Key Points
Physiologic O2 levels in vitro enhance the propagation and function of mesenchymal stromal cells from chronic lymphocytic leukemia patients.
Abstract
Chronic lymphocytic leukemia (CLL) cells interact in the marrow with mesenchymal stromal cells (MSCs), which can enhance CLL-cells' resistance to spontaneous or drug-induced apoptosis. Here we examined the effect of oxygen on the growth and function of MSCs from marrow aspirates of CLL patients. Cultures in ambient oxygen provided for poor recovery and growth of MSCs, which developed features of cell-senescence. However, MSCs were propagated readily from the same cells when they were cultured at a physiologic oxygen concentration of 5%. Such MSCs promoted short-term CLL-cell survival in either 5% or ambient O2. However, longer-term CLL-cell survival was enhanced when the cocultures were maintained in 5% O2 versus 21% O2 because of increased MSC proliferation and production of soluble prosurvival factors, such as CXCL12. This study establishes the importance of physiologic oxygen concentration in the propagation and function of MSCs derived from marrow aspirates of CLL patients in vitro.
Introduction
The marrow of chronic lymphocytic leukemia (CLL) patients invariably is infiltrated with leukemia cells to an extent that inversely correlates with clinical stage and prognosis.1 In the marrow, CLL cells come in contact with mesenchymal stromal cells (MSCs)2 and/or nurselike cells (NLCs),3 which can provide survival factors enhancing CLL-cells' resistance to spontaneous or chemotherapy-induced apoptosis.4 The prosurvival factors identified to date cannot fully recapitulate stromal cell support, highlighting the need for continued use of MSC/NLC cocultures to interrogate the influence of the microenvironment on CLL-cell survival in vitro.
The marrow microenvironment in CLL is typically studied using human stromal cell lines or primary MSCs from healthy individuals.5 Some investigators have developed techniques to expand MSCs from bone biopsies of patients with CLL,6-8 but such samples are not commonly available. Less attention has been given to MSCs derived from marrow aspirates of patients with CLL, in part because of the difficulty in obtaining homogeneous preparations of MSCs,9 which typically are present at only minute proportions in the marrow aspirates of CLL patients compared with those of healthy individuals.10-12 This is compounded by the low fraction of MSCs that can form colonies in vitro, estimated at ∼ 15%11 and by the reduction in MSC “fitness” associated with aging, which might predispose MSCs to premature replicative senescence.13
The difficulty in culturing MSCs from marrow aspirates of CLL patients also could be because of the toxic effects of ambient oxygen (21% O2) on MSCs in vitro.14 In the marrow, the cells are exposed to 1% to 9% O2, which is lower than the level encountered in vitro.15 Such low O2 concentrations can impact the biology of various cell types in vitro, especially stem cells, which are sensitive to oxidative stress that can impair self-renewal.15 Here we examined the effect of O2 concentration on MSCs cultured from CLL marrow aspirates.
Methods
Collection of biologic samples from CLL patients and MSC culture
Blood and marrow aspirates were collected from CLL patients at University of California San Diego (UC San Diego) Moores Cancer Center, in compliance with the Declaration of Helsinki16 and UC San Diego institutional review board. Mononuclear cells were isolated by Ficoll-Hypaque gradient (Pharmacia) and used fresh or viably frozen in liquid nitrogen for later use. For MSC generation, marrow mononuclear cells were seeded at 2 × 106 cells/mL in DMEM (Mediatech), containing 10% fetal bovine serum (FBS; Omega Scientific), 10mM HEPES (Gibco/BRL), 100U/mL penicillin and 100μg/mL streptomycin (Gibco/BRL). The cultures were exposed to 21% O2 in a standard incubator or to 5% O2 in a MCO-18M O2/CO2 incubator (Sanyo Scientific), where nitrogen gas injection maintains a set O2 concentration. MSC outgrowth was monitored using a Nikon TE300 microscope (10× objective, 0.3 numeric aperture) and a 5MHz CCD camera (Princeton Instrument) using Metamorph Version 7.7.1.0 software (Molecular Devices). For subculturing, MSCs were detached with trypsin-EDTA (Gemini) and replated at 1000 cells/cm2. Media was renewed weekly. Proliferation was monitored by 5-bromo-2′-deoxy-uridine (BrdU) incorporation by ELISA (Roche), and expression of (SA)–β-galactosidase (SA-β-Gal) was monitored as a marker of cell-senescence (Cell Signaling).17
CLL-cell viability measurement
CLL-cell viability was determined based on the mitochondrial transmembrane potential (ΔΨm) using 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Invitrogen) and membrane permeability to propidium iodide (Sigma-Aldrich), as described.18 We excluded from these analyses any contaminating MSCs (< 5%) by forward and side-angle light-scatter gating.
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for immunophenotyping, CXCL12-secretion measurement, and immunoblotting procedures.
Results and discussion
Effect of O2 concentration on growth of MSCs from marrow aspirates of CLL patients
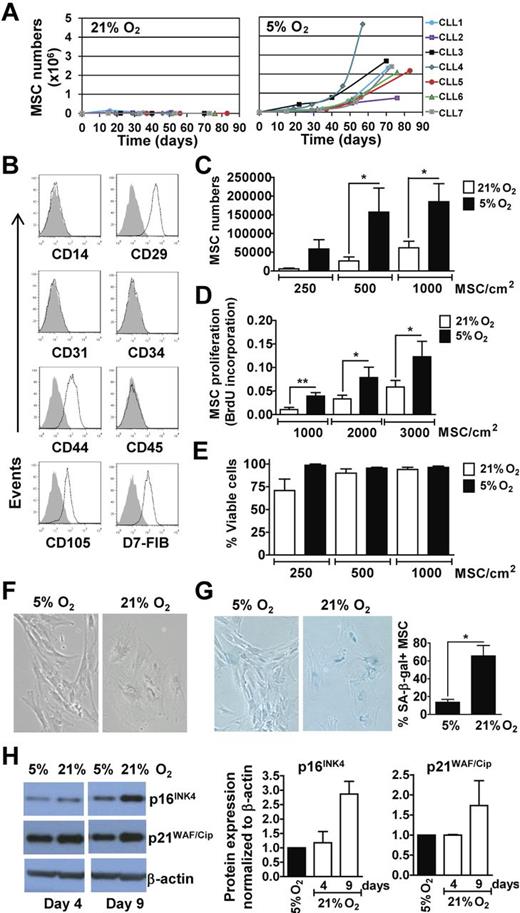
We examined for the in vitro outgrowth of MSCs from the CLL marrow aspirates cultured in ambient oxygen (21% O2) or 5% O2. The latter represents the mid-range concentration of O2 present in the marrow15 and the O2 concentration found optimal for hematopoietic stem-cell growth in vitro.19 The aspirates of 7 different patients were examined who had CLL cells that were either ZAP-70Neg (CLL1, 3, and 5) or ZAP-70Pos (CLL2, 4, 6, and 7). Two of these 7 samples (CLL1 and 2) generated MSC colonies at 21% O2, but these cells did not expand further with subsequent passages (Figure 1A). In contrast, MSC colonies developed from all 7 cultures in 5% O2 and showed a steady propagation over time (Figure 1A). MSCs generated in 5% O2 coexpressed CD29, CD44, CD105, and D7-FIB, but lacked expression of CD14, CD31, CD34, or CD45, as noted for MSCs in prior studies (Figure 1B).11,20 Further testing of marrow aspirates from each patient in a larger cohort (n = 22) indicated that physiologic O2 generally allows for propagation of MSCs from marrow aspirates in vitro, as 19 of 22 samples produced an expandable pool of MSCs at 5% O2.
MSCs From marrow aspirates of CLL patients propagate better and resist senescence in vitro when cultured in 5% O2 rather than in ambient O2. (A) Marrow mononuclear cell suspensions from 7 different CLL patients were cultured in DMEM-10%FBS, at 5% CO2 in 21% O2 or 5% O2 at 37°C. MSCs outgrowth and expansion was monitored over time by microscopy and viability determined via Trypan blue exclusion. From the 7 samples tested, only 2 (CLL1 and CLL2) generated MSC colonies at 21% O2. The expansion curves of MSCs in 21% O2 are depicted on the left and those generated in 5% O2 are depicted on the right. (B) MSCs generated in 5% O2 were examined for expression of selected surface antigens by flow cytometry. The phenotypic characterization of MSCs from a representative patient sample is shown. The shaded histogram is the fluorescence of MSCs stained with an isotype control antibody, whereas the open histogram depicts the fluorescence of MSCs stained with fluorochrome-conjugated antibody specific for the antigen listed below each histogram. (C-E) MSCs generated from marrow aspirates of CLL patients in 5% O2 (between passage 2 and 5) were plated at various seeding densities, as indicated at the bottom of the histograms, in parallel cultures that subsequently were placed in 21% O2 or 5% O2. The black bars provide the data of cultures exposed to 5% O2 and the open bars depict the data from MSCs cultured in 21% O2. (C) Provides the mean number of viable MSCs obtained after 20 days in each of 4 independent experiments, performed using MSCs from the marrow aspirates of each of 3 different patients (mean ± SEM). (D) Provides the BrdU incorporation of MSCs after 6 days (n = 3; mean ± SEM) as indicated on the y-axis representing the absorbance at 450 to 690 nm. (E) Provides the viability of the MSCs at each condition in (C) based on Trypan blue exclusion (mean ± SEM). Statistically significant differences for each panel are indicated by the asterisk (*P < .05; Student t test). (F-H) MSCs generated in 5% O2 were plated in parallel cultures at 300 cells/cm2 and exposed to either 21% O2 or 5% O2. (F) Provides representative pictures of MSCs cultured in 5% or 21% O2 after 9 days (100× magnification). (G) Provides representative photomicrographs of MSCs cultured in 5% or 21% O2 for 16 days after staining for SA-β-Gal, an indicator of senescent MSCs (left panels) and the fraction of SA-β-Gal+ stained cells over the total cell number counted in 5 microscopic fields per O2 concentration (n = 3; mean ± SD; far right panel; *P < .05). (H) Provides the immunoblot of lysates prepared from MSCs cultured in 5% or 21% O2, as indicated at the top of each lane. The blots were probed with antibodies specific for p16INK4 (top row), p21WAF/Cip (middle row), or β-actin (bottom row), which was used to control for the amount of protein added. The histograms (right) provide densitometry analyses on immunoblots for p16INK4 and p21WAF/Cip in lysates of MSCs grown in 5% O2 (black bars) or 21% O2 (open bars) for 4 and 9 days. p21WAF/Cip and p16INK4 band intensities were normalized to β-actin levels, and are expressed relative to the level of each protein found in 5% O2 at the same time point. Error bars indicate ± SD of the mean.
MSCs From marrow aspirates of CLL patients propagate better and resist senescence in vitro when cultured in 5% O2 rather than in ambient O2. (A) Marrow mononuclear cell suspensions from 7 different CLL patients were cultured in DMEM-10%FBS, at 5% CO2 in 21% O2 or 5% O2 at 37°C. MSCs outgrowth and expansion was monitored over time by microscopy and viability determined via Trypan blue exclusion. From the 7 samples tested, only 2 (CLL1 and CLL2) generated MSC colonies at 21% O2. The expansion curves of MSCs in 21% O2 are depicted on the left and those generated in 5% O2 are depicted on the right. (B) MSCs generated in 5% O2 were examined for expression of selected surface antigens by flow cytometry. The phenotypic characterization of MSCs from a representative patient sample is shown. The shaded histogram is the fluorescence of MSCs stained with an isotype control antibody, whereas the open histogram depicts the fluorescence of MSCs stained with fluorochrome-conjugated antibody specific for the antigen listed below each histogram. (C-E) MSCs generated from marrow aspirates of CLL patients in 5% O2 (between passage 2 and 5) were plated at various seeding densities, as indicated at the bottom of the histograms, in parallel cultures that subsequently were placed in 21% O2 or 5% O2. The black bars provide the data of cultures exposed to 5% O2 and the open bars depict the data from MSCs cultured in 21% O2. (C) Provides the mean number of viable MSCs obtained after 20 days in each of 4 independent experiments, performed using MSCs from the marrow aspirates of each of 3 different patients (mean ± SEM). (D) Provides the BrdU incorporation of MSCs after 6 days (n = 3; mean ± SEM) as indicated on the y-axis representing the absorbance at 450 to 690 nm. (E) Provides the viability of the MSCs at each condition in (C) based on Trypan blue exclusion (mean ± SEM). Statistically significant differences for each panel are indicated by the asterisk (*P < .05; Student t test). (F-H) MSCs generated in 5% O2 were plated in parallel cultures at 300 cells/cm2 and exposed to either 21% O2 or 5% O2. (F) Provides representative pictures of MSCs cultured in 5% or 21% O2 after 9 days (100× magnification). (G) Provides representative photomicrographs of MSCs cultured in 5% or 21% O2 for 16 days after staining for SA-β-Gal, an indicator of senescent MSCs (left panels) and the fraction of SA-β-Gal+ stained cells over the total cell number counted in 5 microscopic fields per O2 concentration (n = 3; mean ± SD; far right panel; *P < .05). (H) Provides the immunoblot of lysates prepared from MSCs cultured in 5% or 21% O2, as indicated at the top of each lane. The blots were probed with antibodies specific for p16INK4 (top row), p21WAF/Cip (middle row), or β-actin (bottom row), which was used to control for the amount of protein added. The histograms (right) provide densitometry analyses on immunoblots for p16INK4 and p21WAF/Cip in lysates of MSCs grown in 5% O2 (black bars) or 21% O2 (open bars) for 4 and 9 days. p21WAF/Cip and p16INK4 band intensities were normalized to β-actin levels, and are expressed relative to the level of each protein found in 5% O2 at the same time point. Error bars indicate ± SD of the mean.
Atmospheric O2 concentration induces MSC senescence
We generated MSCs in 5% O2 and then divided the cells into 2 cultures maintained in either 21% O2 or 5% O2. MSCs cultured in 21% O2 had significantly lower rates of growth (Figure 1C) and DNA synthesis (Figure 1D) than MSCs cultured in 5% O2, but maintained similar levels of viability in short-term culture (Figure 1E). Moreover, MSCs cultured in 21% O2 displayed the wide-spread cytoplasm and enlarged nucleus typically noted for senescent cells, which contrasted with the elongated morphology of MSCs cultured in 5% O2 (Figure 1F). Most MSCs cultured in 21% O2 stained positively for SA-β-Gal, a feature of cell senescence.17 However, such staining was rarely observed for MSCs cultured in 5% O2 (Figure 1G). Finally, MSCs cultured in 21% O2 expressed higher levels of p16INK4 and p21WAF/Cip, features associated with cell-cycle arrest and senescence (Figure 1H).21,22 These results indicate that atmospheric O2 can induce MSC senescence, which could compromise the capacity of MSCs to form colonies in vitro.
MSCs generated in 5% O2 provide survival support to CLL cells
We noted that the MSCs generated in 5% O2 were competent in producing soluble prosurvival factors, as MSC-conditioned media (CM) enhanced the survival of CLL cells in a dose-dependent fashion in either 5% or 21% O2 (Figure 2A). We generated CM from cultures of MSCs in 21% O2 or 5% O2 and examined each CM for its ability to support CLL-cell survival. We observed that the CM from MSCs cultured in 21% O2 were less potent in promoting CLL-cell survival than the CM of MSCs cultured in 5% O2 (Figure 2B), suggesting that MSCs cultured in ambient oxygen may produce fewer prosurvival factors over time than MSCs cultured in 5% O2. Consistent with this notion, we found that the CM produced by MSCs in 5% O2 had significantly higher concentrations of CXCL12 than did CM of MSCs cultured in ambient oxygen (Figure 2C left). Moreover, the secretion rate of CXCL12 per MSC was higher for MSCs cultured in 5% than in 21% O2 (Figure 2C right). On the other hand, CLL cells cultured in 5% O2 had the same expression levels of CXCR4 than did CLL cells cultured in 21% O2 (Figure 2D).
MSCs can provide survival support to CLL cells in vitro. (A) CLL cells were cultured in the presence of increasing concentrations of MSC conditioned media (CM), which was prepared in 5% O2, and exposed to either 21% or 5% O2 for 3 days, at which point the CLL cells were collected for viability measurements by flow cytometry. Data from 4 different CLL patients are presented (mean ± SEM), which were normalized to day 0. The initial absolute viability of all samples at day 0 was of 66% ± 6% (mean ± SEM). Asterisks indicate significant difference between CM conditions and media control values in 5% O2 and 21% O2 (1-way ANOVA and Tukey posthoc test; ***P < .001, **P < .01, *P < .05). (B) CLL cells were cultured for 3 days in the presence of increasing amounts of MSC CM, which was prepared by incubating MSCs either in 5% O2 or 21% O2 for 6 days. CLL-cell viability was assessed by flow cytometry. Data on cells obtained from each of 3 different CLL patients are presented (mean ± SEM), which were normalized to day 0. The initial absolute viability of all samples at day 0 was of 87% ± 2% (mean ± SEM). Asterisks indicate significant difference measured by 1-way ANOVA and Tukey posthoc test (**P < .01, *P < .05). (C) The presence of CXCL12 was quantified in MSC CM prepared by incubating MSCs either in 5% O2 or 21% O2 for 6 days by ELISA. Left: CXCL12 concentration in the CM; right: CXCL12 secretion rate per 1 × 105 MSCs was estimated by dividing CXCL12 concentration in the CM by the number of MSCs collected at the end of the 6-day culture period. The data presented were obtained from MSCs derived from each of 2 patients, each tested in 2 independent experiments (mean ± SEM). Asterisks indicate significant difference measured by Student t test. (*P < .05). (D) CXCR4 expression levels on CLL cells (n = 6) cultured for 24 hours in 5% or 21% O2 were assessed by flow cytometry, which was gated on CD19PosCD5Pos cells. CXCR4 expression is presented as the absolute mean fluorescence intensity (MFI), which was the MFI of CD19+CD5+ cells stained for CXCR4 minus the MFI of the same cells stained with an isotype control antibody. Student t test was used to determine statistical significance. (E-F) MSCs generated in 5% O2 were plated in parallel cultures at 1000 cells/cm2 and subsequently placed in 5% or 21% O2 for 2 to 3 days before the addition of CLL cells (1 × 106 cells/mL). CLL cells from 4 different patients (3 ZAP-70Neg, 1 ZAP-70Pos) were seeded in duplicate either alone, directly on MSCs (E), or separated from the MSCs by transwell (TW) porous membrane (0.4 μm; F). CLL-cell viability was assessed after 0, 3, 6, 10, or 14 days and normalized to that of the cells on day 0 (mean ± SEM; n = 4). The initial absolute viability of all samples at day 0 was of 74% ± 5% (mean ± SEM). In panel E an asterisk indicates the significant difference between the percentage live CLL cells on MSCs in 21% O2 and 5% O2 (Student t test; P < .05). In panel F asterisks (*) or (**) indicate a P < .05 or P < .01, respectively (Student t test) for the differences between CLL cells cultured alone or with MSCs. (G) CLL cells from 4 different patients (3 ZAP-70Neg and 1 ZAP-70Pos) were cocultured with MSCs that were derived from 2 different ZAP-70Neg patients (bottom) or 2 ZAP-70Pos CLL patients (top) for 14 days, at which point the cells were collected for viability assessment by flow cytometry. The viability data presented have been normalized to day 0 (mean ± SEM). The initial absolute viability of all samples at day 0 was 74% ± 6% for the 4 samples plated on MSCs from ZAP-70Pos patients, and of 73% ± 10% for the 4 samples plated on MSCs from ZAP-70Neg patients (mean ± SEM). Asterisks indicate significant difference measured by Student t test. (*P < .05; **P < .01).
MSCs can provide survival support to CLL cells in vitro. (A) CLL cells were cultured in the presence of increasing concentrations of MSC conditioned media (CM), which was prepared in 5% O2, and exposed to either 21% or 5% O2 for 3 days, at which point the CLL cells were collected for viability measurements by flow cytometry. Data from 4 different CLL patients are presented (mean ± SEM), which were normalized to day 0. The initial absolute viability of all samples at day 0 was of 66% ± 6% (mean ± SEM). Asterisks indicate significant difference between CM conditions and media control values in 5% O2 and 21% O2 (1-way ANOVA and Tukey posthoc test; ***P < .001, **P < .01, *P < .05). (B) CLL cells were cultured for 3 days in the presence of increasing amounts of MSC CM, which was prepared by incubating MSCs either in 5% O2 or 21% O2 for 6 days. CLL-cell viability was assessed by flow cytometry. Data on cells obtained from each of 3 different CLL patients are presented (mean ± SEM), which were normalized to day 0. The initial absolute viability of all samples at day 0 was of 87% ± 2% (mean ± SEM). Asterisks indicate significant difference measured by 1-way ANOVA and Tukey posthoc test (**P < .01, *P < .05). (C) The presence of CXCL12 was quantified in MSC CM prepared by incubating MSCs either in 5% O2 or 21% O2 for 6 days by ELISA. Left: CXCL12 concentration in the CM; right: CXCL12 secretion rate per 1 × 105 MSCs was estimated by dividing CXCL12 concentration in the CM by the number of MSCs collected at the end of the 6-day culture period. The data presented were obtained from MSCs derived from each of 2 patients, each tested in 2 independent experiments (mean ± SEM). Asterisks indicate significant difference measured by Student t test. (*P < .05). (D) CXCR4 expression levels on CLL cells (n = 6) cultured for 24 hours in 5% or 21% O2 were assessed by flow cytometry, which was gated on CD19PosCD5Pos cells. CXCR4 expression is presented as the absolute mean fluorescence intensity (MFI), which was the MFI of CD19+CD5+ cells stained for CXCR4 minus the MFI of the same cells stained with an isotype control antibody. Student t test was used to determine statistical significance. (E-F) MSCs generated in 5% O2 were plated in parallel cultures at 1000 cells/cm2 and subsequently placed in 5% or 21% O2 for 2 to 3 days before the addition of CLL cells (1 × 106 cells/mL). CLL cells from 4 different patients (3 ZAP-70Neg, 1 ZAP-70Pos) were seeded in duplicate either alone, directly on MSCs (E), or separated from the MSCs by transwell (TW) porous membrane (0.4 μm; F). CLL-cell viability was assessed after 0, 3, 6, 10, or 14 days and normalized to that of the cells on day 0 (mean ± SEM; n = 4). The initial absolute viability of all samples at day 0 was of 74% ± 5% (mean ± SEM). In panel E an asterisk indicates the significant difference between the percentage live CLL cells on MSCs in 21% O2 and 5% O2 (Student t test; P < .05). In panel F asterisks (*) or (**) indicate a P < .05 or P < .01, respectively (Student t test) for the differences between CLL cells cultured alone or with MSCs. (G) CLL cells from 4 different patients (3 ZAP-70Neg and 1 ZAP-70Pos) were cocultured with MSCs that were derived from 2 different ZAP-70Neg patients (bottom) or 2 ZAP-70Pos CLL patients (top) for 14 days, at which point the cells were collected for viability assessment by flow cytometry. The viability data presented have been normalized to day 0 (mean ± SEM). The initial absolute viability of all samples at day 0 was 74% ± 6% for the 4 samples plated on MSCs from ZAP-70Pos patients, and of 73% ± 10% for the 4 samples plated on MSCs from ZAP-70Neg patients (mean ± SEM). Asterisks indicate significant difference measured by Student t test. (*P < .05; **P < .01).
CLL cells cocultured with MSCs in either 5% or 21% O2 had enhanced viability relative to the same CLL cells cultured alone (Figure 2E, n = 4). However, CLL-cell viability in such cocultures was higher at later time points (≥ 6 days) in cultures maintained in 5% O2 relative to those maintained in 21% O2. This also was apparent for CLL cells cultured with MSCs on different sides of transwell membranes, as the cultures in 5% O2 provided a significantly longer survival support to CLL cells than those in 21% O2 (Figure 2F). We did not observe any differences in the ability of MSCs derived from marrow aspirates of patients with ZAP-70Neg or ZAP-70Pos CLL to support CLL-cell survival in vitro (Figure 2G). Furthermore, the supportive functions of these MSCs were comparably altered by modulating the O2 concentrations, as CLL cells from either subgroup had significantly higher long-term viability when cocultured with MSCs in 5% O2 than in 21% O2 (Figure 2G). These observations suggest that MSCs generated in 5% O2 can support CLL cell survival in both 5% and 21% O2, but long-term viability of CLL cells cocultured with MSCs is enhanced in 5% O2, probably because of reduced induction of MSC senescence, increased MSC proliferation, and production of soluble prosurvival factors at this lower O2 concentration.
Overall, our results indicate that maintaining physiologic O2 concentrations is a key parameter for successful growth of MSCs from marrow aspirates of patients with CLL in vitro. Such MSCs can enhance the resistance to spontaneous apoptosis of CLL cells, especially when maintained in 5% O2, suggesting for the first time the importance of a physiologic O2 concentration for the function of such accessory cells generated from the marrow mononuclear cells of patients with CLL. Attention to maintaining culture condition in physiologic O2 should facilitate the generation of MSCs that could further assist studies on the role these cells play in the pathophysiology of CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andrew Abriol Santos Ang for his excellent technical assistance.
This work was supported by National Institutes of Health grant for the CLL Research Consortium (P01-CA081534) to T.J.K., by the Blood Cancer Research Fund and Le Fond de la Recherche en Santé du Québec to J.-F.F.
National Institutes of Health
Authorship
Contribution: J.-F.F. designed and performed research, analyzed data, and wrote the paper; D.M. designed research, analyzed data and revised the paper; S.Z., B.C., and L.C. performed research, contributed to scientific discussion, data interpretation, and paper revision; and T.J.K. supervised the study, designed research, provided patient samples, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.M. is Inception Sciences, San Diego, CA.
Correspondence: Thomas J. Kipps, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Dr, Rm 4307, La Jolla, CA 92093-0820; e-mail: tkipps@ucsd.edu.