In this issue of Blood, Smith and colleagues report on the functional role of the interaction between these 2 proteins by studying the involved binding sites responsible for clot stabilization.1
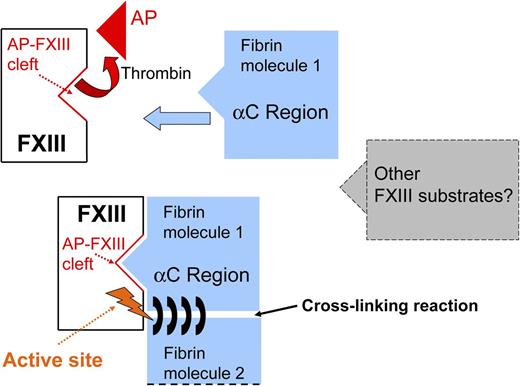
Simplified description of the interaction between activated FXIII and fibrin(ogen). Thrombin induces release of AP-FXIII and exposure of the AP-FXIII cleft. The fibrin αC region binds to the AP-FXIII cleft leading to an ideal position for cross-linking of fibrin chains by the FXIII active site. The same mechanism may also apply to other FXIII substrates.
Simplified description of the interaction between activated FXIII and fibrin(ogen). Thrombin induces release of AP-FXIII and exposure of the AP-FXIII cleft. The fibrin αC region binds to the AP-FXIII cleft leading to an ideal position for cross-linking of fibrin chains by the FXIII active site. The same mechanism may also apply to other FXIII substrates.
Fibrinogen and coagulation factor XIII (FXIII) are the key proteins involved in clot formation producing a stable clot resistant to degradation. Formation of a stable fibrin clot involves the activation of a complex cascade of plasma proteins. The final steps within this cascade lead to a clot that is resistant to degradation. Major key players right at the end of this cascade are FXIII, a transglutaminase, and fibrinogen, whereby fibrinogen provides the matrix that is modified by activated FXIII (FXIIIa), acting as cross-linker. In its role as a transglutaminase, FXIIIa not only cross-links fibrin chains for clot stabilization, but FXIIIa also cross-links other plasma proteins involved in clot formation and fibrinolysis to fibrin(ogen).2
Smith et al have now taken the understanding of FXIII/fibrinogen interaction and binding a big step forward. Using recombinant proteins, a variety of functional assays, inhibitory synthetic peptides, mass spectrometry, and molecular modeling, the authors characterized the functional implications of this interaction and identified the interacting residues on FXIII, as well as the contact sites between the 2 proteins. In a recent publication, a key residue on the α-chain of fibrin involved in the binding of the FXIII A-subunit has already been identified by the same group3 ; however, the binding site on the FXIII A-subunit was not known. In the present work, this binding site could now be characterized. The authors localized the binding site on FXIII at the FXIII activation peptide cleft, which is exposed to the fibrinogen α-chain after the release of the FXIII activation peptide (AP-FXIII) (see figure). The small AP-FXIII consisting of 37 amino acids is released from FXIII after activation by thrombin, and thrombin also activates fibrinogen. That the AP-FXIII cleft plays such an important role in regulating fibrin cross-linking and therefore clot stabilization is indeed an interesting new finding. Only a few years ago, the question on whether AP-FXIII was actually released on cleavage by thrombin was still under debate. Evidence from crystal structure analysis had suggested that cleaved AP-FXIII may stick to its parent protein.4 Then, my group developed the first enzyme-linked immunosorbent assay method using antibodies directed specifically toward free AP-FXIII and showed that AP-FXIII is indeed released into plasma following FXIII activation by thrombin.5,6 We were also able to present first measurements of in vivo–generated free AP-FXIII circulating in plasma of patients with an acute thrombotic event.7 Now, Smith et al1 present the next milestone in the research on the role of AP-FXIII, showing that AP-FXIII release is not optional but, on the contrary, is a prerequisite for FXIIIa to function optimally and efficiently recognize and localize its substrate(s).
Why are the new findings by Smith et al1 important in a wider context? Thrombotic disorders represent one of the most common medical conditions, with a high rate of morbidity and mortality worldwide. Every thrombotic event in the venous vascular tree such as deep vein thrombosis and in the arterial vascular system such as stroke or myocardial infarction is characterized by the formation of fibrin clots, whereby FXIII and fibrinogen are the key players in this acute event. The characterization of the processes on the molecular level has implications for the better understanding of these processes and also for development of new therapeutic strategies.
In addition to that, there may be further implications. Over the last decade, several putative novel intra- and extravascular functions of FXIII have been unveiled because of its unique role as a transglutaminase among the other serine protease coagulation factors.2,8 The somewhat “neglected coagulation factor” in the past became known as a unique and multifunctional protein involved in a wide range of mechanisms beyond coagulation and fibrinolysis. Several proteins beside fibrinogen are FXIII substrates, such as adhesive and extracellular matrix proteins and intracellular cytoskeletal proteins. The interactions between FXIII and the mentioned proteins are also of great interest, and it is possible that the AP-FXIII cleft plays also a central role for binding of these substrates. The knowledge of these binding sites and their implications for substrate interaction might be of interest in many areas of clinical medicine.
In conclusion, the work by Smith et al1 showed that the AP-FXIII cleft plays a central role in substrate binding and extends our knowledge of the interaction between FXIII and fibrin(ogen). This might indeed be the basis to develop targeting molecules that inhibit this interaction and could be used in the therapy of acute arterial and venous thrombotic events.
Conflict-of-interest disclosure: The author declares no competing financial interests.