Key Points
The Rac effector p21-activated kinase (Pak) regulates hematopoietic engraftment.
Pak integrates cytoskeletal changes and proliferation pathways.
Abstract
The p21-activated kinases (Paks) are serine/threonine kinases that are major effectors of the Rho guanosine 5′\x{2011}triphosphatase, Rac, and Cdc42. Rac and Cdc42 are known regulators of hematopoietic stem and progenitor cell (HSPC) function, however, a direct role for Paks in HSPCs has yet to be elucidated. Lin-Sca1+c-kit+ (LSK) cells from wild-type mice were transduced with retrovirus expressing Pak inhibitory domain (PID), a well-characterized inhibitor of Pak activation. Defects in marrow homing and in vitro cell migration, assembly of the actin cytoskeleton, proliferation, and survival were associated with engraftment failure of PID-LSK. The PID-LSK demonstrated decreased phosphorylation of extracellular signal-regulated kinase (ERK), whereas constitutive activation of ERK in these cells led to rescue of hematopoietic progenitor cell proliferation in vitro and partial rescue of Pak-deficient HSPC homing and engraftment in vivo. Using conditional knock-out mice, we demonstrate that among group A Paks, Pak2−/− HSPC show reduced homing to the bone marrow and altered cell shape similar to PID-LSK cells in vitro and are completely defective in HSPC engraftment. These data demonstrate that Pak proteins are key components of multiple engraftment-associated HSPC functions and play a direct role in activation of ERK in HSPCs, and that Pak2 is specifically essential for HSPC engraftment.
Introduction
Hematopoietic stem and progenitor cell (HSPC) engraftment is a highly orchestrated series of biological processes that result in the ability of intravenously administered cells to lodge in the bone marrow (BM) medullary cavity. Subsequent retention and proliferation in the bone marrow space leads to reconstitution of the hematopoietic system. The capacity of the HSPC to transit out of the circulation into the bone marrow, commonly referred to as BM homing, somewhat resembles the migration of leukocytes to sites of inflammation. In the case of leukocytes, this process includes an initial phase of tethering and rolling along the vessel wall followed by firm adhesion to the endothelium via integrin activation, transendothelial migration, and finally chemotaxis through the extracellular matrix to the site of inflammation.1-3 HSPCs migrate toward the medullary space in response to stimulation of the chemokine receptor CXCR4 by binding of stromal-derived factor 1α (SDF-1α). On arrival in the medullary cavity, cell surface molecules on HSPCs interact in specific microenvironment locations termed the stem cell “niche.”4-6 Identifying the signaling pathways that regulate and integrate these HSPC processes is necessary to improve the engraftment efficiency of HSPCs, produce more effective mobilization of these cells, and develop better therapies for the treatment of hematological diseases.
Rac proteins are members of the Rho guanosine triphosphatase (GTPase) family of proteins that have been shown to integrate multiple extracellular signals and play important regulatory roles in HSPC functions such as cell migration, adhesion, proliferation, and survival.7,8 Although, Rac1 and Rac2 proteins have been shown to regulate distinct biological processes, simultaneous deletion of both Rac1 and Rac2 reveal overlapping functions in HSPC survival, engraftment, retention and mobilization.9 Whether Rac proteins use distinct or shared downstream effector proteins to carry out these functions has yet to be determined.
The best characterized Rac effector proteins are the group A p21-activated kinases (Paks). Paks are a family of serine/threonine kinases consisting of 6 isoforms divided into 2 groups: group A (Pak1, Pak2, Pak3) and group B (Pak4, Pak5, Pak6) based on homology.10,11 Among the group A Paks, Pak1 is highly expressed in brain, muscle, and spleen cells; Pak2 is ubiquitously expressed; and Pak3 is specifically expressed in the brain.11 Group A Pak proteins are characterized by 2 canonical N-terminal proline-rich motifs that mediate interactions with Src-homology 3–containing proteins, a p21-binding domain that binds activated (guanosine triphosphate [GTP]-bound) Rac or Cdc42, and a C-terminal kinase domain. Pak A proteins exist as inactive homodimers. This inactive conformation results from the association of the autoinhibitory domain of 1 Pak molecule binding to and interfering with the C-terminal kinase cleft of the second Pak molecule. Activation of Pak is achieved though Rho GTPase binding to the p21-binding domain, relieving autoinhibition through a series of conformational changes allowing the protein to adopt a catalytically active state. The group B Pak proteins bind activated Cdc42, but not Rac, and use a distinct mechanism of activation in cells.11-14 Paks have been implicated in tumor cell proliferation and survival and are being exploited as therapeutic targets in cancer several cancers.15,16
Previously, we have shown that Rac proteins have both unique, as well as overlapping, HSPC functions and are activated by stromal-derived factor 1/CXCR4, SCF/c-kit and fibronectin/β1 integrins, all ligands/receptors critical for HSPC engraftment. Paks have been shown to prime Mek1 for activation by Raf1,17,18 thus integrating activation by cytokine receptors and mediating directional sensing in response to SDF-1α in migrating T lymphocytes19 and macrophages.20 Now we present evidence that Pak A proteins have multiple roles in HSPC functions.
We show that activation of Pak A proteins is required for HSPC engraftment and inhibition of Pak activity is associated with aberrant regulation of diverse cellular functions such as chemokine-induced reorganization of the actin cytoskeleton, migration, proliferation, and survival. Using a genetic approach to further define this pathway, we demonstrate that Pak2-deficient, but not Pak1-deficient cells, show a complete lack of HSPC engraftment. Thus, Pak-dependent signaling pathways appear to coordinately regulate both cytoskeletal and proliferative pathways in HSPC. Dissection of these separate pathways using individual Pak KO mice may offer new targets for modulating normal and leukemic stem cell functions.
Methods
Mice
All procedures involving mice followed Children’s Hospital Boston Institutional Animal Care and Use Committee guidelines. C57Bl/6J, B6.SJL, and non-obese diabetic/severe combined immuno-deficiency (NOD/SCID) mice were obtained from Jackson Laboratories. C57Bl/6J mice were mated with B6.SJL mice to generate wild-type (WT) (CD45.2+/CD45.1+) heterozygous donor mice. Pak1−/− mice have been previously described,21 and Pak2flox/flox conditional knock-out mice were generated by homologous recombination using standard techniques, the derivation of which will be reported in detail in a subsequent publication.
Isolation and transduction of murine lineage negative LSK cells
Isolation and transduction of primary lin-Sca1+c-kit+ (LSK) were performed according to our previously published methods,22 and also see supplemental “Methods.” Retroviral vector constructs and antibodies can be found in supplemental “Methods.”
Bone marrow transplantation experiments and in vivo homing
Bone marrow transplantation experiments on transduced LSK were performed according to our previously published methods,22 and also see supplemental “Methods.”
In vitro chemotaxis and transwell migration
In vitro chemotaxis in response to stromal derived factor-1 α (SDF1α) was analyzed by time-lapse microscopy as previously described7 and further described in the supplemental “Methods.” Cell tracks were visualized using Velocity software (PerkinElmer, Waltham, MA); total cell displacement (eucleadian distance) was calculated using ImageJ software (National Institutes of Health, Bethesda, MD). Transwell assays were performed as previously described7,23 and described in detail in the supplemental “Methods.”
F-actin staining and quantification of cell morphological changes in response to chemokine stimulation
Green fluorescent protein (GFP)+ LSK cells were layered onto fibronectin-coated coverslips and stimulated with 100 ng/mL of SDF-1α. Cells were subsequently fixed and stained according to previously described methods and are further described in the supplemental “Methods.”
Proliferation and apoptosis analysis
For the determination of proliferation/cell-cycle LSK cells were cultured in the presence of 5-bromo-2′-deoxy-uridine (BrdU) for 1 hour then fixed, permeabilized, and stained using the APC BrdU Flow Kit (BD Biosciences, San Jose, CA), following the manufacturer’s instructions. To determine differences in apoptosis, cells were stained with Annexin V-APC (BD Biosciences) and 7AAD (Invitrogen, Carlsbad, CA).
Intracellular phospho-flow
The 1.25 × 105 cells were fixed and stained according to previously published methods24 using Alexa647-conjugated p-ERK (p44/42) and pAKT (Cell Signaling Technology, Danvers, MA) and the analysis is further described in supplemental “Methods.”
Statistical analysis
Datasets were compared by two-tailed t tests and P values less than 0.05 were considered statistically significant.
Results
Inhibition of Pak proteins in HSPC impairs directed cell migration
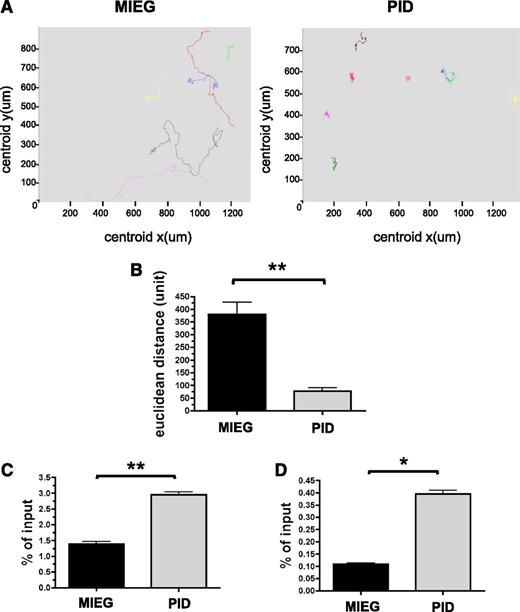
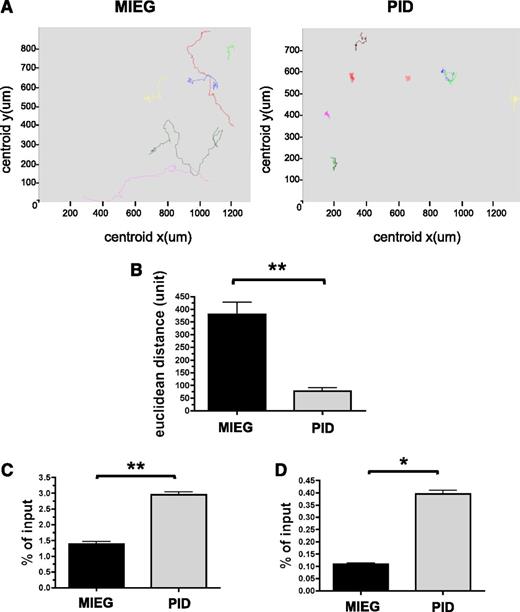
Pak proteins regulate cell shape, adhesion, and migration, and these processes are critical for HSC homing and engraftment. To examine the role of Pak activation in HSPC, we measured the capacity of Pak inhibitory domain (PID)-expressing LSK cells to migrate in response to an SDF-1α gradient using a Dunn chamber and time-lapse video microscopy. We have previously demonstrated the expression and subsequent phosphorylation of Pak in HSPC cells after treatment with SCF and SDF-1α.8,25 To inhibit Pak A proteins, we expressed the PID in WT HSPC cells. PID binds to the autoinhibitory domain of group A Pak proteins and inhibits subsequent activation.26,27 Inhibition of Pak in HSPCs via expression of the PID results in simultaneous inhibition of all three Pak A proteins reducing the confounding variable of compensation by other highly homologous family members. PID was inserted into MSCV-IRES-enhanced green fluorescent protein (3) (MIEG3) retrovirus28 to yield MIEG3-PID vector and NIH/3T3 cells were transduced with either MIEG3 expressing EGFP alone or MIEG3-PID co-expressing EGFP and PID. PDGF-stimulated transduced NIH/3T3 cells expressing PID led to significant inhibition of Pak activation as measured in an in vitro kinase assay (supplemental Figure 1). Freshly isolated LSK cells from WT (CD45.1+/CD45.2+) BM were transduced with MIEG3-PID or MIEG3 as a control. PID-expressing HSPC showed abnormal directed migration in response to an SDF-1α gradient (Figure 1A and supplemental Movies 1 and 2). The total cell displacement by PID-expressing cells in 1 hour was significantly reduced compared with MIEG3 controls (76.9 ± 62.1 vs 380.3 ± 142.5 units; mean ± SEM; PID vs MEIG; P < .01) (Figure 1B). PID-GFP+ LSK cells were also found to simultaneously display the presence of 2 or more lamellipodia in distinct regions around the cell (supplemental Movies 1 and 2) in contrast to MIEG3 controls.
Increased random migration of PID-transduced HSPC in response to SDF-1α. (A-B) Time-lapse microscopy of GFP+ cells from PID or MIEG3-transduced LSK cells on a fibronectin-coated coverslip in the presence of an SDF-1α gradient. (A) Paths followed by individual cells in 1 hour. Representative fields (325 × 325 μm); data shown represents 1 of 2 independent experiments. (B) Total displacement of migration in 1 hour. Data represent mean ± SEM (N = 9-19 cells analyzed per group). **P < .01. (C-D) Chemokinesis of LSK measured after 2 hours in a transwell chamber assay as a result of (C) uniform concentration of SDF-1α or (D) 2% bovine serum albumin alone. Data represent the mean ± SEM, of the percentage of migrated cells (in bottom chamber) normalized to input (total number of cells plated to top chamber), performed in triplicate (N = 3 independent experiments). *P < .05; **P < .01.
Increased random migration of PID-transduced HSPC in response to SDF-1α. (A-B) Time-lapse microscopy of GFP+ cells from PID or MIEG3-transduced LSK cells on a fibronectin-coated coverslip in the presence of an SDF-1α gradient. (A) Paths followed by individual cells in 1 hour. Representative fields (325 × 325 μm); data shown represents 1 of 2 independent experiments. (B) Total displacement of migration in 1 hour. Data represent mean ± SEM (N = 9-19 cells analyzed per group). **P < .01. (C-D) Chemokinesis of LSK measured after 2 hours in a transwell chamber assay as a result of (C) uniform concentration of SDF-1α or (D) 2% bovine serum albumin alone. Data represent the mean ± SEM, of the percentage of migrated cells (in bottom chamber) normalized to input (total number of cells plated to top chamber), performed in triplicate (N = 3 independent experiments). *P < .05; **P < .01.
To address whether inhibition of Pak proteins led to a loss of cytoskeletal machinery required for cell migration, we examined HSPC chemokinesis or random cell migration. We used in vitro transwell assays in which a uniform concentration of SDF-1α was added to both the top and bottom of transwells. We found a greater than 2-fold increase in chemokinesis in PID-expressing HSPC compared with MIEG3 controls (3.0 ± 0.2% vs 1.4 ± 0.2% of input cells, respectively; mean ± SEM; P < .01) (Figure 1C). Random migration in the absence of chemokine was also increased in PID-expressing HSPC compared with MIEG3 controls (0.40 ± 0.04% vs 0.11 ± 0.01% of input cells, respectively; mean ± SEM; P < .05) (Figure 1D). To determine if these defects in directed cell migration were the result of different levels of the chemokine receptor, CXCR4, on LSK transduced with PID compared with MIEG3 controls, we performed flow cytometry for expression of CXCR4 levels and found no significant differences (supplemental Figure 2). Taken together, the decrease in directed migration and increase in chemokinesis in PID-expressing HSPC suggests that appropriate activation of Pak A is required for directional migration in HSPC.
Inhibition of Pak leads to disruptions in cytoskeletal organization in response to chemokine stimulation
Regulation of cellular migration is dependent on the dynamics of actin reorganization and F-actin stabilization.29 To determine the role Pak proteins play in coordinated organization of the cytoskeleton, we examined the change in cell morphology in response to chemokine stimulation in PID-expressing HSPC. In contrast to MIEG3 controls, PID-expressing HSPCs demonstrated exaggerated uropod-like structures after stimulation with SDF-1α (Figure 2A), similar to structures we have previously characterized in Rac-deficient neutrophils.30 We quantified disruptions in the actin cytoskeleton by observing changes in cell shape and membrane protrusions after stimulation with SDF-1α using cell surface area and total cell perimeter as a measure of cell spreading. We found significant increases in both surface area and cell perimeter of PID-expressing LSK compared with controls (1949 ± 490.8 vs 1312 ± 202.6 uM2 and 194.5 ± 36.3 vs 136.8 ± 16.6 uM; mean ± SEM; PID vs MIEG3, respectively; P < .01) (Figure 2B-C). These data, together with live imaging microscopy results, demonstrate that PID-expressing HSPC display aberrant reorganization of the actin cytoskeleton in response to chemokine-stimulated migration and suggest a role for Pak A proteins in regulating actin dynamics during directed HSPC migration.
Inhibition of PAK in HSPC leads to excessive cell spreading and protrusions in response to SDF-1 chemokine stimulation. WT LSK was transduced with PID or MIEG3 retrovirus. GFP+ LSK were isolated 48 hours later using fluorescence-activated cell sorter, and were stimulated with SDF-1α and plated on fibronectin-coated coverslips. (A) PID transduced LSK showed aberrant cell morphology compared with MIEG3 controls in response to stimulation with SDF-1α for 5 minutes and staining of F-actin with rhodamine-labeled phalloidin (red) and DAPI-stained nuclei (blue). The changes in cell shape were quantified by (B) total cell area (uM2) and (C) cell perimeter (uM). Data represent mean ± SEM (N ≥ 40 cells analyzed per group). **P < .001.
Inhibition of PAK in HSPC leads to excessive cell spreading and protrusions in response to SDF-1 chemokine stimulation. WT LSK was transduced with PID or MIEG3 retrovirus. GFP+ LSK were isolated 48 hours later using fluorescence-activated cell sorter, and were stimulated with SDF-1α and plated on fibronectin-coated coverslips. (A) PID transduced LSK showed aberrant cell morphology compared with MIEG3 controls in response to stimulation with SDF-1α for 5 minutes and staining of F-actin with rhodamine-labeled phalloidin (red) and DAPI-stained nuclei (blue). The changes in cell shape were quantified by (B) total cell area (uM2) and (C) cell perimeter (uM). Data represent mean ± SEM (N ≥ 40 cells analyzed per group). **P < .001.
Pak proteins are important for HSPC proliferation and survival
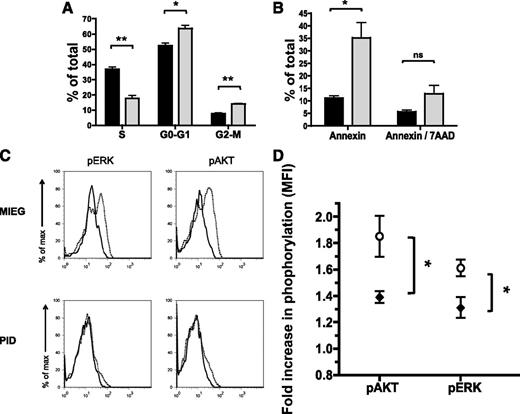
We have observed defects in HSPC directed cell migration and cytoskeletal re-organization as a result of Pak inhibition. However Paks are complex, multi-domain signaling molecules that mediate activation of several signaling pathways.12,14 In other cell systems, Pak proteins have been demonstrated to regulate cell proliferation and survival.12,31 To measure differences in proliferation in PID-expressing vs control HSPC, we examined transduced and cytokine-stimulated HSPC for BrdU incorporation. We found a significant decrease in proliferation of HSPC in PID-expressing LSK compared with control transduced cells (17.7 ± 3.4 vs 36.8% ± 2.4 of cells in S-phase; mean ± SEM; P < .01) (Figure 3A). Consistent with the decrease in the percentage of cells entering S-phase of the cell cycle, there was a corresponding increase of cells in G0-G1 and G2-M phases of the cell cycle (Figure 3A). PID-expressing HSPC also demonstrated a significant increase in cells undergoing apoptosis (Annexin V+) compared with controls (35.2 ± 10.6 vs 11.1 ± 1.6%; mean ± SEM; P < .05) (Figure 3B). Taken together, these data demonstrate that Pak A proteins regulate multiple HSPC functions important for HSPC engraftment. These functions not only involve directed cell migration and organization of the actin cytoskeleton but also HSPC proliferation and survival.
Inhibition of Pak in HSPC results in impaired proliferation and increased apoptosis in vitro. WT LSK was transduced with PID (gray) or MIEG3 (black) retrovirus, and then GFP+ LSK were isolated by fluorescence-activated cell sorter. (A) Cell cycle analysis was performed by culturing the sorted cells in the presence of BrdU and subsequent staining using an anti-BrdU antibody and 7AAD. Data represent the mean ± SEM, percent of cells in S, G0/G1, or G2/M phase of cell cycle (N = 3 independent experiments). *P < .05; **P < .01. (B) The percent of cells undergoing apoptosis was measured using Annexin V/7AAD and flow cytometry. Data represent mean ± SEM (N = 3 independent experiments). *P < .05. ns, not significant. (C-D) Activity of signaling pathways were assessed using intracellular fluorescence-activated cell sorter and phospho-specific antibodies. (C) Representative flow cytometric analysis of levels of phospho-AKT, and ERK in MIEG3 or PID-transduced LSK, after serum starvation (solid line) or stimulation (dotted line) with cytokines for 5 minutes. Representative data from 1 of 3 independent experiments. (D) Composite data from MIEG3 (○) and PID (♦) transduced LSK cells. Data shows fold increase of phosphorylation for each group after stimulation, (MFI) ± SEM (N = 3 independent experiments). P < .05 for pAKT and pERK.
Inhibition of Pak in HSPC results in impaired proliferation and increased apoptosis in vitro. WT LSK was transduced with PID (gray) or MIEG3 (black) retrovirus, and then GFP+ LSK were isolated by fluorescence-activated cell sorter. (A) Cell cycle analysis was performed by culturing the sorted cells in the presence of BrdU and subsequent staining using an anti-BrdU antibody and 7AAD. Data represent the mean ± SEM, percent of cells in S, G0/G1, or G2/M phase of cell cycle (N = 3 independent experiments). *P < .05; **P < .01. (B) The percent of cells undergoing apoptosis was measured using Annexin V/7AAD and flow cytometry. Data represent mean ± SEM (N = 3 independent experiments). *P < .05. ns, not significant. (C-D) Activity of signaling pathways were assessed using intracellular fluorescence-activated cell sorter and phospho-specific antibodies. (C) Representative flow cytometric analysis of levels of phospho-AKT, and ERK in MIEG3 or PID-transduced LSK, after serum starvation (solid line) or stimulation (dotted line) with cytokines for 5 minutes. Representative data from 1 of 3 independent experiments. (D) Composite data from MIEG3 (○) and PID (♦) transduced LSK cells. Data shows fold increase of phosphorylation for each group after stimulation, (MFI) ± SEM (N = 3 independent experiments). P < .05 for pAKT and pERK.
Pak A proteins regulate phosphorylation of ERK and AKT in response to cytokine stimulation
Next, to determine which signaling pathways are responsible for the observed Pak-dependent HSPC functions, we examined activation of kinase pathways in HSPC in response to cytokines. PID- and MIEG3-transduced LSK cells were isolated using fluorescence-activated cell sorter, starved, and then stimulated with growth cytokines. After stimulation, cells were fixed and stained for intracellular flow analysis using fluorescent tagged phospho-specific AKT and ERK antibodies. We found cytokine-induced increase in the levels of phospho-AKT and phospho-ERK in MIEG3-transduced LSK (Figure 3C). PID-expressing HSPC showed a significant impairment of activation of AKT and ERK (1.85 vs 1.38 and 1.61 vs 1.3 fold-increase in pAKT and pERK in MIEG3 vs PID-expressing LSK, respectively; 3 independent experiments; P < .05) (Figure 3D). These data suggest that Pak regulates both AKT and MAPK pathway activation in HSC in response to cytokines.
Group A Pak proteins are essential for HSPC engraftment
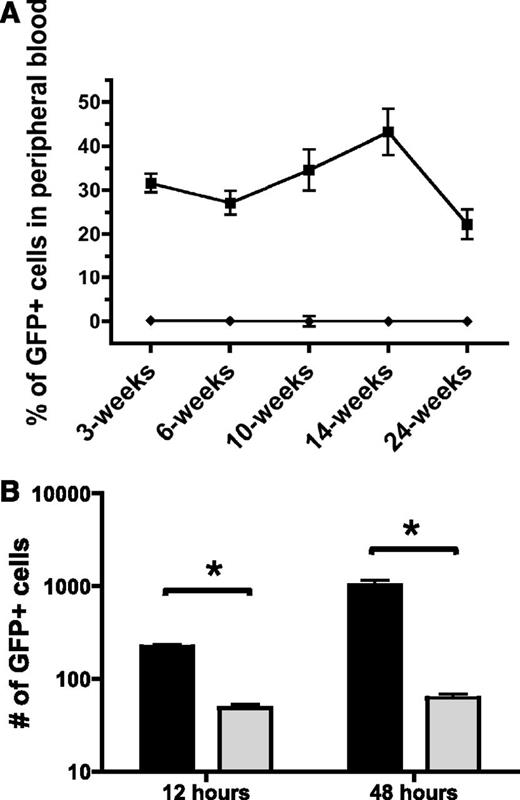
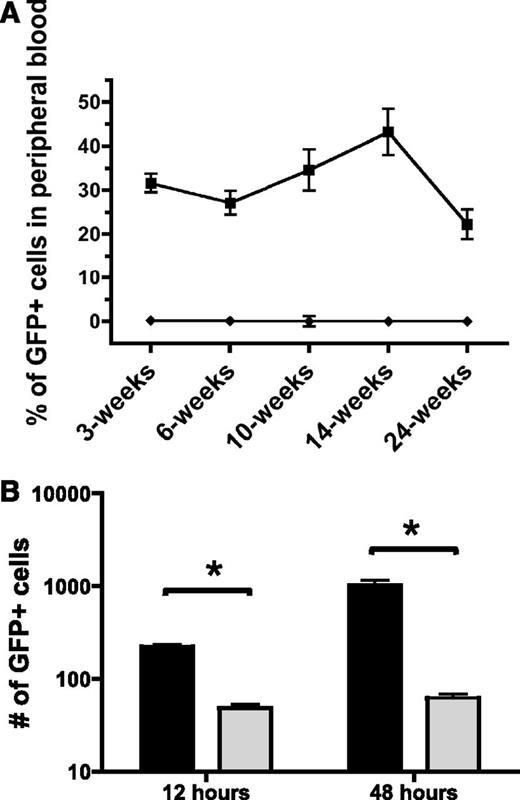
Next, to determine if Pak proteins are critical for HSPC engraftment, we tested whether Pak inhibition in HSPC altered in vivo reconstitution of hematopoiesis in a transplant model. 1.0 × 105 GFP+-transduced LSK cells were isolated and co-transplanted with 5.0 × 105 WT BoyJ (CD45.1+) whole BM into lethally irradiated WT C57Bl/6J (CD45.2+) recipients. Although animals transplanted with WT cells transduced with MIEG3 showed a sustained level of engraftment up to 22.2 ± 10.7% chimerism at 24-weeks posttransplant, animals transplanted with PID-transduced cells showed only 0.22 ± 0.19% donor chimerism at 3 weeks, decreasing to 0.07 ± 0.06% at 6 weeks with no detectable engraftment of these cells from 10-weeks postinfusion and onward (Figure 4A). To verify that PID-transduced LSK retained the capacity to differentiate into multiple lineages in progenitor-derived colonies in vitro, we performed colony forming unit assays. We found no significant differences in the number or type of colony forming units from PID-transduced LSK compared with MIEG3 controls (supplemental Figure 3). To determine if cells capable of full hematopoietic reconstitution were transduced in this protocol, we assessed the lineage contribution of MIEG3- and PID-transduced cells at 24-weeks post-BMT in the peripheral blood, spleen, and BM of recipient mice. Mice transplanted with MIEG3-transduced LSK cells demonstrated donor cell contribution to erythroid, myeloid, B-lymphoid, and T-lymphoid lineages, whereas PID-transduced cells failed to contribute to measurable engraftment in any of these lineages (supplemental Figure 4). The near complete absence of PID-transduced cells in recipient mice at early and later time points suggests Pak proteins play an important role in both short- and long-term hematopoietic reconstitution.
Inhibition of group A Pak proteins leads to decreased bone marrow engraftment and homing. (A) Engraftment of control and PID-transduced cells. WT LSK were transduced with MIEG3 (▪) or PID (♦) retrovirus and transplanted into lethally irradiated B6.SJL recipient mice. Percentage of engrafted, GFP+ cells in peripheral blood of recipient at indicated time points post-bone marrow transplantation. Data represent mean ± SEM (N = 10 recipients per group). P < .01 for all time points of 2 independent experiments. (B) Homing of control (black) and PID (gray) transduced cells. At 48 hours posttransduction GFP+ LSK were isolated using fluorescence-activated cell sorter. 2.5 × 105 GFP+ cells were transplanted into lethally irradiated B6.SJL recipient mice. The number of GFP+ LSK determined by flow cytometry that homed to the bone marrow at 12 hours and were retained 48 hours postinfusion was determined using cells collected from the femur, tibia, and iliac crest. Data represent equivalent numbers of live events per group per time point (mean ± SEM; N = 6 recipient mice per group per time point). One representative experiment is shown of 2 independent experiments. *P < .05 (t test).
Inhibition of group A Pak proteins leads to decreased bone marrow engraftment and homing. (A) Engraftment of control and PID-transduced cells. WT LSK were transduced with MIEG3 (▪) or PID (♦) retrovirus and transplanted into lethally irradiated B6.SJL recipient mice. Percentage of engrafted, GFP+ cells in peripheral blood of recipient at indicated time points post-bone marrow transplantation. Data represent mean ± SEM (N = 10 recipients per group). P < .01 for all time points of 2 independent experiments. (B) Homing of control (black) and PID (gray) transduced cells. At 48 hours posttransduction GFP+ LSK were isolated using fluorescence-activated cell sorter. 2.5 × 105 GFP+ cells were transplanted into lethally irradiated B6.SJL recipient mice. The number of GFP+ LSK determined by flow cytometry that homed to the bone marrow at 12 hours and were retained 48 hours postinfusion was determined using cells collected from the femur, tibia, and iliac crest. Data represent equivalent numbers of live events per group per time point (mean ± SEM; N = 6 recipient mice per group per time point). One representative experiment is shown of 2 independent experiments. *P < .05 (t test).
Functional inhibition of group A Pak proteins results in impaired BM homing
Engraftment is a complex process involving the coordinated regulation of multiple physiological processes including cellular migration, localization to the appropriate niche in the medullary cavity, adhesion, and subsequent proliferation and differentiation.32 To address the potential role(s) of Pak proteins in these processes, we first tested the capacity of the PID-expressing HSPC to home to the BM at 12 hours, and to be retained at 48 hours postinfusion compared with an equivalent number of MIEG3-transduced LSK cells (Figure 4B). The number of GFP+ cells that homed to the BM per hind limb was determined by flow analysis. At 12 hours postinfusion, there was a 78% reduction in PID-expressing cells that homed to the BM compared with control transduced cells. At 48 hours, the proportion of PID-GFP+ cells remaining in the BM was reduced by 94% compared with control cells. Thus, expression of PID was associated with reduced initial homing and reduced numbers of PID-transduced cells subsequently found in the BM compared with controls.
Constitutive activation of ERK increases proliferation and partially rescues the short-term engraftment defect of PID-expressing HSPC
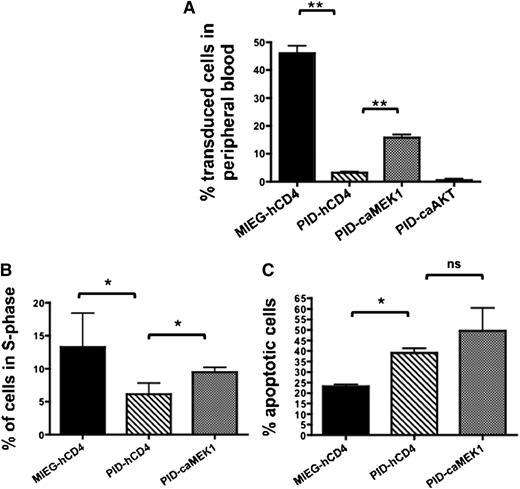
To determine if the defect in proliferation and engraftment of PID-expressing cells was related to defects in AKT or MAPK activation, we next transduced LSK with retroviral vectors expressing constitutively active (ca) forms of AKT or MEK1 (ERK) and co-expressing a truncated human-CD4 (hCD4) epitope, along with PID-GFP or MIEG3 constructs. GFP+ and hCD4+ cells were injected into lethally-irradiated C576BL/6J mice. At 3-weeks post-BMT we found that expression of caMEK1 but not caAKT was capable of partially rescuing the engraftment defect of PID-expressing HSPC (percentage of donor cells for PID + empty vector [hCD4], 3.1 ± 1.4%; PID + caMEK1, 15.8 ± 3.4%, and PID + caAKT, 0.5 ± 0.1%; mean ± SEM; P < .05 for empty vector vs caMEK1) (Figure 5A). However, by 6-weeks post-BMT, PID + caMEK1 cells were no longer significantly different than PID+ empty vector (hCD4) (supplemental Figure 5). When 250 000 sorted LSK were injected into lethally irradiated BoyJ recipients, there was also a trend toward rescue of the homing defect when PID-transduced HSPC expressing caMEK1 (GFP+/hCD4+) were compared with HSPC expressing PID alone at 12 hours postinjection (37.3 vs 52.6% of control; PID vs PID+caMEK1; N = 5-10). To determine if MEK1-induced rescue of short-term engraftment was also contributed to by increased proliferation and/or survival of PID-expressing, we performed proliferation and apoptosis assays on fluorescence-activated cell sorted cells 3 days posttransduction. Expression of caMEK1 was associated with a modest increase in proliferation in PID-expressing LSK compared with controls (PID + empty vector [hCD4] 6.1 ± 2.9% vs PID+ caMEK1; 9.5 ± 1.2% BrdU+ cells; mean ± SEM; N = 3 independent experiments; P < .05) (Figure 5B). This increase in proliferation occurred with a concomitant trend toward increased apoptosis that did not reach statistical significance (PID + empty vector [hCD4], 39.1 ± 3.7% and PID+ caMEK1 49.53 ± 18.8% Annexin V+ cells; mean ± SEM; N = 3 independent experiments; P = not significant) (Figure 5C). These results suggest that the PID-associated defect in MEK1-ERK signaling in response to cytokines was associated with reduced cell proliferation and loss of early engraftment of PID-expressing cells.
Constitutively active MEK1 (caMEK1) transiently rescues defects in Pak-deficient HSPC engraftment and proliferation. (A) 1.5 × 105 PID or MIEG3 (GFP+) cells co-transduced with caMEK, caAKT, or hCD4 empty vector control were co-transplanted with 5.0 × 105 CD45.1 whole bone marrow into lethally irradiated C57Bl/6J (CD45.2) recipient mice. Data represent mean ± SEM (N = 8-10 recipients per group). 2 independent experiments (**P < .01). Data shows the percentage of GFP cells in the peripheral blood at 3 weeks. (B-C) WT LSK were transduced with MIEG3 (black) or PID (gray) retrovirus and co-transduced with caMEK or hCD4 empty vector (hatched) control. At 36 hours posttransduction cells were stained using an anti-human CD4-PE Cy7 antibody, GFP+/PE Cy7+ double- positive LSK were then isolated by fluorescence-activated cell sorter. (B) Cell cycle analysis was performed 3 days postsort by culturing the isolated cells in the presence of BrdU for 1 hour and subsequent staining using an anti-BrdU antibody and 7AAD. Data represent the mean ± SEM;, percent of cells in the S phase of the cell cycle (N = 3 independent experiments), *P < .05. (C) Percentage of cells undergoing apoptosis was measured using Annexin V antibody and flow cytometry 3 days postsort. Data represent mean ± SEM (N = 3 independent experiments). *P < .05. ns, not significant.
Constitutively active MEK1 (caMEK1) transiently rescues defects in Pak-deficient HSPC engraftment and proliferation. (A) 1.5 × 105 PID or MIEG3 (GFP+) cells co-transduced with caMEK, caAKT, or hCD4 empty vector control were co-transplanted with 5.0 × 105 CD45.1 whole bone marrow into lethally irradiated C57Bl/6J (CD45.2) recipient mice. Data represent mean ± SEM (N = 8-10 recipients per group). 2 independent experiments (**P < .01). Data shows the percentage of GFP cells in the peripheral blood at 3 weeks. (B-C) WT LSK were transduced with MIEG3 (black) or PID (gray) retrovirus and co-transduced with caMEK or hCD4 empty vector (hatched) control. At 36 hours posttransduction cells were stained using an anti-human CD4-PE Cy7 antibody, GFP+/PE Cy7+ double- positive LSK were then isolated by fluorescence-activated cell sorter. (B) Cell cycle analysis was performed 3 days postsort by culturing the isolated cells in the presence of BrdU for 1 hour and subsequent staining using an anti-BrdU antibody and 7AAD. Data represent the mean ± SEM;, percent of cells in the S phase of the cell cycle (N = 3 independent experiments), *P < .05. (C) Percentage of cells undergoing apoptosis was measured using Annexin V antibody and flow cytometry 3 days postsort. Data represent mean ± SEM (N = 3 independent experiments). *P < .05. ns, not significant.
Pak2, not Pak1, plays an essential role in HSPC migration, homing, and engraftment
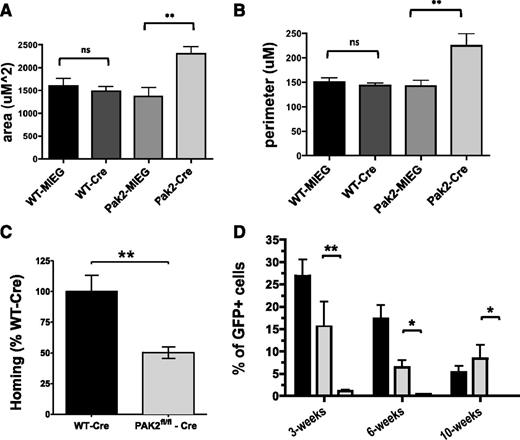
Next, because PID expression indiscriminately inhibits all Pak A proteins, we used mouse knockout lines to determine the relative role of Pak 1 and Pak 2 in the engraftment process. Pak1−/− mice are viable and healthy,21 and we found no significant differences in engraftment between Pak1−/− vs Pak1wt/wt donor chimerism at any time point from as early as 3 weeks (35.8 ± 5.3% vs 37.4 ± 3.6% donor chimerism; mean ± SEM; N = 6; P = not significant) up to 24 weeks (50.7 ± 8.3 vs 52.1 ± 12.4; mean ± SEM; N = 10; P = not significant). These animals were not studied further. Pak2 germline knock-out mice are embryonic lethal. Therefore, we used mice homozygous for floxed Pak2 alleles (Pak2flox/flox) for subsequent studies. The derivation and genotyping of these animals will be described in detail in a subsequent publication. Freshly isolated Pak2fl//fl and Pak2WT/WT LSK cells were transduced with Cre-GFP or MIEG3 control retrovirus. LSK-transduced and GFP-sorted Pak2fl/fl cells showed near complete deletion of Pak2 sequences (supplemental Figure 6). Transduced LSK cells were assayed for migration and analyzed for cell shape changes. Similar to LSK-expressing PID, analysis of Pak2Δ/Δ cells demonstrated excessive cell spreading and protrusions (Figure 6A–B). Sorted GFP+ cells were injected into lethally irradiated C57BL/6J recipient mice. Pak2Δ/Δ cells showed ∼50% reduction in homing to the bone marrow compared with Pak2WT/WT cells transduced with Cre when analyzed 12 hours after injection (100+/−13 vs 50.4+/−15%; WT vs Pak2Δ/Δ; P < .05; N = 9) (Figure 6C). Finally, GFP+ cells were then co-transplanted with 5.0 × 105 WT BoyJ (CD45.1+) WBM into sublethally irradiated (2.8 Gy) NOD/SCID recipients. The use of immunodeficient mice as recipients was necessary because the Pak2flox/flox mice were on a mixed genetic background. Compared with Pak2WT/WT cells transduced with Cre recombinase, Pak2flox/flox + Cre recombinase cells failed to contribute to recipient hematopoiesis, as demonstrated by near complete absence of donor chimerism in the peripheral blood in all time points examined posttransplant (Figure 6D). To verify Pak2Δ/Δ cells retain the ability to differentiate into multiple lineages, as well as the capacity to form progenitor-derived colonies, we performed colony forming assays and found no significant differences in the number of colonies or in the frequency of the colony type after expression of Cre-recombinase compared with Cre-transduced WT bone marrow and noted by the presence of erythroid, myeloid, and mixed colony types (supplemental Figure 7). Previously, we noted, along with others, that expression of Cre-recombinase in LSK leads to some toxicity, even in WT cells, however, Pak2-deficient LSK display a significant decrease in engraftment beyond what was seen with WT-LSK transduced with Cre-recombinase and no decrease in colony formation compared with these cells. These data strongly suggest that Pak2 is required for HSPC engraftment.
Pak2 is required for HSPC engraftment. (A-D) In vitro assays and homing of Pak2 cells. WT and Pak2fl/fl LSK were transduced with MIEG3 empty vector (MIEG3) or Cre-MIEG3+ (Cre) retrovirus. GFP+ LSK were isolated 48 hours later using fluorescence-activated cell sorter, and were stimulated with SDF-1α and plated on fibronectin-coated coverslips. Cre-transduced Pak2fl/fl LSK showed aberrant cell morphology compared with Pak2fl/fl transduced with MIEG3 and WT controls in response to stimulation with SDF-1α. The changes in cell shape were quantified by (A) total cell area (uM2) and (B) cell perimeter (uM). Data represent mean ± SEM (N = 7-19 cells analyzed per group). **P < .01. (C) LSK cells from WT and Pak2 fl/fl were transduced with MIEG3-Cre-EGFP virus. The 250 000 sorted GFP+ cells were injected into lethally irradiated mice. Homing of the transplanted cells was measured at 12 hours posttransplantation using bone marrow from the femur, tibia, and iliac crest. Pak2 homing efficiency is graphed as a percent of WT-Cre-transduced LSK. (N = 9). **P < .001. (D) 1.0 × 105 Pak2fl/fl + MIEG3 (black) cells, Pak2WT/WT +Cre-GFP (gray) cells, or Pak2fl/fl + Cre-GFP (white) cells were co-transplanted with 5.0 × 105 CD45.1 whole bone marrow into sublethally irradiated NOD-SCID recipient mice. Data represent mean ± SEM (N ≥ 6 recipients per genotype). *P < .05; **P < .01 of 2 independent experiments.
Pak2 is required for HSPC engraftment. (A-D) In vitro assays and homing of Pak2 cells. WT and Pak2fl/fl LSK were transduced with MIEG3 empty vector (MIEG3) or Cre-MIEG3+ (Cre) retrovirus. GFP+ LSK were isolated 48 hours later using fluorescence-activated cell sorter, and were stimulated with SDF-1α and plated on fibronectin-coated coverslips. Cre-transduced Pak2fl/fl LSK showed aberrant cell morphology compared with Pak2fl/fl transduced with MIEG3 and WT controls in response to stimulation with SDF-1α. The changes in cell shape were quantified by (A) total cell area (uM2) and (B) cell perimeter (uM). Data represent mean ± SEM (N = 7-19 cells analyzed per group). **P < .01. (C) LSK cells from WT and Pak2 fl/fl were transduced with MIEG3-Cre-EGFP virus. The 250 000 sorted GFP+ cells were injected into lethally irradiated mice. Homing of the transplanted cells was measured at 12 hours posttransplantation using bone marrow from the femur, tibia, and iliac crest. Pak2 homing efficiency is graphed as a percent of WT-Cre-transduced LSK. (N = 9). **P < .001. (D) 1.0 × 105 Pak2fl/fl + MIEG3 (black) cells, Pak2WT/WT +Cre-GFP (gray) cells, or Pak2fl/fl + Cre-GFP (white) cells were co-transplanted with 5.0 × 105 CD45.1 whole bone marrow into sublethally irradiated NOD-SCID recipient mice. Data represent mean ± SEM (N ≥ 6 recipients per genotype). *P < .05; **P < .01 of 2 independent experiments.
Discussion
HSPC engraftment is a complex process that requires the coordination of multiple pathways regulating distinct cellular processes, such as chemotaxis, extravasation from the circulation to the BM microenvironment, lodgment to the stem cell niche and subsequent survival, proliferation, and differentiation.32 This process requires HSPC to integrate extracellular cues from chemokines, cytokines, and integrin receptors. Alterations in signaling pathways downstream of these receptors can result in multiple HSPC abnormalities, and in some cases malignant transformation. Here we studied the potential role of group A Paks, major downstream targets of Rac, and Cdc42 Rho GTPases,12 in HSPC functions. Although Paks have been studied in a variety of cell lines, a direct role for Paks in HSPC biology has yet to be identified. In other model systems, Pak proteins regulate multiple cellular functions such as transcription, translation, migration, focal adhesion turnover, organization of the actin cytoskeleton, proliferation, and survival.10,12,19,33,34 In the present work, we used 2 approaches – transgenic expression of the PID to inhibit all group A Paks, as well as individual gene knockouts of Pak1 and Pak2 – to establish that group A Paks are required for HSPC functions critical for engraftment including directed migration, homing and survival/proliferation and that Pak2, but not Pak1, is the critical isoform in engraftment of these cells post-transplant. In particular, we demonstrate that Pak2-deficient HSPC fail to reconstitute hematopoiesis in irradiated murine recipients. Although the analyses of the differential roles of Pak1 and Pak2 were done using different experimental systems, these data imply that Paks, and particularly Pak2, coordinate both cytoskeletal and kinase pathways involved in the complex processes of HSC engraftment. Additional analysis of the relative contributions of other group A Paks, including Pak1, to engraftment are underway.
Here we demonstrate in HSPC that Pak-inhibition leads to defective ERK and AKT activation, resulting in reduced proliferation and increased apoptosis in response to growth factors. Alterations in ERK activation likely contributes directly to the engraftment defect in Pak-deficient cells, because expression of caMEK increases proliferation of these cells in vitro and partially restores homing and engraftment of Pak-deficient cells in vivo. It is not clear whether the lack of full rescue of caMEK expression in Pak-deficient cells results from the insufficient levels of ERK/MEK1 activation to rescue the concomitant increased apoptosis that we noted in these cells, or because multiple domains of Pak, and therefore multiple downstream pathways, are required to restore full engraftment activity. Studies to resolve these issues are underway.
Transducing LSK with retrovirus containing the PID domain, we demonstrate a marked defect in directed migration of HSPC in response to SDF-1α and preserved or even enhanced actin assembly leading to excessive cell spreading and adhesion. Some functions of Pak, especially certain cytoskeleton effects are independent of Pak kinase activity.33,35 Thus, although not well characterized in hematopoietic cells, in other cells the actin and cytoskeletal effects of Pak activation appear to be mediated by a scaffold function that is dependent on proline-rich domains and interaction with Src-homology 3-domain containing proteins such as Nck and PIX.10,12 PID inhibition of Pak activity may leave this scaffold function intact, possibly explaining the excessive cell spreading and adhesion. In addition, phosphorylation of Pak and its regulation of actin remodeling in some cells is dependent on the presence of Nck-binding sites on Pak.36 We are currently investigating the potential scaffolding role of Pak2 in HSPC functions.
The Rho GTPases are regulated by guanine exchange factors and GTPase-activating proteins (GAPs) in a cell and agonist-specific fashion and result in the activation of 1 or more effector proteins. Dissecting the complex regulation of these pathways and defining the functional interactions between GTPases and their effector proteins in specific HSPC functions remains a major challenge. We have recently demonstrated that Vav1, a Rac guanine exchange factor, is critical for HSPC perivascular homing and retention in the bone marrow.25 Along with the data presented here, we have thus defined a Rac-signaling axis that includes the chemokine receptor CXCR4, c-kit, and β1 integrins, the guanine exchange factors Vav1, Rac/Cdc42, and Pak2,7-9 which is essential for HSPC engraftment and retention in the hematopoietic microenvironment. A more precise understanding of the roles of the individual components and their regulation of this pathway that are critical for engraftment and sustained hematopoiesis, will likely lead to the identification of new targets for therapeutic intervention and manipulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of our laboratory and the laboratories of Scott Armstrong and Ben Ebert for helpful discussions and advice. We thank Harvey Lodish and Jing Zhang for the constitutively active MEK1, AKT, and MICD4 constructs.
This work was supported by grants from the National Institutes of Health 5R01DK062757-11 (D.A.W.), R01CA142928-3, and R01CA117884-2 (J.C.), 5 T32 HL 007574-29, and 5 T32 HL 066987-08 (A.M.D).
Authorship
Contribution: A.M.D., M.D.M., J.C., and D.A.W. designed research. A.M.D., R.K., S.D.V., P.N.G.R., M.K.M., R.M., C.E.H., M.R., and S.W.L. performed research. A.M.D., M.D.M., and D.A.W. analyzed and interpreted data. R.M. and J.C. contributed new reagents and provided advice. A.M.D., J.C., and D.A.W. wrote the manuscript. All the authors reviewed versions of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Williams, Division of Hematology/Oncology, Children’s Hospital Boston, 300 Longwood Ave, Karp Family Research Laboratories 08125.3, Boston, MA 02115; e-mail: dawilliams@childrens.harvard.edu.
References
Author notes
S.D.V., M.R., J.C., and D.A.W. contributed equally to this study.