Abstract
SPIB is an Ets transcription factor that is expressed exclusively in mature B cells, T-cell progenitors, and plasmacytoid dendritic cells. In the present study, we developed a novel mAb against the SPIB protein and characterized its expression in major hematolymphoid neoplasms, including a series of 45 cases of blastic plasmacytoid dendritic cell (BPDC) neoplasms and their potential cutaneous mimics. We found that SPIB is expressed heterogeneously among B- and T-cell lymphoma types. Interestingly, SPIB is expressed in a large proportion of nongerminal center type DLBCLs. In cutaneous neoplasms, SPIB is overexpressed in all BPDC neoplasms, but none of its cutaneous mimics. SPIB remains overexpressed in all cases that lack 1 or 2 of the markers used for BPDC neoplasms (ie, CD4, CD56, TCL1, and CD123). We conclude that SPIB expression can be used as a tool for diagnosing BPDC neoplasms, but it needs to be tested in conjunction with the growing arsenal of markers for human plasmacytoid dendritic cells.
Key Points
SPIB is an Ets transcription factor involved in plasmacytoid dendritic cell differentiation.
SPIB protein expression can be used as a marker for human blastic plasmacytoid dendritic cell neoplasms.
Introduction
SPIB is an Ets family transcription factor that is expressed exclusively in mature B cells, T-cell progenitors, and plasmacytoid dendritic cells.1-3 SPIB is required for full BCR signaling and T cell–dependent Ab responses.4 SPIB is central to the germinal center (GC) reaction because those in SPIB-deficient mice are smaller, persist for less time after immunization, and contain more apoptotic cells than those of wild-type animals.4 During B-cell maturation, it is first induced in the pre-B- to immature B-cell transition,5 overexpressed during the GC reaction,3 and repressed by PRDM1/Blimp1 once plasmacytic differentiation has been initiated.6 Interestingly, SPIB has also been shown to target Blimp1 directly, providing a regulatory loop essential for the maintenance of GC and memory B cells, thereby preventing the initiation of the plasma cell program of differentiation.7
Under neoplastic conditions, the SPIB locus has been found to be affected by chromosomal imbalances comprising gains/amplifications and translocations in activated B-cell (ABC)–type diffuse large B-cell lymphoma (DLBCL) cell lines and clinical samples.8,9 In fact, SPIB is one of the components of the Wright algorithm that classifies DLBCLs into the GC and ABC types according to their expression profile.10 In addition, knock-down of SPIB by RNAi is toxic to ABC DLBCL cell lines, but not to other DLBCL subtypes or myeloma, suggesting a role for this putative oncogene in the pathogenesis of ABC-type DLCBL.8,11
Together with its role during B-cell differentiation, SPIB has been shown to be a key regulator of human plasmacytoid dendritic cell development1,12 and a component of the signature of blastic plasmacytoid dendritic cell (BPDC) neoplasms,13 which were formerly known as blastic natural killer neoplasms.14 It is also up-regulated at the gene-expression level when BPDC neoplasms are compared with myelomonocytic leukemia, a common diagnostic problem in cutaneous hematolymphoid infiltrates.13
The aim of the present study was to characterize the immunohistochemical expression of the SPIB protein using a novel mAb in major hematolymphoid neoplasms. A specific set of 45 BPDC neoplasms was also evaluated with regard to the differential diagnosis with other mimics in clinical diagnostic samples.
Methods
For a description of SPIB mAb production and validation, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Western blot analysis
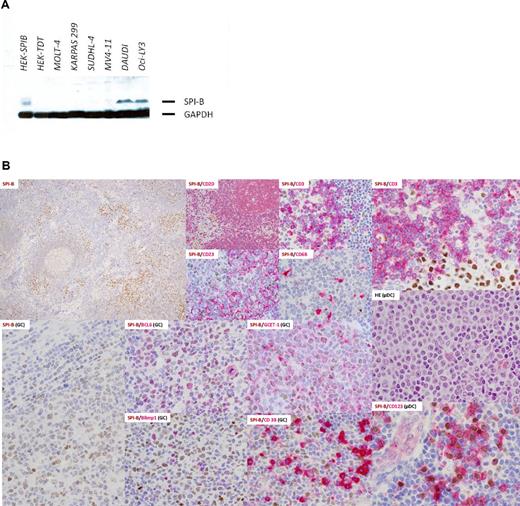
Western blot was performed with selected human cell lines. As shown in Figure 1A, SPIB (clone 235 D/C7) was overexpressed by Western blot in the Burkitt lymphoma and ABC DLBCL cell lines DAUDI and OCILY-3, respectively. Details of total protein extraction and blot visualization can be found in supplemental Methods.
Immunohistochemical expression in FFPE samples and image capture
SPIB immunohistochemical staining was performed following conventional automated methods. All H&E and immunohistochemistry slides were examined under an Olympus Bx41 microscope. Protein expression was evaluated in all cases by visual (semiquantitative) methods (see details in supplemental Methods).
The study protocol and sampling procedure were approved by the Carlos III Institutional Review Board. Informed consent was obtained when necessary in accordance with the Declaration of Helsinki.
Results and discussion
SPIB protein expression in normal hematolymphoid tissues
SPIB was found to be expressed by 2 hematolymphoid cell subsets, mature B cells in the pre-plasma cell stage of maturation and plasmacytoid dendritic cells. No expression was found in mature T-cell subsets, post-GC B cells, mature plasma cells, or other BM-derived cell lineages (Figure 1B). SPIB mAb positively stains the nuclei of the cells. The intensity of these normal GC B cells is used as the threshold for scoring the neoplastic cases (Figure 2E). Cases without any signal were considered negative, those with an intensity below the normal GC B-cell blast were considered weakly positive, and those with an intensity equal or higher to the GC B-cell blast were considered moderately or intensely positive, respectively.
SPIB protein expression in selected human cell lines and normal hematolymphoid populations. (A) Western blot in selected human cell lines revealed overexpression of SPIB (clone 235 D/C7) in the Burkitt lymphoma and ABC-DLBCL cell lines DAUDI and OCILY-3, respectively. None of the other cell lines studied, including the GC-type DLBCL, T-cell lymphoma, or monocytic leukemia cell lines, showed positivity with SPIB mAb. (B) SPIB immunohistochemical expression in reactive lymph node hyperplasia. SPIB mAb stained the nuclei of mature B cells and plasmacytoid dendritic cells. The SPIB protein was expressed at low levels in mantle zone B cells and up-regulated in the GC reaction. In the GC, it stains centroblasts and centrocytes (see coexpression with BCL6 and GCET1); however, late centrocytes that enter the plasma cell differentiation program do not express SPIB (see double immunostains with Blimp1 and CD38). Normal plasmacytoid dendritic cells express SPIB intensely (see double immunostains with CD123). The intensity of the staining in this specific subpopulation was higher than that in the mature B-cell compartment. No expression was found in other subpopulations of the myelomonocytic cell lineage (ie, macrophages, follicular dendritic cells, and reticular and interdigitating cells).
SPIB protein expression in selected human cell lines and normal hematolymphoid populations. (A) Western blot in selected human cell lines revealed overexpression of SPIB (clone 235 D/C7) in the Burkitt lymphoma and ABC-DLBCL cell lines DAUDI and OCILY-3, respectively. None of the other cell lines studied, including the GC-type DLBCL, T-cell lymphoma, or monocytic leukemia cell lines, showed positivity with SPIB mAb. (B) SPIB immunohistochemical expression in reactive lymph node hyperplasia. SPIB mAb stained the nuclei of mature B cells and plasmacytoid dendritic cells. The SPIB protein was expressed at low levels in mantle zone B cells and up-regulated in the GC reaction. In the GC, it stains centroblasts and centrocytes (see coexpression with BCL6 and GCET1); however, late centrocytes that enter the plasma cell differentiation program do not express SPIB (see double immunostains with Blimp1 and CD38). Normal plasmacytoid dendritic cells express SPIB intensely (see double immunostains with CD123). The intensity of the staining in this specific subpopulation was higher than that in the mature B-cell compartment. No expression was found in other subpopulations of the myelomonocytic cell lineage (ie, macrophages, follicular dendritic cells, and reticular and interdigitating cells).
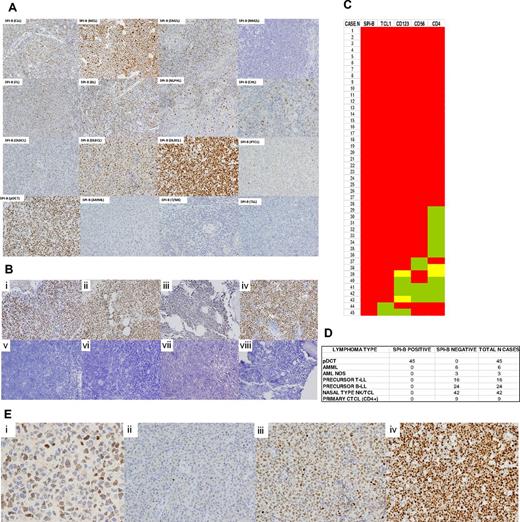
SPIB protein expression in major hematolymphoid neoplasms and human BPDCs. (A) The SPIB protein is overexpressed in BPDC neoplasms and is expressed with variable intensity among B- and T-cell lymphoma types. Cases of BPDC neoplasms show clear-cut nuclear expression of the SPIB protein. However, other cutaneous infiltrates that can mimic this specific tumor type lack SPIB protein expression (a case of acute myelomonocytic leukemia, AMML, is shown for comparison). (B) SPIB is overexpressed in BPDC neoplasms irrespective of the localization of the infiltrate. Cutaneous (i-ii), BM (iii), and lymph node (iv) infiltrates show a similar intensity and distribution of the expression in the neoplastic population. SPIB was found to be negative in all 24 cases of B-cell acute lymphoblastic leukemia tested (v-viii). (C) Heat map showing the distribution of immunohistochemical expression of conventional markers used in the diagnosis of 45 cases of BPDC neoplasms. As depicted, BPDC neoplasms showed significant phenotypic diversity: 29 of 45 (64%) expressed all 4 markers (ie, TCL1, CD123, CD4, and CD56), 11 of 45 (24%) lacked 1 marker (8 cases CD4, 2 cases CD56, 1 case TCL1), 3 of 45 (6%) lacked 2 markers, and 2 of 45 (4%) lacked 3 markers. SPIB continued to be overexpressed in all cases of BPDC neoplasms. Red indicates cases with intense homogeneous expression, yellow indicates cases with partial expression, and green indicates cases that were negative for a given marker. (D) SPIB is overexpressed in all BPDC neoplasms but is negative in all of the tumor types studied (including acute myelomonocytic leukemia, acute myeloid leukemia not otherwise specified, precursor T- and B-lymphoblastic lymphoma/leukemia, nasal type natural killer/T-cell neoplasms, and primary cutaneous T-cell CD4+ lymphoma). (E) SPIB immunohistochemical expression scoring. (i) SPIB mAb positively stains the nuclei of the normal GC B cells. The intensity of these GC B cells is used as the threshold for scoring the neoplastic cases. (ii) DLBCL case with weakly positive SPIB expression. (iii) DLBCL case with moderate intensity SPIB expression. (iv) DLBCL with intense expression of SPIB protein.
SPIB protein expression in major hematolymphoid neoplasms and human BPDCs. (A) The SPIB protein is overexpressed in BPDC neoplasms and is expressed with variable intensity among B- and T-cell lymphoma types. Cases of BPDC neoplasms show clear-cut nuclear expression of the SPIB protein. However, other cutaneous infiltrates that can mimic this specific tumor type lack SPIB protein expression (a case of acute myelomonocytic leukemia, AMML, is shown for comparison). (B) SPIB is overexpressed in BPDC neoplasms irrespective of the localization of the infiltrate. Cutaneous (i-ii), BM (iii), and lymph node (iv) infiltrates show a similar intensity and distribution of the expression in the neoplastic population. SPIB was found to be negative in all 24 cases of B-cell acute lymphoblastic leukemia tested (v-viii). (C) Heat map showing the distribution of immunohistochemical expression of conventional markers used in the diagnosis of 45 cases of BPDC neoplasms. As depicted, BPDC neoplasms showed significant phenotypic diversity: 29 of 45 (64%) expressed all 4 markers (ie, TCL1, CD123, CD4, and CD56), 11 of 45 (24%) lacked 1 marker (8 cases CD4, 2 cases CD56, 1 case TCL1), 3 of 45 (6%) lacked 2 markers, and 2 of 45 (4%) lacked 3 markers. SPIB continued to be overexpressed in all cases of BPDC neoplasms. Red indicates cases with intense homogeneous expression, yellow indicates cases with partial expression, and green indicates cases that were negative for a given marker. (D) SPIB is overexpressed in all BPDC neoplasms but is negative in all of the tumor types studied (including acute myelomonocytic leukemia, acute myeloid leukemia not otherwise specified, precursor T- and B-lymphoblastic lymphoma/leukemia, nasal type natural killer/T-cell neoplasms, and primary cutaneous T-cell CD4+ lymphoma). (E) SPIB immunohistochemical expression scoring. (i) SPIB mAb positively stains the nuclei of the normal GC B cells. The intensity of these GC B cells is used as the threshold for scoring the neoplastic cases. (ii) DLBCL case with weakly positive SPIB expression. (iii) DLBCL case with moderate intensity SPIB expression. (iv) DLBCL with intense expression of SPIB protein.
SPIB protein expression in major mature B- and T-cell lymphoma subtypes
The SPIB protein was expressed heterogeneously among B-cell lymphoma types (Figure 2A and supplemental Figure 1). A detailed study of SPIB protein expression in a series of 100 cases of DLBCL (Figure 2A-E) showed that SPIB was expressed at higher intensity and the percentage of cells in the non–GC-type DLBCL15 (37 of 55 non–GC-type DLBCL cases had moderate/intense expression in > 50% of the neoplastic population vs 21 of 45 GC-type DLBCL cases; χ2 test, P = .038). Fourteen (18%) of the T-cell lymphomas studied showed weak immunohistochemical expression of SPIB.
SPIB protein expression in BPDC neoplasms and their potential mimics
A series of 45 cutaneous BPDC neoplasms, 9 myelomonocytic leukemia/acute myeloid leukemia, not otherwise specified, 40 precursor B- and T-lymphoblastic leukemias/lymphomas, 42 nasal type natural killer/T-cell neoplasms, and 9 primary cutaneous T CD4+ cell lymphomas of small pleomorphic cells were studied with SPIB mAb. Minimal immunophenotypic criteria used for the diagnosis of BPDC neoplasms included coexpression of CD4, CD56, CD123, and TCL1 in the absence of B-, T-, and myeloid/myelomonocytic cell lineage markers.14,16,17
As shown in Figure 2, SPIB was overexpressed in all BPDC neoplasms, but in none of the other cutaneous neoplastic infiltrates that simulate BPDC neoplasms. Interestingly, there was considerable phenotypic diversity among the BPDC neoplasms when a combination of 4 markers (TCL1, CD123, CD56, and CD4) was used. Therefore, 29 of 45 cases (64%) expressed all 4 markers, 11 of 45 (24%) lacked 1 marker (8 cases CD4, 2 cases CD56, and 1 case TCL1), 3 of 45 (6%) lacked 2 markers, and 2 of 45 (4%) lacked 3 markers. Of these 4 markers, CD4 was negative in 12 of 45 cases (26%), CD56 in 5 of 45 (11%), CD123 in 4 of 45 (8%), and TCL1 in 2 of 45 (4%). These results roughly coincide with those from a recently published series of 33 cases of BPDC neoplasms by Cota et al,18 although those investigators did not use the SPIB mAb.
Interestingly, SPIB remains overexpressed in all cases of BPDC neoplasms that lack 1 or 2 of the most commonly used markers of this type of tumor, TCL1 and CD123 (Figure 2C). Furthermore, the SPIB protein is overexpressed to the same extent irrespective of the localization of the infiltrate. Cases with skin, lymph node, and BM involvement by BPDC neoplasms had an equivalent intensity of expression of the SPIB protein (Figure 2C).
We conclude that BPDC neoplasms show significant phenotypic diversity (34%-36% of cases have a defective phenotype) and additional (and specific) markers are required to better identify this rare type of aggressive neoplasm. Clinical features were retrieved for BPDC neoplasms. Most cases presented with systemic involvement with lymph node and/or BM infiltration (21 cases 77 78%). In cases with disease limited to the skin, 5 (18, 52%) cases had multifocal skin involvement (> pT2cN0M0) and 1 single case (3, 7%) had localized skin disease (< pT2N0M0). Median follow-up for 24 cases with available data were 16 months (range, 1-61). Overall survival at the median follow-up was 60%, and progression-free survival was 33%. Overall survival in young patients (< 20 years of age) was 100% in the median time of follow-up, despite a progression rate of 80% (P < .05 by log-rank test compared with the rest of patients). These results in young patients are in agreement with previously published data.19 SPIB protein expression was homogeneously overexpressed in all cases and no relationship was found with clinical features, including outcome.
In conclusion, SPIB expression, in the appropriate clinicopathologic context, can be used as a tool for diagnosing BPDCN, but needs to be tested in conjunction with the growing arsenal of novel markers for human plasmacytoid dendritic cells, such as BDCA-4, IRF-8, BCL11A, and CD2AP.20
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura Cereceda and CNIO's Tumor Bank Unit (M.J. Artiga and Manuel Morente) for their skillful retrieval and handling of clinical data and samples from different clinical institutions and all of the clinical colleagues who kindly submitted cases for centralized review.
This study was supported by grants from the Asociación Española contra el Cancer and the Ministerio de Ciencia e Innovación (SAF 2008-03871), Spain.
Authorship
Contribution: S.M.-M. designed and performed the research, analyzed the data, and wrote the manuscript; R.R.-M. designed and performed the research and analyzed the data; A.M.-L. analyzed the data; C.B.C., S.M.R.P., R.P., A.P.C., S.Q.-T., and L.S.-V. performed the research; G.R. designed the research and contributed new reagents; J.M.-T. contributed new reagents; and M.A.P. designed the research, contributed new reagents, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Santiago Montes Moreno, MD, Pathology Department, Hospital Universitario Marqués de Valdecilla/IFIMAV, Avda Valdecilla 25, 39008 Santander, Spain; e-mail: smontes@humv.es.
References
Author notes
S.M.-M. and R.R.-M. contributed equally to this work.