact primary HPCs isolated from control mice (TFflox/flox mice) or HPCΔTF mice. FXa generation by HPCs was assessed in the absence of exogenous FVIIa (see Methods). FXa levels are expressed as mean + SEM (pM/min) from 3 independent experiments. FXa generation was not detectable (ND) in cells from HPCΔTF mice. (B) PCA of intact control or HPCΔTF primary HPCs determined using a single-stage clotting assay using normal human pooled plasma or FVII-deficient human plasma. Data are expressed as mean fold change + SEM of control HPCs clotted with normal human pooled plasma from 3 independent experiments. *Significantly different from control HPCs clotted with normal human pooled plasma (P < .05).
HPC TF PCA does not require exogenous FVIIa. (A) Assessment of FXa generation by intact primary HPCs isolated from control mice (TFflox/flox mice) or HPCΔTF mice. FXa generation by HPCs was assessed in the absence of exogenous FVIIa (see Methods). FXa levels are expressed as mean + SEM (pM/min) from 3 independent experiments. FXa generation was not detectable (ND) in cells from HPCΔTF mice. (B) PCA of intact control or HPCΔTF primary HPCs determined using a single-stage clotting assay using normal human pooled plasma or FVII-deficient human plasma. Data are expressed as mean fold change + SEM of control HPCs clotted with normal human pooled plasma from 3 independent experiments. *Significantly different from control HPCs clotted with normal human pooled plasma (P < .05).
Isolated primary mouse HPCs express encrypted TF on the cell surface.
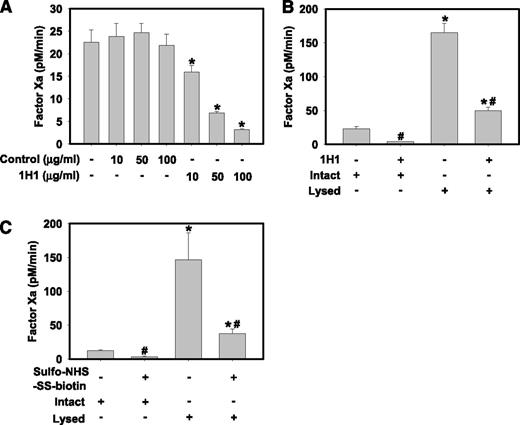
HPCs are persistently exposed to plasma in vivo and it is therefore likely that the majority of cell surface TF PCA is encrypted. Compared to incubation with isotype control antibody, incubation of intact control HPCs with various concentrations of an inhibitory rat anti-mouse TF antibody (clone 1H1) inhibited FXa generation by intact control HPCs in a concentration-dependent manner (Figure 3A). This strongly suggests that a portion of TF on the surface of isolated HPCs has PCA. Next, we determined the effect of control HPC lysis with the detergent N-octyl-β-D-glucopyranoside on FXa generation. Compared with intact cells, HPC lysis dramatically increased FXa generation, suggesting that the majority of the total TF PCA was encrypted prior to lysis (Figure 3B-C). To determine whether lysis increased the PCA of cell surface TF, similar studies were performed in which intact control HPCs were incubated with 100 μg/mL 1H1, then washed prior to lysis, to target only cell surface TF with 1H1. Using this approach, the 1H1 antibody inhibited ∼70% of total FXa generation by lysed HPCs (Figure 3B). Next, intact control HPCs were incubated with 15 mM sulfo-NHS-SS-biotin, a membrane-impermeable compound that reacts with free amines in proteins, including the side chain of lysine. Previous studies have shown that several lysine residues in the TF molecule are critical for activation of FX by the TF:FVIIa complex,28-30 and sulfo-NHS-SS-biotin was previously used to inhibit surface TF PCA.31-33 Similar to 1H1, sulfo-NHS-SS-biotin significantly reduced FXa generation by intact control HPCs and also dramatically reduced the lysis-dependent increase in FXa generation (Figure 3C). Taken together, the results indicate that a small portion of TF in isolated HPCs has PCA and that lysis increases the PCA of TF expressed on the HPC cell surface.
Isolated primary mouse HPCs express encrypted TF on the cell surface. Primary HPCs were isolated from control mice (TFflox/flox mice). (A) Intact HPCs were incubated with various concentrations of inhibitory rat anti-mouse TF antibody (clone 1H1, 10-100 μg/mL) or isotype control antibody (clone 54447) for 15 min prior to determination of FXa generation. (B) Intact HPCs were incubated with rat anti-mouse TF antibody (clone 1H1, 100 μg/mL) for 15 min, the unbound antibody was removed by washing, and FXa generation by intact and detergent-lysed (see Methods) HPCs was determined. (C) Intact HPCs were incubated with 15 mM sulfo-NHS-SS-biotin for 15 min, excess reagent removed by washing, and FXa generation by intact and detergent-lysed (see Methods) HPCs was determined. FXa generation by HPCs was assessed in the absence of exogenous FVIIa (see Methods). FXa levels are expressed as mean + SEM (pM/min) from 3 independent experiments. For panel A, *significantly different from respective isotype control group. For panels B-C,* significantly different from respective treatment without lysis. #Significantly different from respective group without TF inhibitor (P < .05).
Isolated primary mouse HPCs express encrypted TF on the cell surface. Primary HPCs were isolated from control mice (TFflox/flox mice). (A) Intact HPCs were incubated with various concentrations of inhibitory rat anti-mouse TF antibody (clone 1H1, 10-100 μg/mL) or isotype control antibody (clone 54447) for 15 min prior to determination of FXa generation. (B) Intact HPCs were incubated with rat anti-mouse TF antibody (clone 1H1, 100 μg/mL) for 15 min, the unbound antibody was removed by washing, and FXa generation by intact and detergent-lysed (see Methods) HPCs was determined. (C) Intact HPCs were incubated with 15 mM sulfo-NHS-SS-biotin for 15 min, excess reagent removed by washing, and FXa generation by intact and detergent-lysed (see Methods) HPCs was determined. FXa generation by HPCs was assessed in the absence of exogenous FVIIa (see Methods). FXa levels are expressed as mean + SEM (pM/min) from 3 independent experiments. For panel A, *significantly different from respective isotype control group. For panels B-C,* significantly different from respective treatment without lysis. #Significantly different from respective group without TF inhibitor (P < .05).
HPC TF is required for early thrombin generation after administration of a hepatotoxic dose of APAP
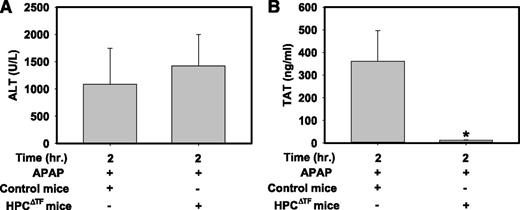
We previously showed that the coagulation cascade is activated in mice given a hepatotoxic dose of APAP (300 mg/kg), as indicated by an increase in plasma TAT levels that peaks ∼2 h after APAP administration.13,34 Of interest, TF mRNA expression was not increased in livers of APAP-treated mice at this time (data not shown). Serum ALT activity was similarly elevated at 2 h in both APAP-treated control mice and APAP-treated HPCΔTF mice (Figure 4A) compared with serum ALT activity in naïve mice (∼25-50 U/L). In agreement with previous studies,13,34 control mice given APAP had elevated plasma TAT levels 2 h after APAP administration (Figure 4B). Plasma TAT levels were dramatically lower in APAP-treated HPCΔTF mice (Figure 4B). Of importance, plasma TAT levels in APAP-treated HPCΔTF mice (13.2 ± 1.2 ng/mL) were similar to APAP-treated low TF mice (17.2 ± 6.2 ng/mL) at 2 h. The data suggest that HPCs are the primary cellular source of TF required for generation of thrombin in APAP-induced liver injury.
HPC TF is required for early thrombin generation in mice given a hepatotoxic dose of APAP. Fasted control mice (TFflox/flox mice) and HPCΔTF mice were treated with 300 mg/kg APAP. (A) Serum ALT activity and (B) plasma TAT levels were determined 2 h after APAP administration. Data are expressed as mean + SEM. *Significantly different from control mice (P < .05). n = 5 to 10 mice/group.
HPC TF is required for early thrombin generation in mice given a hepatotoxic dose of APAP. Fasted control mice (TFflox/flox mice) and HPCΔTF mice were treated with 300 mg/kg APAP. (A) Serum ALT activity and (B) plasma TAT levels were determined 2 h after APAP administration. Data are expressed as mean + SEM. *Significantly different from control mice (P < .05). n = 5 to 10 mice/group.
Donor HPC TF triggers the activation of coagulation after portal venous HPC injection
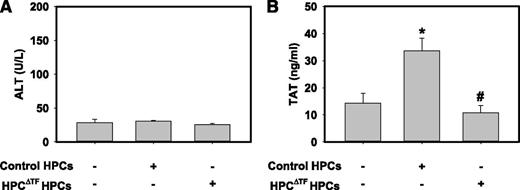
Transplantation of HPCs or HPC-like cells derived from mesenchymal stem cells offers an attractive approach to treat both chronic and acute liver diseases.35,36 Clinical transplantation of HPCs has been accomplished by the injection of donor cells into the portal vein to facilitate hepatic engraftment.36 Contact of transplanted HPCs with blood can precipitate a response termed the instant blood-mediated inflammatory reaction (IBMIR), a component of which is a marked procoagulant response.12,17 Using an ex vivo tubing loop model, a previous study suggested that HPCs were capable of mediating a procoagulant response in whole blood.12 However, the role of TF in this response has yet to be analyzed in vivo. We tested the hypothesis that HPC TF mediates thrombin generation in a model of HPC transplantation. Wild-type recipient mice receiving donor HPCs from control mice or HPCΔTF mice did not develop liver injury as assessed by serum ALT (Figure 5A). However, compared with mice injected with HBSS, plasma levels of TAT were significantly increased in wild-type mice transplanted with control HPCs (Figure 5B). Of importance, the increase in TAT was absent in wild-type mice receiving HPCs from HPCΔTF mice (Figure 5B). The data indicate that donor HPCs are the cellular source of TF mediating the procoagulant response after portal venous injection of HPCs in mice.
Donor HPC TF triggers systemic thrombin generation in a model of HPC transplantation. Anesthetized mice were given 2 × 105 primary control HPCs, HPCΔTF HPCs, or vehicle (100 µL sterile HBSS) via a portal vein injection. (A) Serum levels of ALT activity and (B) plasma TAT were determined 15 min after HPC injection. Data are expressed as mean +SEM. *Significantly different from control mice injected with vehicle (P < .05). #Significantly different from control mice injected with control primary HPCs (P < .05). n = 6 to 7 mice/group.
Donor HPC TF triggers systemic thrombin generation in a model of HPC transplantation. Anesthetized mice were given 2 × 105 primary control HPCs, HPCΔTF HPCs, or vehicle (100 µL sterile HBSS) via a portal vein injection. (A) Serum levels of ALT activity and (B) plasma TAT were determined 15 min after HPC injection. Data are expressed as mean +SEM. *Significantly different from control mice injected with vehicle (P < .05). #Significantly different from control mice injected with control primary HPCs (P < .05). n = 6 to 7 mice/group.
Discussion
Utilizing a genetic approach, we selectively reduced TF expression and activity in mouse HPCs and found that isolated primary mouse HPCs express procoagulant TF on their cell surface. Moreover, the PCA of a large fraction of TF expressed by isolated HPCs was encrypted prior to lysis with detergent. The selective deletion of TF in HPCs allowed us to identify this cell population as the major cellular source of procoagulant TF in normal mouse liver. Of interest, total liver PCA tended to be lower in livers from low TF mice compared with HPCΔTF mice, suggesting that other cell types in normal liver may also contribute to total liver PCA. Indeed, we previously showed that mouse intrahepatic bile duct epithelial cells, which comprise ∼2% to 3% of the total liver cell population, also express TF.22 Overall, these results add an important dimension to previously published studies indicating that human HPCs also express TF.11,12
HPCs are a primary site of FVII/FVIIa synthesis and are persistently exposed to coagulation factors in the plasma.10 Therefore, it seems highly probable that HPCs could express FVII/FVIIa bound to TF. Consistent with this hypothesis, we found that primary mouse HPCs were capable of clotting FVII-deficient human plasma and generated FXa in a TF-dependent manner without the addition of exogenous FVIIa. These results suggest the potential existence of a TF:FVIIa complex on the surface of primary HPCs. This is not the first suggestion of a “preloaded” TF molecule, with work by Hoffman and colleagues37 demonstrating this in squamous epithelium of the dermis. The presence of a TF:FVIIa complex on the surface of HPCs in the highly leaky liver microvasculature highlights the importance of regulating TF PCA beyond interaction of TF with its ligand. However, at present, the mechanism whereby the TF:FVIIa complex is regulated on the surface of mouse HPCs is not clear.
Given that HPCs are exposed to plasma, expression of encrypted TF by HPCs is likely a physiological necessity. The comparison of intact with detergent-lysed primary HPCs in our studies revealed that ∼90% of HPC TF PCA is encrypted. It is unclear whether the small amount of TF activity observed in intact HPCs after isolation mirrors the status of TF expressed by HPCs in vivo. If so, there must be considerably tight control of hepatic coagulation by anticoagulant pathways in the liver. The alternative is that all TF expressed by HPCs in vivo is encrypted unless otherwise provoked, and that the activation of some TF as a consequence of the HPC isolation process is unavoidable. Targeting only cell surface TF using a function blocking antibody or cell-impermeable lysine-conjugating reagent demonstrated that lysis functionally increases the PCA of encrypted TF on the surface of HPCs. Overall, the results indicate that a substantial pool of TF is expressed on the cell surface of HPCs in an encrypted form. The mechanism regulating HPC TF PCA is currently not known, although primary HPCs may provide an exciting opportunity to understand mechanisms of TF encryption in primary cells.
Irrespective of the mechanism whereby HPC TF achieves full PCA, it seems reasonable to speculate that insults, such as exposure to hepatotoxic chemicals, are likely to trigger a procoagulant response involving HPC TF. Indeed, identifying fundamental mechanisms of HPC TF-mediated coagulation is of importance to both liver toxicologists and hepatologists. To determine whether a hepatotoxic response could prompt a HPC TF-dependent thrombin generation, we used a model of APAP overdose in mice. APAP overdose causes acute HPC injury in mice and humans in conjunction with an increase in plasma TAT levels.13-15 Of importance, peak TAT levels were dramatically lower in APAP-treated HPCΔTF mice compared with APAP-treated control mice. In fact, levels were similar to APAP-treated low TF mice, indicating that HPCs are the primary cellular source of procoagulant TF in vivo in this model of liver injury. Of importance, this massive HPC-dependent procoagulant response occurred in the absence of an increase in hepatic TF mRNA expression 2 h after APAP administration. Moreover, the lack of temporal association between peak thrombin generation (∼2 h) and the maximal liver necrosis (∼24 h) suggests a more complex and immediate mechanism of thrombin generation after APAP overdose. Studies evaluating mechanisms of both initiation and expansion of coagulation cascade activation after APAP overdose are ongoing in our laboratory.
In addition to serving as a critical trigger of coagulation after liver damage in vivo, the expression of TF by isolated HPCs has another hugely important clinical indication. Transplantation of HPCs is currently being explored as an approach to treat acute liver injury, chronic liver diseases, and various genetic disorders.35,36 Initiation of the IBMIR by HPCs injected into the portal vein may involve TF.12,17,18 Indeed, we established an in vivo model of portal venous HPC injection resulting in coagulation cascade activation, as determined by increased plasma TAT levels in recipient mice. Of importance, we found that TF expressed by donor HPCs was the sole cellular source of TF required for thrombin generation in this model. Expansion of this model may yield novel strategies to limit HPC-mediated IBMIR in HPC transplantation. Moreover, we posit that pharmacologic approaches selectively limiting TF activity on donor HPCs may ablate the requirement for global anticoagulation in recipients by blocking the primary procoagulant trigger.
In summary, we identified HPCs as the primary cellular source of TF in mouse liver. Moreover, we found that TF on the surface of primary mouse HPCs was bound to FVII/FVIIa and was largely encrypted. HPC TF was essential for coagulation cascade activation in a model of APAP hepatotoxicity in mice and donor HPC TF was required for coagulation in a model of HPC transplantation. Taken together, the data reveal novel aspects of TF regulation in liver and a role of HPC TF in procoagulant responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr. Bruno Hagenbuch and Dr. Amanda Hays at the University of Kansas Medical Center for providing methodology and consulting advice for cell surface lysine conjugation and Dr. Bryan Copple at Michigan State University for technical training of mouse HPC isolation. Additionally, the authors would like to thank Daniel Kirchhofer (Genentech) for the generous gift of anti-TF 1H1 antibody used in these studies.
This work was supported by grants from the National Institutes of Health (R01-DK087886 and R01-ES017537).
National Institutes of Health
Authorship
Contribution: B.P.S. designed and performed research, analyzed and interpreted data, performed statistical analysis and wrote the manuscript; A.K.K. designed and performed research, analyzed and interpreted data, and wrote the manuscript; N.J. designed and performed research, collected and interpreted data, and edited the manuscript; H.C. performed research and edited the manuscript; J.A.B. performed research, analyzed and interpreted data, and edited the manuscript; S.C.B. designed and performed research, analyzed and interpreted data and edited the manuscript; K.M.K. performed research; C.R. performed research and analyzed and interpreted data; N.M. designed research, analyzed and interpreted data, and edited the manuscript; and J.P.L. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James P. Luyendyk, Department of Pathobiology and Diagnostic Investigation, Michigan State University, 348 Food Safety and Toxicology Building, 1129 Farm Lane, East Lansing, MI 48824; e-mail: luyendyk@msu.edu.