Key Points
AE induces hematopoietic self-renewal through a COX/prostaglandin E2/β-catenin signaling pathway.
Clinically available COX inhibitors may target AML stem cells and suppress AML of various karyotypes.
Abstract
Developing novel therapies that suppress self-renewal of leukemia stem cells may reduce the likelihood of relapses and extend long-term survival of patients with acute myelogenous leukemia (AML). AML1-ETO (AE) is an oncogene that plays an important role in inducing self-renewal of hematopoietic stem/progenitor cells (HSPCs), leading to the development of leukemia stem cells. Previously, using a zebrafish model of AE and a whole-organism chemical suppressor screen, we have discovered that AE induces specific hematopoietic phenotypes in embryonic zebrafish through a cyclooxygenase (COX)-2 and β-catenin–dependent pathway. Here, we show that AE also induces expression of the Cox-2 gene and activates β-catenin in mouse bone marrow cells. Inhibition of COX suppresses β-catenin activation and serial replating of AE+ mouse HSPCs. Genetic knockdown of β-catenin also abrogates the clonogenic growth of AE+ mouse HSPCs and human leukemia cells. In addition, treatment with nimesulide, a COX-2 selective inhibitor, dramatically suppresses xenograft tumor formation and inhibits in vivo progression of human leukemia cells. In summary, our data indicate an important role of a COX/β-catenin–dependent signaling pathway in tumor initiation, growth, and self-renewal, and in providing the rationale for testing potential benefits from common COX inhibitors as a part of AML treatments.
Introduction
Cancer therapies have historically depended on the use of cytotoxic agents that nonspecifically kill proliferating cells. Although these treatments are effective in inducing a remission in more than half of patients with acute myelogenous leukemia (AML), most remissions are not sustained. Overall, 75% of AML patients relapse within 2 years of remission, and most die of the disease.1
In recent years, growing research and clinical evidence has highlighted the role of a small population of leukemia stem cells (LSCs) in causing AML relapses after a short period of remission after chemotherapies. LSCs in AML are mostly quiescent, thus, they are resistant to common antiproliferation agents.2,3 Nevertheless, LSCs possess several important features, including increased self-renewal and dysregulated differentiation, distinguishing them from normal hematopoietic stem/progenitor cells (HSPCs). These features are caused by the expression of some of the leukemia oncogenes, such as AML1-ETO (AE).4
The AE oncogene is a fusion product of the (8;21) chromosomal translocation and one of the most common leukemia oncogenes associated with AML.5 AML-1 (also known as RUNX-1 or core binding factor–α2B) is a component of a heterodimeric complex called the core-binding factor that regulates the expression of many hematopoietic genes.6 Several lines of evidence suggest that AE plays an important role in the genesis of LSCs. First, transcripts of AE can be found in hematopoietic cells of nonmyeloid lineages in some patients, suggesting that the AE mutation is present in the hierarchal, multipotent progenitor cells.7 Second, it has been shown that AE can promote self-renewal of mouse and human HSPCs.4,8,9 Thus, identifying the mechanisms by which AE promotes self-renewal of HSPCs may facilitate the development of targeted therapies against LSCs.
The development of targeted therapies against LSCs is hampered by poor understanding of the underlying signaling mechanisms and by the difficulty of purifying these cells in vivo and culturing them in vitro. Phenotype-based chemical suppressor screens in zebrafish have proven to be a powerful method for identifying effective therapeutic agents without prior knowledge of efficacious therapeutic targets.10 We have shown that embryonic zebrafish possess a well-characterized population of HSPCs, enabling facile detection of oncogenic effects on hematopoietic differentiation in vivo.11,12 We have previously reported that expression of AE results in hematopoietic differentiation phenotypes in zebrafish embryos, which exhibit both cytological and transcriptional hallmarks of human AML associated with the AE oncogene.11 Using this zebrafish model, we conducted an unbiased chemical suppressor screen and found that inhibitors of cyclooxygenase-2 (COX-2) can reverse the hematopoietic differentiation phenotypes of AE in zebrafish.12 Subsequently, we showed that AE upregulates COX-2, which leads to increased β-catenin–dependent signaling in zebrafish hematopoietic cells and in human myeloid leukemia K562 cells.12
The COX enzymes, including COX-1 and COX-2, convert arachidonic acid to prostanoids, which can be further processed to become important lipid mediators, including prostaglandins, prostacyclin, and thromboxanes.13 Both COX activities and β-catenin signaling have been implicated in enhancing hematopoieitc self-renewal.14,15 For example, prostaglandin E2 (PGE2), 1 of the major metabolites downstream of both COX-1 and COX-2, has been shown to activate β-catenin–dependent signaling in hematopoietic stem cells (HSCs) and promote HSC expansion.14 Together with our previous results, these findings raised a few important questions. Are both COX-2 activity and β-catenin signaling involved in the self-renewal of AE-expressing HSPCs? Can COX inhibitors impede AE-mediated leukemogenesis or even abolish the initiation of AML? Finally, do other leukemia oncogenes function through COX-2 activity? Thus, we embarked the current study to answer some of these questions.
Methods
See supplemental materials for additional methods.
Isolation and culture of mouse BM cells
Four-week old AE/Mx1-Cre mice obtained from the crosses of AML1-ETO-stop mice and Mx1-Cre mice (both were kindly provided by Dr James R. Downing)8 were injected with 500 μg of polyinosinic-polycytidylic acid (pI-pC, Sigma-Aldrich, St. Louis, MO) 7 times at 2-day intervals intraperitoneally to induce the expression of Cre. Four weeks after pI-pC injection, the mice were used for BM isolation. Age matched C57BL/6 mice were used as control. All experiments were done in full compliance with institutional guidelines and with the Institutional Animal Care and Use Committee and Institutional Review Board approval.
Eight week-old C57BL/6 and AE/Mx1-Cre mice were used for BM isolation. BM cells were isolated from femurs and tibias by flushing with phosphate-buffered saline. The cell suspension was then layered onto HISTOPAQUE-1083 (Sigma-Aldrich) and centrifuged at 400 × g for 30 minutes at room temperature. The mononuclear cells at the interface were collected, washed with phosphate-buffered saline twice, and resuspended in Iscove modified Dulbecco medium (STEMCELL Technologies, Vancouver, BC, Canada).
Lineage depletion was performed in some sets of colony-forming unit assays to enrich the HSPCs from the BM. Mononuclear cells isolated from mouse BM were labeled with biotinylated mouse lineage depletion cocktail (BD Biosiences, San Jose, CA) and were then incubated with streptavidin particles and placed in the magnetic field to remove the lineage-committed cells from progenitor cells. The lineage-negative (lin−) fraction of cells was resuspended in Iscove modified Dulbecco medium for colony-forming unit assay.
Results
AE induces the expression of the COX genes in mouse BM cells
We have previously shown that AE induces Cox-2 expression in zebrafish hematopoietic cells.12 In addition, human myelogenous leukemia K562 cells stably transduced with AE-green fluorescent protein (GFP) also exhibit a higher level of Cox-2 expression than the cells transduced with GFP.12 To test whether Cox-1 and Cox-2 are regulated by AE in mammalian HSPCs, we took the following 2 approaches.
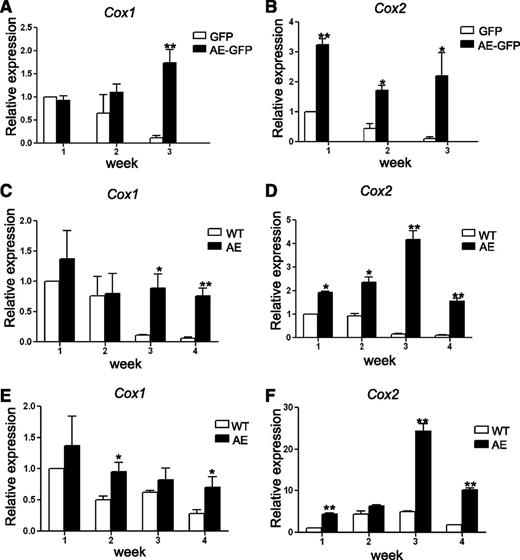
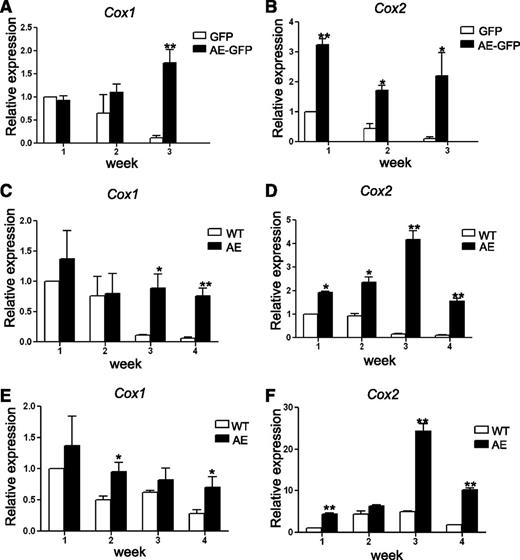
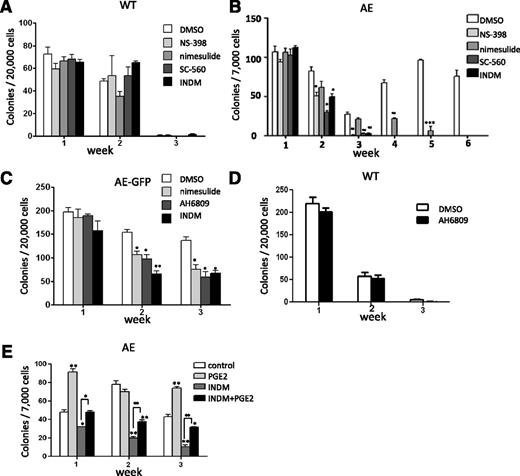
First, wild-type (WT) mouse BM cells were transduced with retroviruses containing either MIGR1 control vector (that expresses GFP) or MIGR1 vector (that expresses both AE and GFP). The GFP-positive cells were grown in methylcellulose-based medium containing cytokines to support clonogenic growth of erythroid, myeloid, and multipotent progenitors. The colonies were pooled, disaggregated, and a same number of cells were replated once a week. As anticipated, although BM cells expressing GFP alone quickly lost their ability to form colonies after 2 passages, BM cells expressing AE-GFP continued to form dense colonies beyond the third week and retained their replating capability even after 6 passages. These results correlate with previous findings indicating that expression of AE enhanced the serial replating efficiency of mouse fetal liver cells, BM cells, and human CD34+ HSPCs.4,8,9 We harvested a portion of the cells from the pooled colonies every week for 3 weeks, and we analyzed Cox-1 and Cox-2 expression by real-time reverse transcription (transcriptase)-polymerase chain reaction (RT-PCR). Interestingly, we found that both Cox-1 and Cox-2 expression gradually decreased in BM cells expressing GFP alone, but not in BM cells expressing AE-GFP (Figure 1A-B). Although Cox-1 expression levels are comparable between GFP and AE-GFP cells in the first week, Cox-1 expression started to increase in AE-GFP cells after 2 more weeks of culture (15.4 ± 2.5-fold in AE-GFP cells as compared with GFP cells in week 3) (Figure 1A). In contrast, Cox-2 expression was higher in AE-GFP cells as compared with GFP cells at all time points tested (3.2 ± 0.18-fold, 4.0 ± 0.4-fold, and 22.2 ± 8.0-fold in weeks 1 to 3, respectively) (Figure 1B).
AE induces COX gene expression in mouse BM cells. (A-B) WT mouse BM cells were transduced with GFP or AE-GFP retrovirus. GFP-positive cells were sorted and plated in methylcellulose medium to form colonies. The cells of the colonies were then pooled for RNA isolation and for replating each week. The expression levels of Cox-1 (A) and Cox-2 (B) were measured by real-time RT-PCR. Relative expression is calculated as fold change compared with GFP-retrovirus transduced BM cells harvested in week 1. (C-D) BM cells isolated from WT and AE/Mx1-Cre mice were cultured, and the expression levels of Cox-1 (C) and Cox-2 (D) were measured and calculated as previously described. (E-F) BM cells isolated from WT and AE/Mx1-Cre mice were depleted of lineage-committed cells. The lin− cells were cultured and the expression levels of Cox-1 (E) and Cox-2 (F) were measured and calculated as previously described. Data represent as mean ± SD; Student t test. *P < .05; **P < .01.
AE induces COX gene expression in mouse BM cells. (A-B) WT mouse BM cells were transduced with GFP or AE-GFP retrovirus. GFP-positive cells were sorted and plated in methylcellulose medium to form colonies. The cells of the colonies were then pooled for RNA isolation and for replating each week. The expression levels of Cox-1 (A) and Cox-2 (B) were measured by real-time RT-PCR. Relative expression is calculated as fold change compared with GFP-retrovirus transduced BM cells harvested in week 1. (C-D) BM cells isolated from WT and AE/Mx1-Cre mice were cultured, and the expression levels of Cox-1 (C) and Cox-2 (D) were measured and calculated as previously described. (E-F) BM cells isolated from WT and AE/Mx1-Cre mice were depleted of lineage-committed cells. The lin− cells were cultured and the expression levels of Cox-1 (E) and Cox-2 (F) were measured and calculated as previously described. Data represent as mean ± SD; Student t test. *P < .05; **P < .01.
In our second approach, we compared WT BM cells to BM cells isolated from the AE/Mx1-Cre mice. The AE/Mx1-Cre mice carry a knockin allele of loxP-stop-loxP-ETO at the endogenous AML1 locus and the Mx1-Cre transgene.8 Serial injections of pI-pC led to induction of Cre recombinases and excision of the stop cassette, resulting in expression of the AE transgene. Total BM cells (Figure 1C-D) or lin− BM cells (Figure 1E-F) were grown on MethoCult and replated each week for 4 weeks. After week 4, we could no longer obtain enough numbers of WT cells for replating, even though AE/Mx1-Cre cells still exhibited robust colony growth at this time. Expression levels of both Cox-1 and Cox-2 were analyzed using real-time RT-PCR. Comparing total BM cells isolated from WT mice with the ones isolated from the AE/Mx1-Cre mice, we observed very similar results to those results from the BM cells transduced with GFP or AE-GFP. Cox-1 levels are higher in AE-expressing BM cells only in week 3 and 4 (8 ± 2.05-fold and 12.8 ± 2.17-fold, respectively) (Figure 1C), whereas Cox-2 levels are higher in AE-expressing BM cells at all time points tested (1.9 ± 0.1-fold, 2.5 ± 0.2-fold, 25 ± 2.5-fold, and 16.9 ± 1.2-fold in weeks 1 to 4, respectively) (Figure 1D). When compared with total BM cells, lin− BM cells from WT mice continued to express elevated levels of Cox-1 and Cox-2 for a longer period of time in culture (Figure 1C-F). In addition, while AE lin− BM cells showed only modest increases of Cox-1 expression over WT lin− BM cells at all time points tested (1.4 ± 0.4-fold, 1.9 ± 0.3-fold, 1.3 ± 0.3-fold and 2.4 ± 0.6-fold in weeks 1 to 4, respectively) (Figure 1E), Cox-2 levels were significantly higher in AE lin− BM cells than in WT lin− BM cells since week 1 (4.4 ± 0.3-fold, 1.4 ± 0.1-fold, 5.0 ± 0.4-fold, and 5.5 ± 0.3-fold in weeks 1 to 4, respectively) (Figure 1F).
In these experiments, we consistently observed Cox-2 upregulation in the first week, whereas Cox-1 increases were only seen in the later weeks. In addition, we have previously shown that expression of AE in the K562 cells caused an upregulation of COX-2, but not COX-1.12 Thus, these data strongly suggest that AE positively regulates Cox-2 expression in mouse HSPCs.
COX inhibitors reduce the serial replating capacity of AE-expressing mouse BM cells
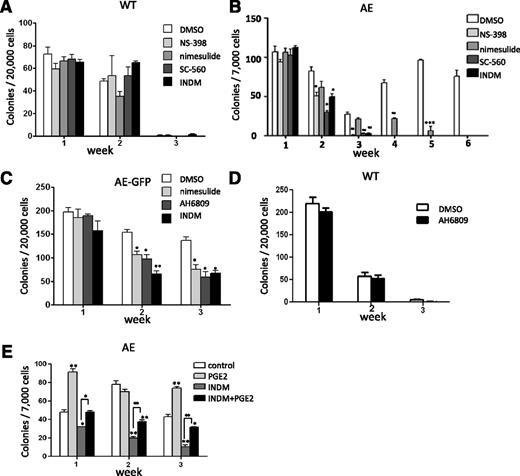
Although it is recently reported that a thrombopoietin/MPL/Bcl-xL pathway is critical for AE-induced self-renewal, there may be other pathways that are also essential for this effect of AE.16 To investigate whether Cox expression plays an important role in AE-mediated self-renewal, we performed a serial replating colony-forming unit assay and examined the effects of various COX inhibitors. The serial replating colony-forming unit assay is often used as an in vitro indicator of self-renewal for HSPCs.8,9 WT and AE/Mx1-Cre mouse BM cells were grown in MethoCult in the presence of the vehicle (dimethylsulfoxide [DMSO]), a nonselective COX inhibitor indomethacin (INDM), COX-2-selective inhibitors (NS-398 and nimesulide) or a COX-1-selective inhibitor (SC-560). As shown in Figure 2A, we found that the colony numbers of WT BM cells declined rapidly and there were very few colonies in week 3. In addition, the presence of various COX inhibitors did not affect colony formation of WT BM cells in all 3 weeks (ANOVA test, P > .05 for all 3 weeks), indicating that these COX inhibitors are not cytotoxic at the concentrations used (Figure 2A). On the other hand, AE/Mx1-Cre BM cells in the presence of the vehicle (DMSO) showed significantly increased serial replating efficiency with colonies growing beyond 6 sequential replatings (Figure 2B). Importantly, we found that treatments of COX inhibitors reduced the serial replating capacity of AE/Mx1-Cre BM cells (Figure 2B). AE/Mx1-Cre BM cells responded to INDM, NS-398 and SC-560 similarly, with colonies diminishing after 3 weeks of treatment. Nimesulide also reduced the serial replating efficiency of AE-expressing cells and inhibited colony formation completely at the end of week 6 (Figure 2B).
COX inhibitors reduce the extended self-renewal capacity of AE-expressing mouse BM cells through a PGE2-dependent pathway. (A) BM cells isolated from WT mice were cultured in MethoCult M3434. Colonies were scored and the cells were serially replated at 7-day intervals. The presence of COX-2 inhibitor NS-398 (50 μM) or nimesulide (50 μM), COX-1 inhibitor SC-560 (50 μM), and a nonselective COX inhibitor indomethacin (INDM, 50 μM) did not affect colony formation of these cells (ANOVA test; P > .05). (B) BM cells isolated from AE/Mx1-Cre mice were cultured, treated, and scored under the same condition as WT mouse BM cells. COX inhibitors reduced the colony forming units of AE-expressing BM cells. (C) COX inhibitors or PGE2 receptor EP2/EP3 dual antagonist AH6809 (30 μM) showed similar inhibitory effects on the colony forming units of AE-GFP-expressing BM cells. (D) EP2/EP3 dual antagonist AH6809 did not affect colony formation of WT mouse BM cells. (E) Treatment of PGE2 partially abrogates the inhibitory effects of INDM on the colony formation of AE/Mx1-Cre BM cells. These results are representatives of at least 2 independent experiments. Data were shown as mean ± SD; Student t test.*P < .05; **P < .01; ***P < .001.
COX inhibitors reduce the extended self-renewal capacity of AE-expressing mouse BM cells through a PGE2-dependent pathway. (A) BM cells isolated from WT mice were cultured in MethoCult M3434. Colonies were scored and the cells were serially replated at 7-day intervals. The presence of COX-2 inhibitor NS-398 (50 μM) or nimesulide (50 μM), COX-1 inhibitor SC-560 (50 μM), and a nonselective COX inhibitor indomethacin (INDM, 50 μM) did not affect colony formation of these cells (ANOVA test; P > .05). (B) BM cells isolated from AE/Mx1-Cre mice were cultured, treated, and scored under the same condition as WT mouse BM cells. COX inhibitors reduced the colony forming units of AE-expressing BM cells. (C) COX inhibitors or PGE2 receptor EP2/EP3 dual antagonist AH6809 (30 μM) showed similar inhibitory effects on the colony forming units of AE-GFP-expressing BM cells. (D) EP2/EP3 dual antagonist AH6809 did not affect colony formation of WT mouse BM cells. (E) Treatment of PGE2 partially abrogates the inhibitory effects of INDM on the colony formation of AE/Mx1-Cre BM cells. These results are representatives of at least 2 independent experiments. Data were shown as mean ± SD; Student t test.*P < .05; **P < .01; ***P < .001.
We then confirmed the effects of COX inhibitors using mouse BM cells transduced with AE-GFP retrovirus. INDM or nimesulide decreased the colony numbers starting from week 2, and the numbers of colonies reduced to approximately 50% compared with DMSO-treated cells in week 3 (Figure 2C). In addition to COX inhibitors, AH6809, a dual antagonist of PGE2 receptors EP2/EP3, also suppressed colony formation of AE-GFP BM cells to a similar degree (Figure 2C). In contrast, AH6809 did not affect colony formation of WT BM cells (Figure 2D). Previously we have shown that nimesulide reverses the hematopoietic phenotypes of AE without affecting AE protein expression in a zebrafish model.12 Similarly, we have found that treatment of INDM or NS-398 did not affect AE protein expression in the BM cells transduced with AE-GFP retrovirus (supplemental Figure 1). Thus, these results suggest that COX inhibitors can antagonize AE function.
To verify that the COX inhibitors antagonize AE function through their on-target effects, namely inhibition of PGE2 biosynthesis, we determined whether supplying PGE2 could reverse the inhibitory effects of INDM on AE function. In this experiment, INDM alone reduced colony formation of AE/Mx1-Cre BM cells after 1 to 3 weeks of treatment (Figure 2E). Treatment of PGE2 alone showed variably positive effects on colony formation during the course of the experiment, consistent with the previously reported role of PGE2 in promoting self-renewal of HSPCs (Figure 2E).17 Co-treatment of PGE2 and INDM rescued the inhibitory effects of INDM on colony formation of AE/Mx1-Cre BM cells (Figure 2E). Therefore, providing exogenous PGE2 abrogated the inhibitory effect of a COX inhibitor on AE function.
Collectively, these data suggest that AE may promote self-renewal of HSPCs through a COX/PGE2–dependent pathway. These data also suggest that inhibiting COX activities or antagonizing PGE2 receptors may suppress AE-mediated leukemogenesis.
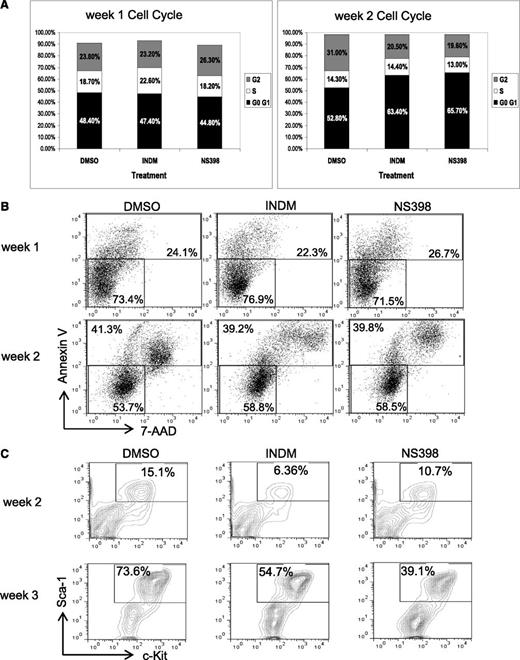
COX inhibitors reduce the percentages of HSPCs in AE-expressing BM cells
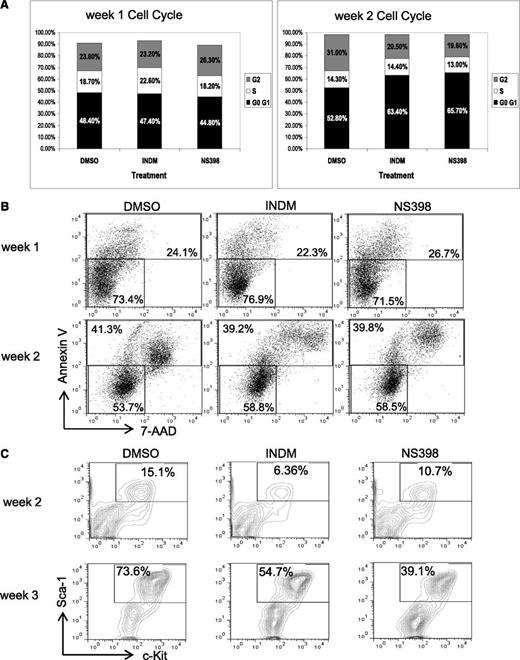
To gain mechanistic insights into the effects of COX inhibitors on AE+ BM cells, we examined whether inhibition of COX affects cell cycle, cell death, or renewal of AE+ HSPCs. In these experiments, we used AE/Mx1-Cre lin− BM cells that had been treated with DMSO, INDM, or NS-398 and grown on MethoCult for analysis. First, we did not find significant changes in the cell cycle profiles of these 3 samples, except that we sometimes detected subtle increases in the G0/G1 population of the samples treated with COX inhibitors (Figure 3A and supplemental Figure 2). Next, we found that the percentages of Annexin V–positive cells, including both apoptotic and necrotic cells, were similar among all 3 samples, suggesting that COX inhibitors do not cause substantial toxicity toward these cells (Figure 3B and supplemental Figure 2). Furthermore, we examined the expression of the HSC markers Sca-1 and c-Kit in these cells after 2 and 3 weeks of treatment. We found that in the DMSO-treated samples, the percentages of Sca-1+c-Kit+ cells increased from 14.6% to 73.6% from week 2 to week 3. However, COX inhibitors reduced the percentages of Sca-1+c-Kit+ cells from 73.6% (DMSO) to 54.7% (INDM) and 39.1% (NS-398) (Figure 3C and supplemental Figure 2). In sum, these data show that COX inhibitors can suppress the expansion of phenotypic HSCs in the presence of AE, which is consistent with the hypothesis that inhibition of COX may impair self-renewal of AE+ HSPCs.
COX inhibitors reduce the percentages of lin-Sca-1+c-Kit+ population in AE-expressing BM cells without inducing apoptosis. The lin− BM cells from AE/Mx1-cre mice were cultured in methylcellulose-based medium in the presence of vehicle (DMSO), INDM (50 μM), or NS-398 (50 μM), and were replated as previously described. These cells were analyzed for cell cycle phases (A), apoptosis (B), and percentages of lin−Sca-1+C-Kit+ cells (C) by flow cytometry after 1 to 3 weeks of treatment. Two independent experiments showed similar results. The results of a duplicate experiment are shown in supplemental Figure 2.
COX inhibitors reduce the percentages of lin-Sca-1+c-Kit+ population in AE-expressing BM cells without inducing apoptosis. The lin− BM cells from AE/Mx1-cre mice were cultured in methylcellulose-based medium in the presence of vehicle (DMSO), INDM (50 μM), or NS-398 (50 μM), and were replated as previously described. These cells were analyzed for cell cycle phases (A), apoptosis (B), and percentages of lin−Sca-1+C-Kit+ cells (C) by flow cytometry after 1 to 3 weeks of treatment. Two independent experiments showed similar results. The results of a duplicate experiment are shown in supplemental Figure 2.
COX inhibitors suppress AE-induced β-catenin signaling
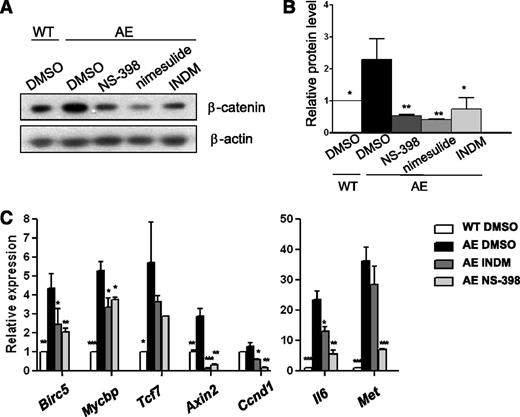
Previously, we have shown that AE activates β-catenin–dependent signaling through COX-2 in zebrafish hematopoietic cells and in human K562 cells.12 In addition, Müller-Tidow et al18 has found that expression of AE induces Wnt signaling in human U937 cells. To test whether expression of AE leads to β-catenin activation in mammalian HSPCs, we first examined β-catenin expression using real-time RT-PCR and found that AE did not affect β-catenin transcript levels in mouse BM cells. However, AE increased β-catenin protein levels by 2.3-fold to 3.6-fold (supplemental Figure 3). In addition, treatments with NS-398, nimesulide and INDM reduced β-catenin levels in AE/Mx1-Cre BM cells, indicating that COX acts upstream of β-catenin activation (Figure 4A-B and supplemental Figure 4). Thus, COX inhibition diminishes β-catenin accumulation induced by AE.
COX inhibitors reduce β-catenin activity in AE-expressing BM cells. (A) Western blot analysis demonstrates that COX inhibitors NS-398, nimesulide, and INDM decreased β-catenin protein levels in AE-expressing mouse BM cells. WT and AE/Mx1-Cre mouse lin− BM cells were cultured with the vehicle (DMSO) or COX inhibitors (50 μM) for 4 weeks. (B) Protein band intensity in (A) was normalized with β-actin. Relative protein level of β-catenin was calculated as fold change compared with WT BM cells in DMSO. In AE/Mx1-Cre BM cells, NS-398, nimesulide, and INDM reduced β-catenin levels to 0.24 ± 0.09-fold, 0.18 ± 0.06-fold and 0.28 ± 0.15-fold of the vehicle control (DMSO), respectively. COX inhibitors also suppressed β-catenin protein expression in AE/Mx1-Cre BM cells after 1 week of treatment (supplemental Figure 4). (C) The expression of β-catenin target genes was measured by real-time RT-PCR and normalized to β-ACTIN expression. Relative expression was calculated as fold change relative to WT vehicle-treated cells (WT DMSO). Data represent mean ± SD; Student t test. *P < .05; **P < .01; ***P < .001 when compared with AE DMSO.
COX inhibitors reduce β-catenin activity in AE-expressing BM cells. (A) Western blot analysis demonstrates that COX inhibitors NS-398, nimesulide, and INDM decreased β-catenin protein levels in AE-expressing mouse BM cells. WT and AE/Mx1-Cre mouse lin− BM cells were cultured with the vehicle (DMSO) or COX inhibitors (50 μM) for 4 weeks. (B) Protein band intensity in (A) was normalized with β-actin. Relative protein level of β-catenin was calculated as fold change compared with WT BM cells in DMSO. In AE/Mx1-Cre BM cells, NS-398, nimesulide, and INDM reduced β-catenin levels to 0.24 ± 0.09-fold, 0.18 ± 0.06-fold and 0.28 ± 0.15-fold of the vehicle control (DMSO), respectively. COX inhibitors also suppressed β-catenin protein expression in AE/Mx1-Cre BM cells after 1 week of treatment (supplemental Figure 4). (C) The expression of β-catenin target genes was measured by real-time RT-PCR and normalized to β-ACTIN expression. Relative expression was calculated as fold change relative to WT vehicle-treated cells (WT DMSO). Data represent mean ± SD; Student t test. *P < .05; **P < .01; ***P < .001 when compared with AE DMSO.
Next, we examined the expression of seven β-catenin target genes in response to AE and COX inhibition using real-time RT-PCR. Among these genes, human baculoviral IAP repeat containing 5 (BIRC5) (also known as survivin) has been shown to be a direct target of β-catenin in colorectal cancer.19 Interestingly, it has been found that AE induces BIRC5 expression in human CD34+ cells, and that genetic knockdown of BIRC5 leads to granulocytic differentiation of AE+ leukemia cells.20 The Wnt/β-catenin signaling also upregulates MYCBP, transcription factor 7 (TCF7) (also known as Tcf1), AXIN2, CCND1, and MET in colorectal cancer and IL6 in 3T3-L1 pre-adipocytes.21-27 As shown in Figure 4C, we detected various degrees of induction of these genes (2.9-fold to 36.3-fold), except Ccnd1 (1.3-fold) in AE/Mx1-Cre BM cells (AE DMSO) as compared with WT BM cells (WT DMSO). Moreover, NS-398 and INDM reduced the expression of all of these genes (0.11-fold to 0.71-fold, and 0.05-fold to 0.78-fold of the vehicle control, respectively) in AE/Mx1-Cre BM cells. For some of the genes such as Il6 and Met, we found that NS-398 was more effective than INDM. It is known that INDM inhibits COX-1 more potently than it inhibits COX-2. Since these 2 genes have the highest fold induction among all of those tested, it could be that they also need a higher degree of COX-2 inhibition than the other genes do to suppress their induction. These results indicate that COX activities act upstream of β-catenin activation in AE+ BM cells.
β-catenin is essential for the clonogenic growth of AE+ HSPCs and leukemic cells
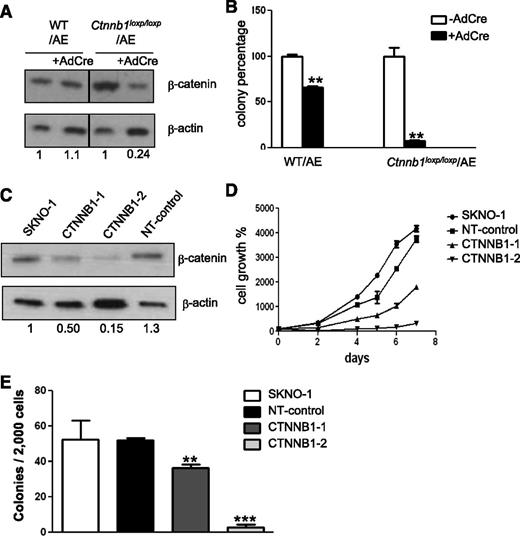
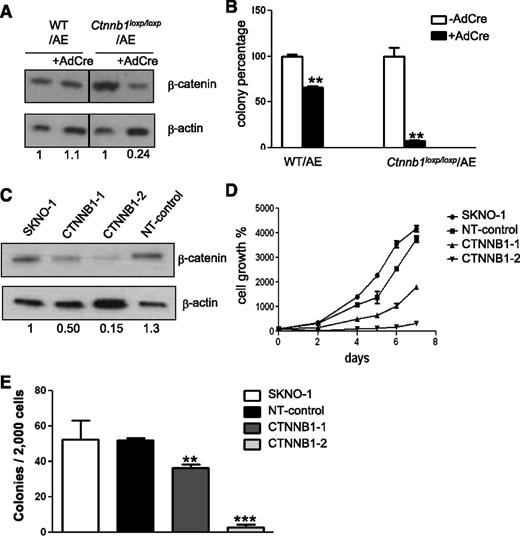
To determine if COX inhibitors impair colony formation of AE+ BM cells via a β-catenin–dependent pathway, we performed genetic knockdown of β-catenin in AE+ BM cells and in SKNO-1 cells, a human myelogenous leukemia cell line established from an AML patient carrying the (8;21) translocation.28 In the first experiment, we isolated BM cells from WT mice and the mice with 2 floxed alleles of the gene encoding β-catenin (Ctnnb1),29 and we transduced these cells with AE-GFP retrovirus. The AE-GFP+ WT BM cells (WT/AE) and the AE-GFP+ floxed-Ctnnb1 BM cells (Ctnnb1loxP/loxP/AE) were then transduced with adenovirus containing the cre gene (AdCre). This procedure led to incomplete disruption of the Ctnnb1 gene in Ctnnb1loxP/loxP BM cells (supplemental Figure 5). As shown in Figure 5A, expression of cre caused a 76% reduction in the β-catenin level of the Ctnnb1loxP/loxP/AE BM cells, but it did not affect the β-catenin level in the WT/AE BM cells. We then plated these cells on MethoCult to determine the effects of β-catenin knockdown on colony formation (Figure 5B). We found that the adenovirus infection procedure by itself caused an appreciable degree of stress and toxicity to the cells. In the WT/AE BM cells, infection with AdCre reduced the colony number to 67% of that formed by their parental cells. However, in the Ctnnb1loxP/loxP/AE cells, infection with AdCre reduced the colony number to only 7% of that formed by their parental cells. These data suggest that β-catenin is critical for the colony forming ability of AE+ HSPCs.
Knockdown of β-catenin reduces colony formation and proliferation of AE-expressing cells. (A) Western blot analysis of β-catenin knockdown efficiency. BM cells isolated from WT and floxed-Ctnnb1 mice were transduced with AE-GFP retrovirus (WT/AE and Ctnnb1loxp/loxp/AE, respectively). Subsequently, cre was introduced by adenovirus infection (AdCre) to disrupt the Ctnnb1 gene. Protein band intensity was quantified, normalized with β-actin and fold change, as compared with the parental cells indicated at the bottom of each lane. (B) Comparison of colony-forming units of AdCre-infected and noninfected AE+ BM cells after 7 days of culture in methylcellulose-based medium. (C) Western blot of β-catenin in SKNO-1 cells and SKNO-1 cells harboring either 2 different Ctnnb1 shRNAs (CTNNB1-1 and CTNNB1-2) or nontargeting control shRNA (NT-control). Fold change is determined as previously described and is indicated at the bottom of each lane. (D) Proliferation of SKNO-1 cells and SKNO-1 cells harboring Ctnnb1 shRNA (CTNNB1-1 and CTNNB1-2) or NT-control shRNA. (E) Comparison of colony forming units after 14 days of culture in methylcellulose-based medium. Data represent mean ± SD; Student t test. **P < .01; ***P < .001.
Knockdown of β-catenin reduces colony formation and proliferation of AE-expressing cells. (A) Western blot analysis of β-catenin knockdown efficiency. BM cells isolated from WT and floxed-Ctnnb1 mice were transduced with AE-GFP retrovirus (WT/AE and Ctnnb1loxp/loxp/AE, respectively). Subsequently, cre was introduced by adenovirus infection (AdCre) to disrupt the Ctnnb1 gene. Protein band intensity was quantified, normalized with β-actin and fold change, as compared with the parental cells indicated at the bottom of each lane. (B) Comparison of colony-forming units of AdCre-infected and noninfected AE+ BM cells after 7 days of culture in methylcellulose-based medium. (C) Western blot of β-catenin in SKNO-1 cells and SKNO-1 cells harboring either 2 different Ctnnb1 shRNAs (CTNNB1-1 and CTNNB1-2) or nontargeting control shRNA (NT-control). Fold change is determined as previously described and is indicated at the bottom of each lane. (D) Proliferation of SKNO-1 cells and SKNO-1 cells harboring Ctnnb1 shRNA (CTNNB1-1 and CTNNB1-2) or NT-control shRNA. (E) Comparison of colony forming units after 14 days of culture in methylcellulose-based medium. Data represent mean ± SD; Student t test. **P < .01; ***P < .001.
Next, we investigated the role of β-catenin in the growth of SKNO-1 cells by short hairpin (shRNA) knockdown. First, we found that 2 shRNAs (CTNNB1-1 and CTNNB1-2) led to different degrees of β-catenin reduction (Figure 5C). Then we compared the growth kinetics of these cells and found that their growth rates showed a positive correlation with their β-catenin levels (Figure 5D). In addition, when these cells were plated on methylcellulose-based medium, we found that knockdown of β-catenin almost completely abolished the colony forming ability of SKNO-1 (Figure 5E). Jointly, these results indicate that β-catenin-mediated signaling is essential for the propagation of AE+ HSPCs and leukemia cells.
Nimesulide suppresses the initiation and progression of xenograft tumors in mouse models
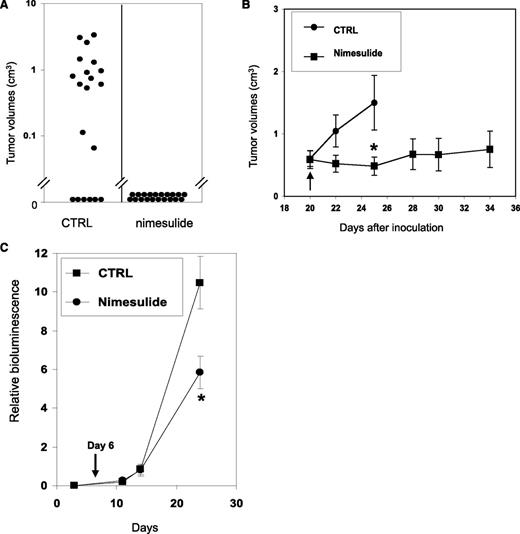
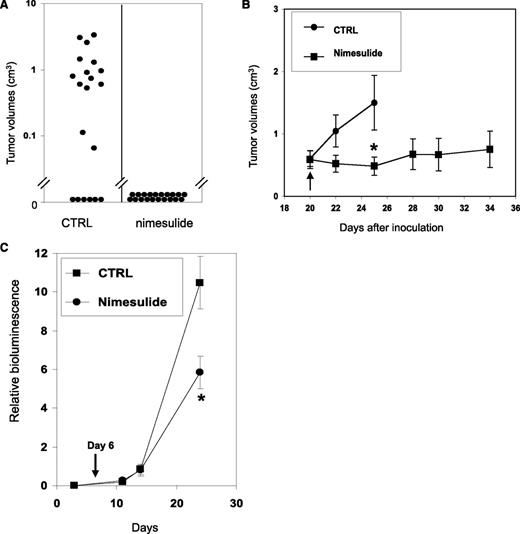
Because COX activity is essential for the clonogenic growth of AE+ HSPCs, we examined whether nimesulide could prevent the initiation of xenograft tumors using SKNO-1 cells. In 2 separate experiments, SKNO-1 cells were inoculated into the bilateral flanks of 10 NCr nude mice and the mice were randomized into control and treatment groups of 5 mice each. From the day of inoculation, the control group was fed with normal food, whereas the treatment group was fed with the same food mixed with 1000 ppm of nimesulide. The average plasma concentration of nimesulide in mice of the treatment group is 22.6 μM (see supplemental Methods and supplemental Table 2). No untoward side effects were observed in the treated mice. The sizes of the xenograft tumors were measured twice a week from day 9 to day 25. Until the end of the experiments, 16 of 20 inoculation sites developed tumors in the control group, whereas no tumor developed from 20 inoculation sites in the treatment group (Figure 6A). The average tumor size measured on day 25 in the control group was 0.85 ± 0.23 cm3. Therefore, nimesulide abolished the formation of xenograft tumors with AE-expressing leukemia cells.
Nimesulide inhibits SKNO-1 in xenograft and orthotopic AML mouse models. (A) Nimesulide suppresses the formation of xenograft tumors with SKNO-1 cells. Treatment with nimesulide was initiated in NCr nude mice (n = 10) on the day of SKNO-1 cell inoculation. The control group (n = 10) did not receive nimesulide. A total of 20 subcutaneous inoculation sites were tracked in each of the treatment and control groups. The tumor sizes measured on day 25 and are shown as black dots. (B) Nimesulide suppresses the progression of xenograft tumors with SKNO-1 cells. Inoculation of tumor cells was performed as in (A). Treatment with nimesulide was initiated on day 20 after inoculation in the treatment group (black arrow). (C) NSG mice were inoculated via tail vein injections with SKNO-1 cells that have been engineered to express luciferase. Injected mice were fed with normal powder food (square) (n = 5) or normal powder food mixed with nimesulide (dot) (n = 6) from day 6 (black arrow). Progression of SKNO-1 cells was monitored by in vivo imaging of bioluminesce (images are shown in supplemental Figure 6). Results are shown as mean ± SEM. Significant difference in values between the control group and the nimesulide group. *P < .05; Mann-Whitney U test.
Nimesulide inhibits SKNO-1 in xenograft and orthotopic AML mouse models. (A) Nimesulide suppresses the formation of xenograft tumors with SKNO-1 cells. Treatment with nimesulide was initiated in NCr nude mice (n = 10) on the day of SKNO-1 cell inoculation. The control group (n = 10) did not receive nimesulide. A total of 20 subcutaneous inoculation sites were tracked in each of the treatment and control groups. The tumor sizes measured on day 25 and are shown as black dots. (B) Nimesulide suppresses the progression of xenograft tumors with SKNO-1 cells. Inoculation of tumor cells was performed as in (A). Treatment with nimesulide was initiated on day 20 after inoculation in the treatment group (black arrow). (C) NSG mice were inoculated via tail vein injections with SKNO-1 cells that have been engineered to express luciferase. Injected mice were fed with normal powder food (square) (n = 5) or normal powder food mixed with nimesulide (dot) (n = 6) from day 6 (black arrow). Progression of SKNO-1 cells was monitored by in vivo imaging of bioluminesce (images are shown in supplemental Figure 6). Results are shown as mean ± SEM. Significant difference in values between the control group and the nimesulide group. *P < .05; Mann-Whitney U test.
We also examined the effects of nimesulide on the progression of xenograft tumors with SKNO-1 cells. The mice were inoculated as previously, and the treatment was started 20 days later. The average tumor size when the treatment started was 0.59 ± 0.14 cm3 (n = 13) in the control group and 0.60 ± 0.13 cm3 (n = 7) in the treatment group (Figure 6B). After 5 days of treatment, nimesulide significantly reduced tumor growth relative to control mice by 68% (P < .05, Mann-Whitney U test). Many of the mice in the control group were killed after day 25 when their tumor sizes exceeded the Institutional Animal Care and Use Committee guidelines. Mice in the treatment group were monitored up to 14 days after treatment, and we found no significant tumor growth on average since beginning treatment (P = .57; Mann-Whitney U test) (Figure 6B). It is noteworthy that in the treatment group, 2 tumors with volumes of 0.38 cm3 and 0.22 cm3 disappeared after 4 and 5 days of the treatment, and another tumor with the volume of 1.15 cm3 decreased its size to 0.11 cm3 after 8 days of treatment.
These data indicate that nimesulide can effectively inhibit both the initiation and the progression of xenograft tumors formed by SKNO-1 cells, supporting the potential use of COX-2 inhibitors in preventing AML relapse and in conjunction with other cytotoxic therapeutics for treating AML.
Nimesulide inhibits progression of SKNO-1 cells in an orthotopic leukemia model
The progression of human leukemia cells may also be evaluated in their natural milieu in mice. In the orthotopic leukemia model, a firefly luciferase gene is introduced into human leukemia cells so that tumor burden may be monitored using whole body imaging of bioluminescence. We further examined the effects of nimesulide on an orthotopic leukemia model of AE+ AML. SKNO-1 cells stably expressing the luciferase gene were intravenously injected into NOD scid gamma (NSG) mice. Treatment with nimesulide was initiated on day 6 after transplantation. At this time, leukemia had been established, as judged by logarithmically increasing bioluminescence signals. In vivo progression of leukemia was monitored and quantified using in vivo bioluminescence imaging (representative images are shown in supplemental Figure 6). After 18 days of treatment, nimesulide significantly reduced tumor burden by 44% compared with control mice (P < .05; Mann-Whitney U test) (Figure 6C). These data indicate that nimesulide impedes the progression of human AE+ AML in a mouse model.
NSAIDs suppress the growth of primary AML samples in vitro and in vivo
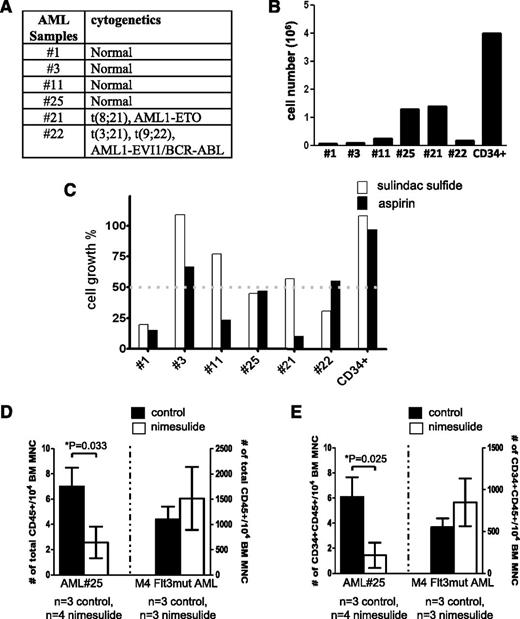
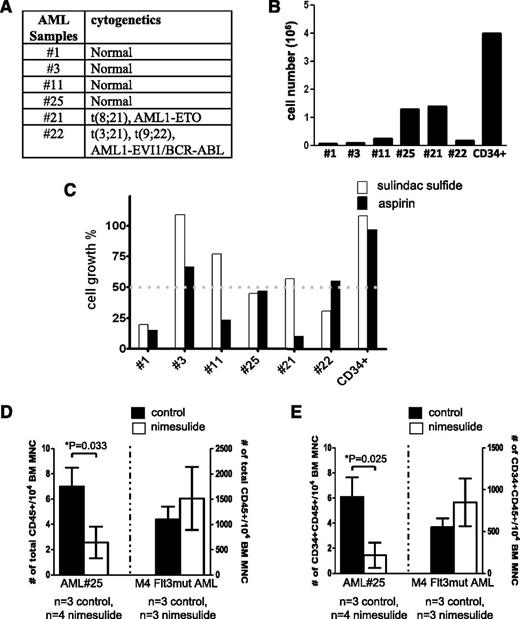
In this study, we have identified a signaling pathway mediated by AE to promote hematopoietic renewal. We wondered whether the COX/β-catenin pathway might be a core signaling pathway used by many AML oncogenes, and whether Food and Drug Administration-approved COX inhibitors, such as nonsteroidal anti-inflammatory drugs (NSAIDs), can impair the growth of primary AML samples harboring various cytogenetic abnormalities. Six AML BM samples of various karyotypes (Figure 7A) were plated at the same density and cultured on methylcellulose medium for 14 days. The total cell numbers after the culture showed that these samples exhibited various rates of growth in culture (Figure 7B). Human BM CD34+ cells were used as a comparison. We used sample #25 to conduct a dose study for aspirin and sulindac (supplemental Figure 7). Although both drugs inhibit COX-1 and COX-2, aspirin, but not sulindac, is an irreversible inhibitor of the cyclooxgenases. Based on the dose study, we treated all 6 samples using 10 μM of sulindac and 500 μM of aspirin. Both doses are within the clinically achievable plasma concentrations in human.30,31 The cell growth percentages in the presence of these drugs were determined as compared with the corresponding DMSO-treated samples. We found that at least 1 of the 2 drugs caused more than 50% of reduction in the growth of 5 of 6 AML samples tested without affecting the growth of human CD34+ cells (Figure 7C). These 5 samples include AML of normal karyotype, t(8;21) and t(3;21)(9;22).
NSAIDs aspirin and sulindac inhibit the growth of primary AML samples in vitro and in vivo. (A) The karyotypes of the AML samples. (B) Twenty thousand cells from AML patients’ BM samples were plated in methylcellulose-based medium and cultured for 14 days. Cell growth was determined by the total cell number after culture. As a comparison, human BM CD34+ cells (STEMCELL Technologies) were cultured under the same condition. (C) Primary AML samples responded to aspirin and sulindac sulfide to various extents. Primary AML cells were plated and cultured as described in (B), except that each well was treated with either DMSO, sulindac sulfide (10 μM),or aspirin (500 μM). The cell growth percentage was determined by comparing the total cell numbers in the drug-treated wells to those in the DMSO-treated wells at the end of culture. In comparison, the growth of human CD34+ cells was unaffected by these 2 drugs. (D-E) Orthotopic xenografts of AML#25 and 1 other AML sample (M4 subtype, Flt3 mutation) in NSG mice. Nimesulide treatment was initiated 1 day after transplantation. After 1 to approximately 3 months (3 months for AML#25 and 6 weeks for M4, Flt3mut AML) the mice were sacrificed and the frequencies of human CD45+ and CD45+CD34+ cells in their BM were determined. Student t test was performed between the control group and the nimesulide-treated group. The comparisons that showed statistical significance (P < .05) were indicated by asterisks. MNC, mononuclear cells; n, the numbers of mice in each group.
NSAIDs aspirin and sulindac inhibit the growth of primary AML samples in vitro and in vivo. (A) The karyotypes of the AML samples. (B) Twenty thousand cells from AML patients’ BM samples were plated in methylcellulose-based medium and cultured for 14 days. Cell growth was determined by the total cell number after culture. As a comparison, human BM CD34+ cells (STEMCELL Technologies) were cultured under the same condition. (C) Primary AML samples responded to aspirin and sulindac sulfide to various extents. Primary AML cells were plated and cultured as described in (B), except that each well was treated with either DMSO, sulindac sulfide (10 μM),or aspirin (500 μM). The cell growth percentage was determined by comparing the total cell numbers in the drug-treated wells to those in the DMSO-treated wells at the end of culture. In comparison, the growth of human CD34+ cells was unaffected by these 2 drugs. (D-E) Orthotopic xenografts of AML#25 and 1 other AML sample (M4 subtype, Flt3 mutation) in NSG mice. Nimesulide treatment was initiated 1 day after transplantation. After 1 to approximately 3 months (3 months for AML#25 and 6 weeks for M4, Flt3mut AML) the mice were sacrificed and the frequencies of human CD45+ and CD45+CD34+ cells in their BM were determined. Student t test was performed between the control group and the nimesulide-treated group. The comparisons that showed statistical significance (P < .05) were indicated by asterisks. MNC, mononuclear cells; n, the numbers of mice in each group.
To test whether COX inhibitors can suppress in vivo progression of primary AML cells, we first transplanted several AML samples into nonobese diabetic/severe combined immunodeficiency IL2Rγc null (NSG) mice. Three months later, only sample #25 resulted in a high level (∼10%) of engraftment. Thus, we used human CD45+ cells isolated from the primary recipients of AML #25 and another AML sample (M4 subtype with Flt3 mutation) also isolated from previous recipient mice for transplantation. Treatment with nimesulide was initiated 1 day after transplantation for the duration of 3 months and 6 weeks for AML #25 and M4 Flt3mut AML, respectively. Mice were then sacrificed and their BM was analyzed for the frequency of human CD45+ and CD45+CD34+ cells. As shown in Figure 7D-E, we found that AML#25, but not M4 Flt3mut AML, responded to nimesulide. For AML#25, nimesulide reduced not only the engraftment of human CD45+ cells in the recipient mice but also the percentage of CD34+ cells in the xenografts. The average percentages of CD34+ cells among hCD45+ cells are 90% and 62% in the control and nimesulide-treated groups, respectively. Furthermore, nimesulide did not affect the frequency of lin−Sca-1+C-Kit+ (LSK) cells in these mice (supplemental Figure 8).
Discussion
In this study, we find that AE causes increased Cox-2 expression and activation of β-catenin signaling in mouse HSPCs. Similarly, AE also leads to increased COX-2 expression in human CD34+ HSPCs.32 Furthermore, we show that COX inhibitors not only suppress AE-induced serial replating efficiency, but also β-catenin activation in AE+ HSPCs. Supplementing PGE2 reverses the inhibitory effects of COX inhibitors. Therefore, these results indicate an epistatic relationship between COX activities/PGE2 production/β-catenin activation in AE+ HSPCs. Moreover, we find that COX inhibition does not elicit cytotoxicity toward AE+ HSPCs, but strongly reduces percentage of Sca-1+c-Kit+ cells. These results suggest a critical role of the COX/β-catenin signaling axis in AE-mediated self-renewal of HSPCs.
We show that β-catenin is essential for the clonogenic growth of AE+ HSPCs and leukemia cells. In accordance with our results, previous studies have demonstrated the important roles of β-catenin in HSCs and in myeloid malignancies. Activation of β-catenin signaling has been shown to enhance self-renewal in HSCs.15 Expression of β-catenin has also been linked to enhanced clonogenic growth of AML cells and poor prognosis.33 Moreover, it has been shown that loss of β-catenin impairs the repopulating efficiency of the LSCs in chronic myelogenous leukemia.34,35 Although several mechanisms have been shown to cause β-catenin activation in AML, Wang et al36 recently reported that the activation of a COX-1–PGE2–β-catenin pathway, mediated by Hoxa9 and Meis1a coexpression or by MLL-AF9, is indispensible for the development of AML LSCs. This report shows that COX-1, but not COX-2, is induced in the presence of these oncogenes. In addition, they show that indomethacin impairs the leukemogenicity of the LSCs expressing these oncogenes.36 In our previous report and the current study, we show that AE activates a COX-2–PGE2–β-catenin pathway in HSPCs and in a leukemia cell line K562.12 Together, these data suggest that it is important to investigate whether there are additional leukemia oncogenes that may contribute to leukemogenesis by inducing COX activities and β-catenin signaling in LSCs. This hypothesis is also supported by our findings that both aspirin and sulindac impede the growth of primary AML cells of various cytogenetic abnormalities. Futhermore, it is recently shown that sulindac sulfide suppresses self-renewal of PML-RARα+ and PLZF-RARα+ mouse HSPCs.37
We find that oral uptake of a COX-2 inhibitor nimesulide completely abrogates the initiation of xenograft tumors formed by human AE+ SKNO-1 cells, correlating with our finding that loss of β-catenin abolishes the clonogenic growth of SKNO-1. Although the origins of the engrafting cells in xenograft tumor models are still controversial, several studies have shown that engraftment of human cancer cell lines is preferentially mediated by a population of tumor-initiating cells within the heterogeneous cell populations in culture.38,39 Thus, our results may suggest a critical role of COX-2 activity in the tumor-initiating cells within the SKNO-1 cell culture. Furthermore, we find that nimesulide also suppresses the growth of established xenograft tumors formed by SKNO-1 cells. In some instances, nimesulide even caused regression of the established tumors in mice. Treatment with nimesulide not only inhibited subcutaneous growth, but also orthotopic progression of SKNO-1 in the mouse model. Therefore, these data indicate that inhibition of COX-2 activity effectively impair the tumorigenicity of human AML SKNO-1 cells.
In orthotopic xenografts of primary AML, we find that nimesulide suppresses the engraftment of an AML sample with a normal karyotype, but not an AML sample with an Flt3 mutation. We cannot be certain from this single sample that Flt3 mutant AMLs are resistant to COX inhibition, and it remains to be determined whether COX inhibition may provide additional therapeutic benefits in combination with Flt3 inhibitors for AML with Flt3 mutations. Nevertheless, these results suggest that non-t(8;21) AML may also respond to COX inhibitors.
Even though β-catenin activation can promote the expansion of normal HSCs, genetic deletion of β-catenin and γ-catenin in adult mice do not elicit hematopoiesis or hematological defects, implying that inhibition of oncogene-mediated β-catenin activation and its signaling may be a valid therapeutic approach for AML.40,41 Interestingly, several epidemiological studies have demonstrated an inverse association between regular use of NSAIDs and incidence of some types of cancers, including AML.42,43 In particular, a case-control study has shown that prescription NSAID use was most evidently associated with decreased risk for the M2 subtype of AML, of which approximately 40% may carry the AE oncogene.44 Jointly, our results, the results from these studies, and the study reported by Wang et al36 support the critical roles of COX activities in the development of LSCs and the potential chemopreventative effects of NSAIDs in AML.
Developing therapeutics against β-catenin–dependent signaling pathways is an area that is currently under intensive investigation. Several exciting strategies and chemical agents are emerging, but they still need to undergo extensive trials to identify potential side effects, drug interactions, and clinical efficacy. In this study, we have identified several β-catenin target genes that are upregulated in AE+ HSPCs. It is possible that some of these genes may also be effective therapeutic targets for AML. For example, both Birc5 and MET are known to be involved in other types of cancers.19,45 In addition, Tcf7 and IL6 have been shown to regulate proliferation, differentiation, and self-renewal of HSCs.46,47 We are currently investigating the roles of these β-catenin target genes in AE-mediated leukemogenesis.
Given that numerous selective and nonselective COX inhibitors are readily available in the clinics, these drugs should be evaluated for their efficacy in treating AML. COX inhibitors, or NSAIDs, are sometimes used on patients for long-term treatments. Interestingly, a COX-2–PGE2–β-catenin signaling axis has been shown to be critical for the development of colorectal cancers, and the efficacies of NSAIDs in reducing risks for these cancers have been reported.48,49 Our data strongly suggest that COX inhibition may not exert significant cytotoxicity toward AML blast cells, but it is likely to impede the development and progression of AML. One potential concern for the use of COX inhibitors in the treatment of leukemia is their anti-platelet activities, as these patients often have periods of thrombocytopenia due to the leukemia or treatment. However, this anti-platelet effect is less with COX-2 inhibition as compared with COX-1 inhibitors or nonspecific COX inhibitors.50 Alternatively, COX inhibitors may be administered after the induction therapy, with the consolidation therapy, and/or as a maintenance treatment. Interestingly, a recent study has shown that indomethacin, when used in combination with imatinib, can effectively suppress chronic myelogenous leukemia stem cells and delay the onset of imatinib-refractory disease in mice.34 Thus, we hypothesize that Food and Drug Administration-approved NSAIDs may be used as a part of AML treatment regimen for suppressing relapse and to improve durable clinical outcomes in AML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr D. M. Langenau for helpful comments on the manuscript.
This work was supported by grants from the National Institutes of Health (K01 AG031300 [J.-R.J.Y.], R01 CA140188 [J.-R.J.Y., D.A.S., R.T.P., and Y.Z.], R01 CA115772 [D.A.S., X.C., and J.W.], and T32 CA071345 [J.W.]); Hundai Hope on Wheels (D.A.S.); the Mattina Proctor Foundation (D.A.S.); and the Massachusetts General Hospital Claflin Distinguished Scholar Award (J.-R.J.Y.).
Authorship
Contribution: Y.Z., J. Wang., J. Wheat., X.C., and S.J. performed research; H.S., A.T.F., and A.L.K. provided critical reagents; Y.Z., J. Wang, J. Wheat, S.J., R.T.P., D.A.S., and J.-R.J.Y. designed experiments, interpreted data, and wrote the manuscript.
Conflict- of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jing-Ruey Joanna Yeh, 149 13th St, 149-4201, Charlestown, MA 02129; e-mail: jyeh1@partners.org; and David A. Sweetser, 175 Cambridge St, Suite 500, Boston, MA 02114; e-mail: dsweetser@partners.org.
References
Author notes
Y.Z. and J.W. contributed equally to this study.