Key Points
VPS45 is a new gene associated with severe infections and bone marrow failure in infancy that can be treated by bone marrow transplantation.
The mutation affects intracellular storage and transport and results in increased programmed cell death in neutrophils and bone marrow.
Abstract
Severe congenital neutropenia as well as primary myelofibrosis are rare in infancy. Elucidation of the underlying mechanism is important because it extends our understanding of the more common adult forms of these disorders. Using homozygosity mapping followed by exome sequencing, we identified a Thr224Asn mutation in the VPS45 gene in infants from consanguineous families who suffered from life-threatening neutropenia, which was refractory to granulocyte CSF, from defective platelet aggregation and myelofibrosis. The mutation segregated in the families, was not present in controls, affected a highly conserved codon, and apparently destabilized the Vps45 protein, which was reduced in the patients’ leukocytes. Introduction of the corresponding mutation into yeast resulted in reduced cellular levels of Vps45 and also of the cognate syntaxin Tlg2, which is required for membrane traffic through the endosomal system. A defect in the endosomal-lysosomal pathway, the homologous system in humans, was suggested by the absence of lysosomes in the patients’ fibroblasts and by the depletion of α granules in their platelets. Importantly, accelerated apoptosis was observed in the patients’ neutrophils and bone marrow. This is the first report of a Vps45-related disease in humans, manifesting by neutropenia, thrombasthenia, myelofibrosis, and progressive bone marrow failure.
Introduction
Severe congenital neutropenia comprises a group of genetically heterogeneous disorders with a common denominator, an increased susceptibility of neutrophil granulocytes and their precursors to undergo apoptosis.1 In many of these disorders, the bone marrow compartment is depleted; however, the severe congenital neutropenias that are caused by mutations in lysosomal-related genes are characterized by myeloid maturation in the bone marrow.
Primary myelofibrosis is rare in children, and the very few patients who presented in infancy originated from consanguineous families, suggesting that congenital myelofibrosis could be transmitted in an autosomal recessive manner.2,3 In adults, primary myelofibrosis is characterized by ineffective hematopoiesis and proliferation of dysfunctional megakaryocytes with reticulin and collagen fibrosis in bone marrow, ineffective extramedullary hematopoiesis, and deregulated production of cytokines. The molecular basis in adults includes cytogenetic abnormalities originating at the progenitor cell level or acquisition of the V617F mutation in the JAK2 gene by the hematopoietic stem cell.4,5 While the JAK2 mutation activates the JAK/signal transducer and activator of transcription pathway, promoting the transcription of a plethora of proproliferative and antiapoptotic genes,6 the fibrosis likely results from the cross-communication among clonal stem cells, which leads to inappropriate cytokine release (reviewed in Lataillade et al7 ).
We now report the clinical course and laboratory findings, including the results of the molecular investigation in infants originating from consanguineous Palestinian families who presented with life-threatening neutropenia, myelofibrosis, and progressive bone marrow failure.
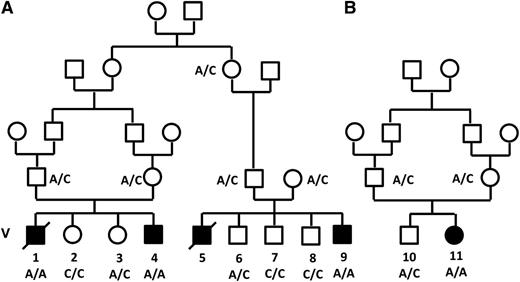
The 3 index patients, 2 males and 1 female, were referred because of recurrent infections since early infancy. They originated from 2 unrelated Palestinian families and were the products of consanguineous marriages (Figure 1). The clinical, hematological, and radiological findings of the 3 index patients and of 2 older siblings, who were similarly affected but died before the study period, are summarized in Table 1. Pregnancy, delivery, birth weight, and development during the early neonatal period were uneventful in all the patients. However, between 1 and 7 months of age they presented with recurrent bacterial infections, typically pneumonia and soft tissue infections. The common pathogens were gram-negative (mainly Pseudomonas) and gram-positive bacteria; invasive fungal infection was documented in 1 patient. Laboratory investigations disclosed profound neutropenia, progressive normocytic anemia with low reticulocyte count, and subsequent development of thrombocytopenia. Peripheral blood smear revealed anisocytosis and poikylocytosis with teardrop cells, neutropenia, and thrombocytopenia with few giant platelets. Hemoglobin F was normal for age. Platelet aggregation was normal with ristocetin but reduced to 5% to 28% of the control in the presence of adenosine 5′-diphosphate, collagen, or epinephrine. Repeated bone marrow aspirations were unsuccessful (dry tap), and biopsy revealed regional acellularity with significant fibrosis. Trilineage hematopoiesis with marked myeloid hyperplasia with maturation and myeloblastic change of the erythroid series was present. Histochemical staining disclosed a significant increase in reticulin fibers. Myelodysplastic syndrome was ruled out, and cytogenetic analysis of the bone marrow was normal. Abdominal ultrasound was normal at 3 months, but bilateral nephromegaly with increased parenchymal echogenicity and mild splenomegaly were observed toward the end of the first year of life. Imaging studies revealed diffuse osteosclerosis with striated hyperintense medulla and hyperintense cortex, diffuse sclerotic changes of the diploic space of the skull base, and uniformly low intensity of the bone marrow of the pelvis (see supplemental Figure 1 on the Blood website). Four treated patients had no response to increasing doses, up to 30 μg/kg, of granulocyte CSF. The oldest among the nontransplanted patients, patient V-5, who was followed until his death at the age of 3, had no signs of extrahematological involvement. Specifically, his cognitive level and communication skills were age adequate.
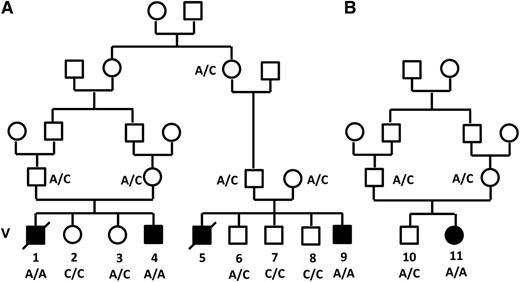
Pedigree of families A and B. Filled symbols denote those affected. The genotype of the c.671 C>A mutation (Thr224Asn) in the VPS45 gene is shown.
Pedigree of families A and B. Filled symbols denote those affected. The genotype of the c.671 C>A mutation (Thr224Asn) in the VPS45 gene is shown.
The disease ran a relentless course, and 2 patients died at 3 months and 3 years, respectively. Two living patients await bone marrow transplantation (BMT), and 1 patient, V-9, underwent BMT from a matched sibling donor at the age of 8 months. Successful engraftment was achieved with full donor chimerism without evidence for graft-versus-host disease; at 3 years of age, blood counts were normal and kidney size returned to normal. Behavioral regression was observed in this patient at 12 months of age, 4 months after BMT, and he was later diagnosed with pervasive developmental disorder. Neurological examination, hearing test, and brain magnetic resonance imaging were normal. No pervasive developmental or any neurological disorders were reported in the other patients.
Molecular investigations performed in patient V-9 revealed no pathogenic mutations in the exons and intronic splice sites of the HAX1, WAS, SBDS, G6PC3, and ELANE genes. The common mutations in JAK2 and BCR/ABL were also excluded.
Of note, the parents and grandparents in families A and B were not aware of any relationship between the 2 families.
Methods
Linkage analysis
Homozygous regions were searched in the DNA samples of patients V-4 and V-9 using the Affymetrix GeneChip Human Mapping 250K Nsp Array (Santa Clara, CA), as previously described in Edvardson et al.8 The homozygous regions in the patients’ samples were identified by inspection. All experiments involving the DNA of the patients, their family members, and anonymous controls were performed after obtaining written informed consent in accordance with the Declaration of Helsinki and were approved by the Ethical Review Boards of Hadassah and the Israeli Ministry of Health.
Whole exome sequencing and bioinformatic analysis
The DNA sample of patient V-9 was enriched with the Agilent Human V.4 51 Mb Exome Capture Kit (Agilent, Santa Clara, CA). Sequencing was carried out on a HiSeq2000 (Illumina, San Diego, CA) as 100-bp paired-end runs. Read alignment and variant calling were performed with the October 2011 release of DNAnexus software (Palo Alto, CA) using the default parameters with the human genome assembly hg19 (GRCh37) as a reference.
Studies in patients’ cells
Cell culture medium consisted of RPMI 1640 (Invitrogen-Gibco, NY) supplemented with l-glutamine and penicillin/streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel). The APOPTEST-FITC Kit containing annexin V–fluorescein isothiocyanate (FITC) was obtained from NeXins Research BV (Hoeven, The Netherlands) or from MBL International (Cambridge, MA). Propidium iodide (PI) was from Molecular Probes (Eugene, OR). DiOC6(3) (3,3′-dihexyloxacarbocyanine iodide) was purchased from Sigma-Aldrich (St. Louis, MO).
Generation of lymphoblast cell lines.
Mononuclear cells were isolated from patient and control donors’ peripheral blood. Cells were then transformed with Epstein-Barr virus (ATCC, VA) for 1 hour, washed and cultured in RPMI 1640, and supplemented with 20% heat-inactivated fetal bovine serum, 2 mM l-glutamine, and 1% penicillin/streptomycin antibiotics. Cells were then monitored daily for viability with trypan blue staining, and media was replaced once a week. Upon reaching a stable cell division rate, cells were passaged 2 to 3 times a week.
Western blot of the VPS45 protein.
Transformed lymphoblasts from patients and healthy controls were lysed using RIPA lysis buffer (150 mM sodium chloride, 50 mM tris(hydroxymethyl)aminomethane, pH 8.0, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, and 1% Triton X-100; Sigma-Aldrich, CA). Total cell lysates were treated with Laemmli sample buffer, boiled to 85°C for 10 minutes, and electrophoresed through a sodium dodecyl sulfate–polyacrylamide gel electrophoresis 12% separating gel. Proteins were then transferred to a nitrocellulose membrane (Millipore, MA). The membrane was blocked using 20% skim milk solution (BD, Franklin Lakes, NJ). Vps45 and actin were traced using rabbit polyclonal anti-Vps45 antiserum (Synaptic Systems, Göttingen, Germany) or mouse monoclonal anti-actin (MP Biomedicals, CA), followed by anti-rabbit or anti-mouse IgG horseradish peroxidase (Promega), respectively. Finally, an enhanced chemiluminescence reaction (Biological Industries, Beit Haemek, Israel) was performed and membranes were exposed to UV irradiation and photographed using an LAS-3000 imaging system (Fuji).
Neutrophil isolation and culturing.
Blood neutrophils were isolated from fresh blood samples obtained from the patients as described in Atallah et al.9 Constitutive apoptosis was induced by resuspending neutrophils with cell culture medium and 10% of autologous plasma. Cells were cultured in 24-well plates at 37°C for 4 hours in a humidified incubator containing 5% carbon dioxide.
Apoptosis assessment in leukocytes.
Cell viability was determined by trypan blue exclusion using a light microscope at varying times after incubation. Programmed cell death of neutrophils, monocytes, and lymphocytes was determined in 2 different ways as described by Edvardson et al.8 Phosphatidylserine exposure was determined by measurement of annexin V–FITC binding, and cells were also stained with PI for detection of necrotic cells.8 Mitochondrial permeability transition was measured as another method for assessment of early apoptosis, based on the ability of intact mitochondria to take up and retain cationic lipophilic fluorescent dyes. To this end, neutrophils were resuspended with 0.3 mL RPMI and loaded with 1.75 nM DiOC6 for 15 minutes at 37°C. Cells were then transferred to 2°C to 8°C, stained with PI for 10 minutes, and analyzed by flow cytometry.
Statistical analysis.
Flow cytometry results were presented as average ± standard deviation. The Student t test was used, and differences were considered statistically significant for P < .05.
Apoptosis assessment in bone marrow.
Formalin-fixed paraffin-embedded bone marrow sections were immunostained for activated caspase-3 (Cell Signaling) using an automated immunostaining system (BenchMark-Ultra, Ventana).
Immunofluorescence analysis.
Sections were deparaffinized and rehydrated by consecutive washes of xylene, ethanol (100% vol/vol, 95% vol/vol, respectively), and water. For antigen unmasking, slides were boiled in 10 mM sodium citrate buffer, pH 6.0, maintained at a subboiling temperature for 10 minutes and cooled at room temperature for 30 minutes. Sections were washed with water and incubated with blocking solution (phosphate-buffered saline, 0.1% Tween 20, 5% normal goat serum) for 1 hour at room temperature. Blocking solution was replaced by primary antibodies (mouse anti-CD177) (clone MEM-166; Abcam, Cambridge, MA) and rabbit anticleaved caspase-3 (Asp1775, clone 5A1E; Cell Signaling Technology, Danvers, MA) diluted 1:200 in SignalStain antibody diluent (Cell Signaling Technology) and incubated overnight at 4°C in a humidified chamber. Antibody solution was removed, and sections were washed with phosphate-buffered saline/0.1% Tween 20. Sections were incubated for 1 hour at room temperature in secondary antibodies (chicken anti-rabbit Alexa 488, chicken anti-mouse Alexa 495) diluted 1:1000 in blocking solution. Sections were washed in wash buffer and covered with DAPI (4,6-diamidino-2-phenylindole)–containing mounting medium (Vectashield, Vector Labs, Burlingame, CA) and coverslips. Digital pictures were captured using an Axiovert 200M microscope (×630 magnification) and Axiovision software 4.8.1 (Zeiss, Jena, Germany).
Presence of lysosomes in cultured skin fibroblasts.
Fibroblasts cultured from a skin biopsy of patient V-11 (obtained with informed consent during central line insertion) were maintained in Dulbecco’s modified Eagle medium (Biological Industries, Beit Haemek, Israel) containing 4.5 g glucose per liter and supplemented with glutamine and 15% fetal calf serum at 37°C, 5% carbon dioxide. Two days before the experiments, cells were seeded on glass-bottomed, 35-mm tissue culture plates. The following day, the medium was replaced with the regular medium or with starvation medium as above but with 1% fetal calf serum for 24 hours. Subsequently, the fibroblasts were incubated with 50 nM LysoTracker Red DND-99 (Molecular Probes, Eugene, OR) for 30 minutes, and thereafter the medium was replaced with or without 200 nM MitoTracker Green FM (Molecular Probes, Eugene, OR) for 20 minutes and visualized live by fluorescent confocal microscopy.
Electron microscopy of platelets.
Blood samples were drawn in tubes with citrate as anticoagulant. Platelet-rich plasma was prepared by centrifugation at 100 × g for 10 minutes at room temperature. The platelet-rich plasma was fixed with 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 2.5 hours, and the platelets were stored in 1% paraformaldehyde in phosphate buffer. Subsequently, the samples were treated with osmium tetroxide, dehydrated in a graded series of ethanol concentrations, and embedded in Epon-Araldite. Sections were stained with uranyl acetate and lead citrate and viewed with a Philips 300 transmission electron microscope.
The effect of the mutation in yeast
Site-directed mutagenesis was used to mutate the 238th codon of yeast VPS45 in the expression construct pCOG07010 to create plasmid pMC007 (encoding Vps45 harboring the THR238ASN mutation, Vps45T238N). Yeast strains used were SEY621011 (containing endogenous Vps45) and a congenic vps45Δ (lacking endogenous Vps45) strain that was constructed by using pNOzY13 to disrupt VPS45, as described in Bryant and James.12
Cellular levels of wild-type Vps45 and Vps45T238N expressed from pCOG070 and pMC007, and endogenous Tlg2 in the same cells, were quantified by densitometry using ImageJ software (National Institutes of Health) in relation to Pgk1 levels (which were used as a loading control) using immunoblot analysis as described in Shanks et al.13
Results
Identification of Thr224Asn mutation in the VPS45 gene
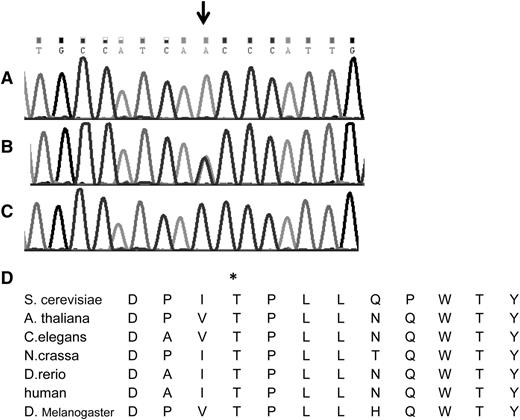
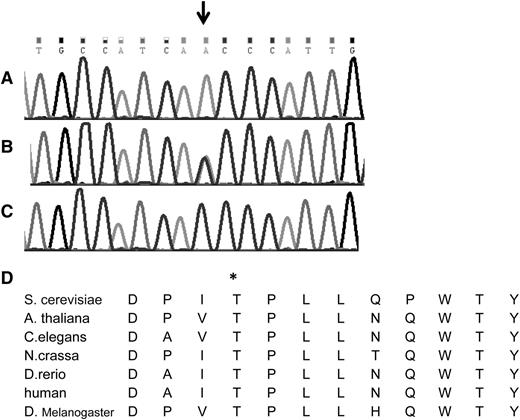
The search for shared homozygous regions in the DNA samples of the remotely related patients V-4 and V-9 disclosed only 1 homozygous region larger than 2 Mb, chr1:145243316-158702189 (numbering according to hg19). This region contained 319 protein coding genes, and after HAX1 was excluded by Sanger sequencing, none of them appeared as an immediate candidate. We therefore opted for whole exome sequencing of patient V-9, which was done at an average depth of ×56. This analysis detected a total of 3178 single nucleotide variants and small insertions/deletions within the coding sequence. We removed those covered less than ×7, those present in dbSNP132 or in the in-house database, and heterozygous and synonymous variants. Sixty-one changes survived this filtering process, but only 2 of them resided in the shared homozygous region: chr1:145556666 A>T corresponding to Asp79Val in the ANKRD35 gene and chr1:150049841 C>A corresponding to c.671 C>A, p.Thr224Asn in the VPS45 gene. Using the pathogenicity prediction software Mutation Taster,14 the ANKRD35 Asp79Val was deemed benign, whereas the VPS45 Thr224Asn mutation was predicted to be pathogenic. VPS45 consists of 15 exons, encoding the 570-residue-long human VPS45 protein. Its transcript is most abundant in peripheral blood mononuclear cells and neutrophils.15 By Sanger sequencing of exon 7, we genotyped the other 2 patients whose DNA were available, V-1 and V-11, and identified homozygosity for the mutation in both of them. The mutation segregated with the disease in the families (Figures 1 and 2A-C). The mutated codon is highly conserved throughout evolution (Figure 2D), and the mutation was not found in 120 anonymous individuals of Palestinian origin, nor was it identified in the exome analyses of 6503 healthy individuals available through the Exome Variant Server (NHLBI GO Exome Sequencing Project, Seattle, WA; http://evs.gs.washington.edu/EVS/).
The Thr224Asn mutation in the VPS45 gene. Shown is the DNA sequence of part of exon 7 of the human VPS45 gene in (A) a patient, (B) an obligate heterozygote, and (C) a healthy control. The mutation is shown by the arrow. (D) The conservation of the human Thr224 residue (asterisk) throughout evolution is shown.
The Thr224Asn mutation in the VPS45 gene. Shown is the DNA sequence of part of exon 7 of the human VPS45 gene in (A) a patient, (B) an obligate heterozygote, and (C) a healthy control. The mutation is shown by the arrow. (D) The conservation of the human Thr224 residue (asterisk) throughout evolution is shown.
Functional characterization of the mutation in yeast
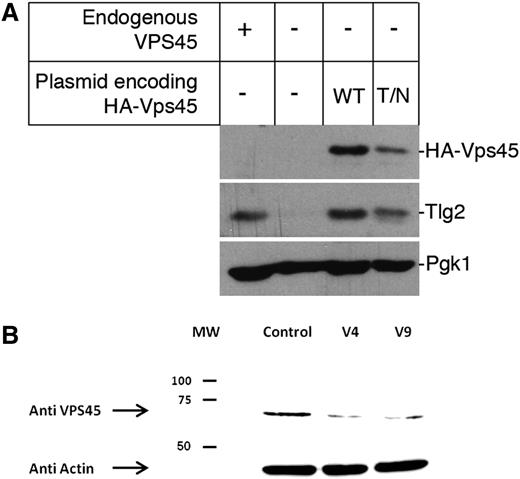
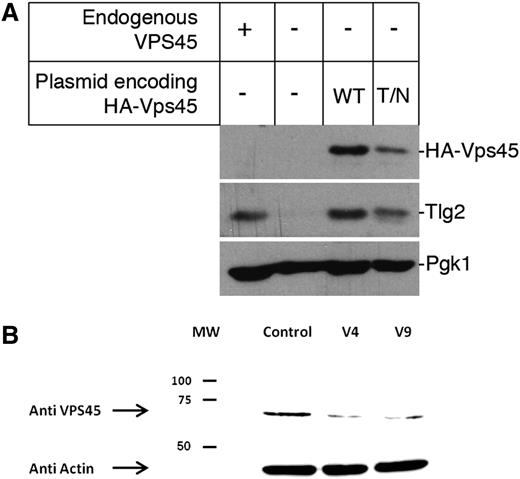
Vps45 function is best characterized in the yeast Saccharomyces cerevisiae, where it regulates the entry of syntaxin Tlg2 into the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes required for traffic through the endosomal system.12 Loss of Vps45 function in yeast results in multiple phenotypes including reduced cellular levels of Tlg2.13 To investigate the effect of the patients’ mutation on Vps45 function, we introduced the analogous mutation, Thr238Asn, into the yeast protein and determined the cellular level of Tlg2. This analysis revealed that yeast cells expressing Vps45T238N from a plasmid in place of endogenous Vps45 have reduced cellular levels of Tlg2 by 44.5 ± 9.4% (n = 3) compared with cells expressing wild-type Vps45 under the same conditions (Figure 3A). It is important to note that although the 2 plasmids encoding the wild-type and the Thr238Asn mutant version of Vps45 are identical apart from the mutation of codon 238 (ACA→AAT), cells harboring the latter have reduced levels of Vps45 by 44.4 ± 7.3% (n = 3). Thus, the data presented in Figure 3A indicate that the Thr238Asn mutation destabilizes the Vps45 protein and thereby abrogates its function in the endosomal pathway.
Decreased cellular levels of Vps45. (A) Mutation Thr238Asn in yeast Vps45 (equivalent to Thr224Asn in the human protein) results in lower cellular levels of Vps45 and Tlg2. Western blot analysis was used to assess cellular levels of Tlg2 in cells containing (+) or lacking (−) endogenous VPS45. In addition, levels of HA-tagged versions of either wild-type (WT) Vps45 or Vps45T238N (T/N) and Tlg2 were assessed in cells lacking endogenous VPS45 but harboring plasmids pCOG070 (encoding WT Vps45) or pMC007 (encoding Vps45T238N). Levels of Pgk1 were assessed as a loading control in all cases. A representative blot of 3 independent experiments is shown. (B) Western blot of the Vps45 protein is shown. Vps45 was detected by rabbit polyclonal anti-VPS45 antiserum and anti-rabbit IgG horseradish peroxidase followed by an enhanced chemiluminescence reaction. Actin, as a housekeeping gene, is shown at the bottom. Shown are lymphoblasts from 2 patients and from a normal control. MW, molecular weight; HA, human influenza hemagglutinin epitope tag.
Decreased cellular levels of Vps45. (A) Mutation Thr238Asn in yeast Vps45 (equivalent to Thr224Asn in the human protein) results in lower cellular levels of Vps45 and Tlg2. Western blot analysis was used to assess cellular levels of Tlg2 in cells containing (+) or lacking (−) endogenous VPS45. In addition, levels of HA-tagged versions of either wild-type (WT) Vps45 or Vps45T238N (T/N) and Tlg2 were assessed in cells lacking endogenous VPS45 but harboring plasmids pCOG070 (encoding WT Vps45) or pMC007 (encoding Vps45T238N). Levels of Pgk1 were assessed as a loading control in all cases. A representative blot of 3 independent experiments is shown. (B) Western blot of the Vps45 protein is shown. Vps45 was detected by rabbit polyclonal anti-VPS45 antiserum and anti-rabbit IgG horseradish peroxidase followed by an enhanced chemiluminescence reaction. Actin, as a housekeeping gene, is shown at the bottom. Shown are lymphoblasts from 2 patients and from a normal control. MW, molecular weight; HA, human influenza hemagglutinin epitope tag.
Reduced expression of the mutant VPS45 protein in patients’ cells
In order to elucidate the effect of the Thr224Asn mutation on the expression of VPS45 protein, we examined its abundance in lymphoblastic cell lines derived from patients V-4 and V-9. As shown in Figure 3B, VPS45 expression was severely reduced in lymphocytes from the patients.
Lack of lysosomes in patients’ fibroblasts
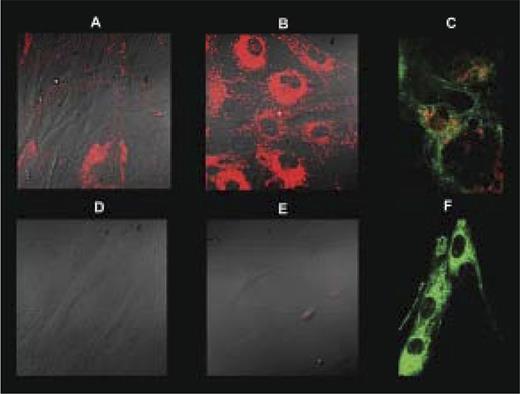
Given that mutations in VPS45 are associated with perturbed trafficking through the endosomal system, we examined the lysosomal compartment in the patients’ fibroblasts. In contrast to control fibroblasts grown in regular medium, LysoTracker stain was absent in a patient’s cells. Upon starvation, an increase in acidic organelle content was clearly observed in the control cells while barely visible in the patients’ cells. In contrast, mitochondrial content visualized by MitoTracker Green was unaffected (Figure 4A-F).
Depletion of lysosomes in fibroblasts. Control (A-C) and patient V-11 (D-F) fibroblasts were grown in regular medium (A,D) or starvation medium (B-C,E-F) and stained with LysoTracker (red) with (C,F) or without (A-B,D-E) subsequent MitoTracker (green) stain. Cells were visualized live by fluorescent confocal microscopy without (C,F) or with differential interference contrast (A-B,D-E) at ×400 magnification.
Depletion of lysosomes in fibroblasts. Control (A-C) and patient V-11 (D-F) fibroblasts were grown in regular medium (A,D) or starvation medium (B-C,E-F) and stained with LysoTracker (red) with (C,F) or without (A-B,D-E) subsequent MitoTracker (green) stain. Cells were visualized live by fluorescent confocal microscopy without (C,F) or with differential interference contrast (A-B,D-E) at ×400 magnification.
Reduced abundance of α granules in patients’ platelets
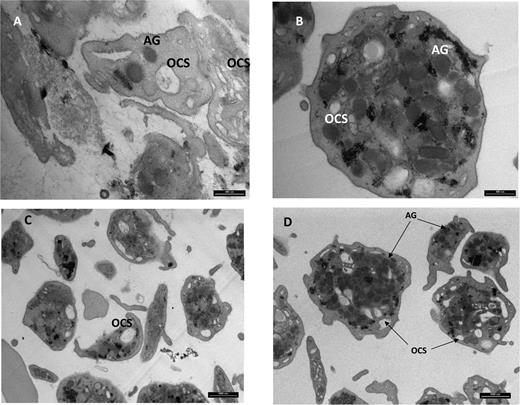
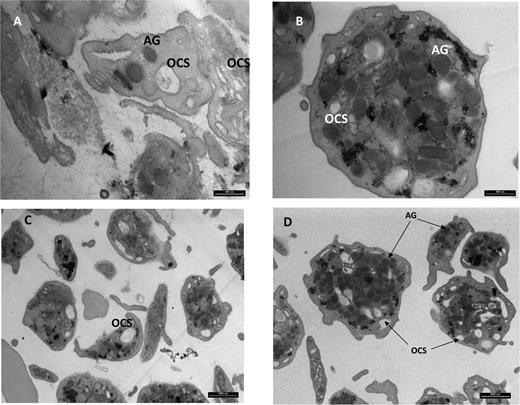
In view of the lysosomes’ depletion in cultured skin fibroblasts, we examined the patients’ platelets for the presence of the lysosome-like α granules. Transmission electron microscopy of a thin section of platelets from the patients disclosed decreased α granule content and a distorted open channel system in comparison with control platelets (Figure 5).
Transmission electron microscopy of platelets. Magnification ×25 000 (A,B) and 8800 (C,D). A and C depict patient platelets and B and D depict control. Shown are decreased alpha-granule (AG) content and distorted open channel system (OCS) in patient platelets when compared to control.
Transmission electron microscopy of platelets. Magnification ×25 000 (A,B) and 8800 (C,D). A and C depict patient platelets and B and D depict control. Shown are decreased alpha-granule (AG) content and distorted open channel system (OCS) in patient platelets when compared to control.
Accelerated apoptosis in patients’ neutrophils and bone marrow myeloid cells
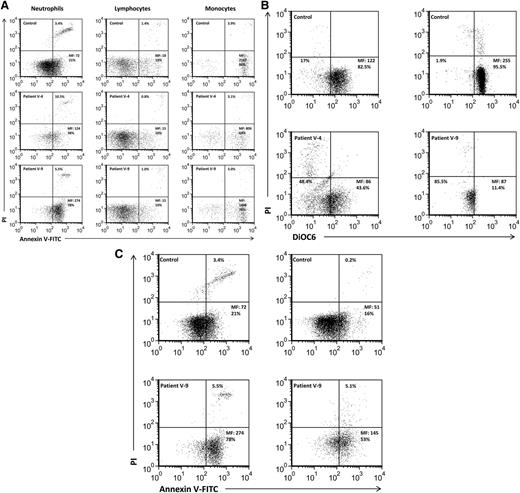
In order to gain insight into the pathomechanism of Vps45 deficiency, leukocyte apoptosis was evaluated using annexin V, PI, and DiOC6 in peripheral blood samples. Bone marrow cells were studied using caspase-3 activation. The results are presented in Table 2 and Figures 6 and 7. Patient V-4 showed median fluorescence (MF) of 160 ± 51 of annexin V–FITC, of which 34 ± 6% were positively gated, whereas the controls’ MF was 68 ± 18 with 21 ± 7% positively gated cells (P < .02, P < .05, respectively, Student t test) (Table 2). In agreement with these results, 21 ± 14% neutrophils from patient V-4 stained with PI as a late apoptotic marker had an MF of 923 ± 992 compared with only 3 ± 1% neutrophils with an MF of 725 ± 480 in the controls (P = nonsignificant for % gated, and P < .04 for MF, Student t test) (Table 2). Neutrophils from patient V-9 demonstrated an MF of 275 ± 16 and 75% of annexin V-FITC–positive early apoptotic cells (P < .0002, P < .0006, vs the controls, respectively, Student t test) (Table 2) and 5 ± 1% gated cells (P < .05) and an MF of 1376 ± 504 (P < .05), for late apoptotic cells. At least 2 samples from each patient were examined using DiOC6 staining with tight correlation to annexin V staining (Figure 6B). When pooled together (Table 2), the results were highly significant for both patients, indicating a higher number of apoptotic neutrophils in patients who are homozygous for the VPS45 mutation.
Accelerated apoptosis in patients’ neutrophils. (A) Freshly isolated neutrophils, monocytes, and lymphocytes were stained for annexin V expression and PI admission. Mean fluorescence (MF) and the percentage of positive cells are indicated. These are representative examples of the samples summarized in Table 2. These are representative examples of the samples summarized in Table 2. (B). Mitochondrial permeability transition evaluation in neutrophils is shown. Neutrophils were resuspended with 0.3 mL RPMI and loaded with 1.75 nM DiOC6 for 15 minutes at 37°C. Cells were then transferred to 2°C to 8°C, stained with PI for 10 minutes, and analyzed by flow cytometry. MF and the percentage of gated cells are shown. The left lower quadrant includes cells that lost mitochondrial potential (apoptotic cells). (C) Accelerated constitutive spontaneous neutrophil apoptosis is shown. Freshly isolated neutrophils were either immediately evaluated (right) or harvested after 4 hours of spontaneous constitutive apoptosis (left). MF is indicated for annexin, and the percentage of PI-positive cells is also indicated.
Accelerated apoptosis in patients’ neutrophils. (A) Freshly isolated neutrophils, monocytes, and lymphocytes were stained for annexin V expression and PI admission. Mean fluorescence (MF) and the percentage of positive cells are indicated. These are representative examples of the samples summarized in Table 2. These are representative examples of the samples summarized in Table 2. (B). Mitochondrial permeability transition evaluation in neutrophils is shown. Neutrophils were resuspended with 0.3 mL RPMI and loaded with 1.75 nM DiOC6 for 15 minutes at 37°C. Cells were then transferred to 2°C to 8°C, stained with PI for 10 minutes, and analyzed by flow cytometry. MF and the percentage of gated cells are shown. The left lower quadrant includes cells that lost mitochondrial potential (apoptotic cells). (C) Accelerated constitutive spontaneous neutrophil apoptosis is shown. Freshly isolated neutrophils were either immediately evaluated (right) or harvested after 4 hours of spontaneous constitutive apoptosis (left). MF is indicated for annexin, and the percentage of PI-positive cells is also indicated.
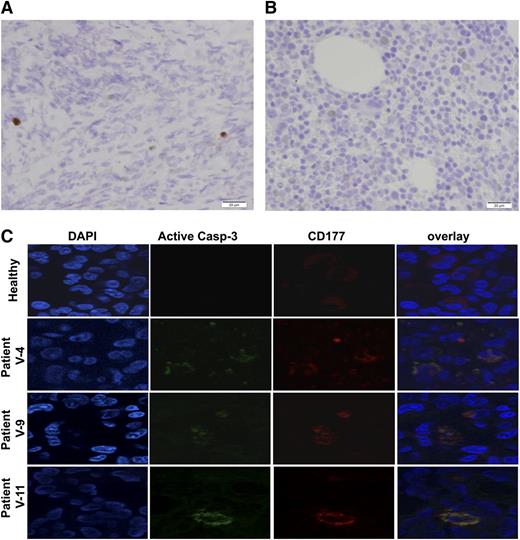
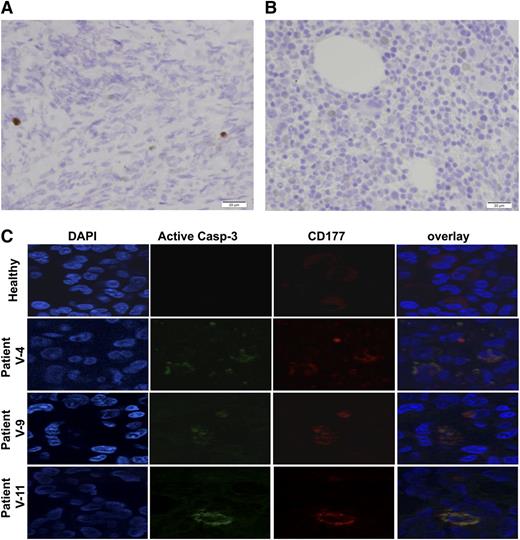
Increased apoptosis in bone marrow. (A) Immunostaining of activated caspase-3 in the bone marrow showed markedly increased apoptosis. The image is representative of 3 patients. (B) Similarly stained normal bone marrow of a patient of the same age is shown. A biopsy was obtained from a patient with Wilms tumor in order to assess the presence of metastases. (C) Increased apoptosis of granulocytes in the bone marrow of patients with mutated VPS45 patients is shown. Bone marrow sections of a healthy person and 3 patients with VPS45 mutations were co-immunostained with specific antibodies against activated caspase-3 (green) and CD177 (red). DNA was counterstained using DAPI.
Increased apoptosis in bone marrow. (A) Immunostaining of activated caspase-3 in the bone marrow showed markedly increased apoptosis. The image is representative of 3 patients. (B) Similarly stained normal bone marrow of a patient of the same age is shown. A biopsy was obtained from a patient with Wilms tumor in order to assess the presence of metastases. (C) Increased apoptosis of granulocytes in the bone marrow of patients with mutated VPS45 patients is shown. Bone marrow sections of a healthy person and 3 patients with VPS45 mutations were co-immunostained with specific antibodies against activated caspase-3 (green) and CD177 (red). DNA was counterstained using DAPI.
We also compared spontaneous constitutive neutrophil apoptosis9 following 4 hours of in vitro incubation, in order to observe apoptosis progression. As shown in Figure 6C (right), the baseline MF of neutrophil annexin V staining was 145 compared with 51 of the control (P < .0001), and 4 hours later, it was 274, whereas in the control it raised only to an MF of 72 (P < .004) (Figure 6B). Taken together, the 2 patients showed accelerated neutrophil apoptosis and accelerated constitutive neutrophil apoptosis, indicating that accelerated apoptosis underlies the severe neutropenia observed in these patients. This abnormality was confined to neutrophils, as only slightly increased apoptosis was seen in monocytes and no increased apoptosis was seen in lymphocytes (Table 2 and Figure 6B). In agreement, peripheral blood leukocyte cell numbers were between 1500 and 5200/μL with a nearly normal lymphocyte number between 1200 and 3900/μL and a normal to high monocyte number (300-1000/μL), contrasting with the severely reduced neutrophil number (100-300/μL) in most complete blood cell counts. Cell counts following cell isolation showed a 50 times reduction in neutrophil numbers as compared with the controls but comparable numbers of mononuclear cells.
To assess the presence of apoptosis in the bone marrow, we stained bone marrow sections with an antibody against activated caspase-3. This analysis demonstrated markedly increased apoptosis in the bone marrow (Figure 7A-B). We further characterized bone marrow apoptosis with immunofluorescence staining using DAPI and anti-CD177. The results of this analysis (Figure 7C) confirmed increased apoptotic cells in the bone marrow, mainly of the myeloid line.
Discussion
The patients reported in this study presented in early infancy with transfusion-dependent anemia, life-threatening thrombocytopenia with significant bleeding tendency, and a prolonged, granulocyte CSF–resistant neutropenia that caused serious opportunistic infections and led to the death of 2 of the 5 patients. The peripheral blood smear was largely abnormal with dakryocytes, nucleated red blood cells, and aniso-/poikilocytosis. Rare myelocytes and promyelocytes were present, and some platelets were large or unusually shaped. Bone marrow biopsy revealed patchy hematopoiesis with marked fibrosis. Imaging studies invariably showed osteosclerosis with periosteal reaction and nephromegaly that was attributed to extramedullary hematopoeisis. Within the limited follow-up period, the disease seemed confined to the hematological system and could be cured by BMT. A longer follow-up may be required, as 1 patient had post-BMT behavioral regression whereas all other patients were neurologically intact.
The parental consanguinity, the presence of the disease in more than 1 child in each nuclear family, and the fact that both sexes were equally affected suggested that this form of infantile myelofibrosis was caused by a founder mutation transmitted in an autosomal recessive manner. The now well-accepted combined approach of homozygosity mapping followed by whole exome sequencing resulted in the identification of a homozygous missense mutation, Thr224Asn, in the VPS45 gene. The mutation is most likely pathogenic because this codon is evolutionarily conserved and the mutation segregated with the disease in the families and was absent from a large cohort of controls. Pathogenicity was confirmed by the low content of the Vps45 protein in the patients’ cells and because the same mutation in the S. cerevisiae VPS45 gene resulted in a clear vacuolar protein sorting phenotype, which is equivalent to the impairment of the endocytic-lysosomal pathway in mammalian cells.
Vps45 is a member of the Sec1/Munc18 protein family. Sec1/Munc18 proteins regulate the assembly of specific SNARE complexes. SNARE complex formation plays a key role in the specificity of membrane trafficking, bridging opposing lipid bilayers, bringing them into close proximity, and providing the energy to mediate their fusion. Vps45 is conserved throughout evolution, and by regulating SNARE proteins it is involved in regulating membrane traffic through the endosomal system. Newly synthesized proteins and recycling proteins from the plasma membrane rely on Vps45 for their proper endosomal sorting.16-20 In yeast, loss of Vps45 function leads to the deceleration of cell growth rate, defective endosomal trafficking concomitant with reduced cellular levels of cognate SNARE proteins, increased sensitivity to osmotic stress, and accumulation of transport vesicles.13,21 Similarly, the absence of Vps45 in Drosophila melanogaster blocks the fusion of endocytic vesicles into the endosome, resulting in vesicle accumulation and the absence of later endocytic structures.19 Human Vps45 was previously shown to regulate vesicular trafficking between the trans–Golgi network and early endosomes and the recycling of plasma membrane receptors.20 The absence of recognizable lysosomes in our patients’ fibroblasts attests to the importance of Vps45 in the biogenesis of the endosomal-lysosomal pathway in humans.
The list of lysosomal-related proteins that are associated with congenital neutropenia includes Chédiak-Higashi syndrome due to mutations in the lysosomal trafficking regulator (LYST) gene; Hermansky-Pudlak syndrome type 2 due to mutations in the AP3B1 gene encoding the B1 subunit of the adaptor-related protein complex-3, a heterotetrameric complex involved in protein trafficking to specialized endosomal-lysosomal organelles; Griscelli syndrome type 2 due to mutations in RAB27A; Cohen syndrome, which is associated with mutations in VPS13B, and a rare form of primary immune deficiency due to a mutation in the endosomal adaptor protein p14 (ROBLD3/p14) gene. The exact mechanism underlying the neutropenia in defects of endosomal-lysosomal proteins is unknown.
The accelerated apoptosis observed in our patients’ neutrophils could be the consequence of the perturbed delivery of cargo from the trans–Golgi network to the endosomal system, which in turn would back up the earlier secretory pathway. This may lead to an overwhelming endoplasmic reticulum stress, which would trigger apoptosis in a somehow similar way to the sequence of events in patients with congenital neutropenia due to ELA2 mutations.22,23 Why neutrophils are more vulnerable than monocytes and lymphocytes is not clear, but it may be related to the short life span of neutrophils and the specific pro-apoptotic features of these cells, including the unique lack of bcl-2.24 The large amount of myeloid apoptotic cells in the bone marrow could also be attributed to their defective clearance, reflecting lysosomal dysfunction.27 Accelerated apoptosis was shown to increase transforming growth factor β (TGF-β) secretion,26 and since the TGF-β signaling pathway is a potent negative regulator of proliferation, elevated levels of TGF-β can promote myelofibrosis.27,28 Whether the accelerated apoptosis observed in Vps45 deficiency enhances TGF-β levels, thereby promoting myelofibrosis, is still a matter of conjecture.
The thrombasthenia seen in our patients may also shed light on the disease mechanism in Vps45 deficiency. In platelets, the endosomal-lysosomal pathway is represented by dense core granules, α granules, and lysosomes, which contain a plethora of bioactive molecules including hemostatic, angiogenic, and growth factors as well as proteases, necrotic factors, and cytokines. Some of these molecules are produced by megakaryocytes and packaged into granules during biosynthesis. Other cargoes are thought to be endocytosed by circulating platelets and then transported to α granules. Impaired granule metabolism manifesting by persistent or intermittent thrombocytopenia or thrombasthenia is common in Chédiak-Higashi syndrome and in Hermansky-Pudlak syndrome type 2. In these disorders, there is dysfunctional sorting of dense core granule proteins (ie, serotonin, adenosine 5′-diphosphate, polyphosphates) during biogenesis with subsequent loss of cargo from the granules.29 Gray platelet syndrome due to a defect in an endoplasmic reticulum protein encoded by NBEAL2 is unique because in this disorder there is a loss of cargo from α granules.30 Similar to the situation in our patients, the disease is confined to the hematological system, and all the patients have myelofibrosis. We now observe depletion of α granules in our patients’ platelets, indicating that the Thr224Asn mutation in the VPS45 gene interferes with normal α granule biosynthesis. Thus, the Vps45-related myelofibrosis could result from a failure of the megakaryocytes to retain synthesized TGF-β, platelet-derived growth factor, and fibroblast growth factor, leading to accelerated apoptosis in the patients’ bone marrow.
In summary, we identified the Thr224Asn mutation in VPS45 in patients with congenital neutropenia, thrombasthenia, and myelofibrosis. The precise disease mechanism is currently unclear, but the reduced level of the Vps45 protein is associated with accelerated apoptosis in neutrophils and bone marrow, defective α granule biogenesis in platelets, and the excess of megakaryocyte-derived fibrosing growth factors in bone marrow. This is reflected by early life-threatening bacterial infections with rapid development of myelofibrosis that can be cured by BMT.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Professor Eli Pikarsky for the study of apoptosis in the patients’ bone marrow and to Dr Eduard Berenstein from the Interdepartmental Unit, The Hebrew University for assistance with electron microscopy. The excellent technical assistance of Yael Sharir and Rachel Dahan is acknowledged.
This work was supported in part by a research grant of the joint fund of the Hebrew University and Hadassah Medical Center (P.S.). N.J.B. is a Prize Fellow of the Lister Institute of Preventive Medicine.
Authorship
Contribution: N.S., Y.B., H.S., M.W., and H.G. performed clinical identification of patients and wrote a draft of the clinical part of the manuscript; P.S. performed clinical identification and evaluation of patients, analyzed data, wrote the clinical section of manuscript, and supervised the writing; A. Saada and N.J.B. designed and performed research and data analysis, wrote the research section of manuscript, and supervised the writing; M.C., A.T., A.K.-L., A. Shaag, U.F., A.B., and S.Z. performed research and data analysis; O.E. designed the genetic approach, performed research, analyzed data, wrote the research section of manuscript, and supervised the writing; and D.M. performed research and data analysis, wrote the research section of manuscript, and supervised the writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Orly Elpeleg, Monique and Jacques Roboh Department of Genetic Research, Hadassah, Hebrew University Medical Center, Jerusalem, Israel; e-mail: elpeleg@hadassah.org.il; Nia Bryant, Institute of Molecular, Cell and Systems Biology, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom; e-mail: nia.bryant@glasgow.ac.uk; and Dror Mevorach, Rheumatology Research Center and Department of Medicine, Hadassah, Hebrew University Medical Center, Jerusalem, Israel; e-mail: mevorachd@hadassah.org.il.
References
Author notes
P.S. and A.S. contributed equally to this study.