Key Points
RhoA-induced actin polymerization promotes nuclear accumulation of MKL1 and transcriptional activation.
Thrombopoietin activates nuclear accumulation of MKL1 and transcriptional activation in primary megakarocytes.
Abstract
How components of the cytoskeleton regulate complex cellular responses is fundamental to understanding cellular function. Megakaryoblast leukemia 1 (MKL1), an activator of serum response factor (SRF) transcriptional activity, promotes muscle, neuron, and megakaryocyte differentiation. In muscle cells, where MKL1 subcellular localization is one mechanism by which cells control SRF activity, MKL1 translocation from the cytoplasm to the nucleus in response to actin polymerization is critical for its function as a transcriptional regulator. MKL1 localization is cell-type specific; it is predominantly cytoplasmic in unstimulated fibroblasts and some muscle cell types and is constitutively nuclear in neuronal cells. In the present study, we report that in megakaryocytes, subcellular localization and regulation of MKL1 is dependent on RhoA activity and actin organization. Induction of megakaryocytic differentiation of human erythroleukemia cells by 12-O-tetradecanoylphorbol-13-acetate and primary megakaryocytes by thrombopoietin promotes MKL1 nuclear localization. This MKL1 localization is blocked by drugs inhibiting RhoA activity or actin polymerization. We also show that nuclear-localized MKL1 activates the transcription of SRF target genes. This report broadens our knowledge of the molecular mechanisms regulating megakaryocyte differentiation.
Introduction
Although megakaryoblastic leukemia 1 (MKL1, also known as MRTF-A, MAL, or BSAC) plays a role in normal megakaryocytopoiesis,1-3 much of what is known about this transcriptional coactivator of serum response factor (SRF) has been defined in fibroblasts and muscle cells. MKL1 promotes muscle-specific gene expression, maintains mammary myoepithelial cell differentiation, and contributes to myocardial infarction–induced fibrosis and myofibroblast activation.4-7 Other members of the MKL1 family include MKL2 and Myocardin. All 3 genes have been implicated in muscle cell differentiation, but have different patterns of cellular and developmental expression, which likely explains some of the differences in their knockout (KO) phenotypes. Although Mkl2- and Myocardin-KO mice are embryonic lethal with severe cardiac abnormalities, Mkl1-KO mice are viable with a less severe phenotype. Female Mkl1-KO mice have premature mammary gland involution that prevents lactation.6,8 In addition, Mkl1-KO mice have impaired megakaryocytopoiesis defined by increased numbers of megakaryocytes in the BM, decreased ploidy of BM megakaryocytes, and low peripheral blood platelet counts.1,3
In fibroblast cell lines, MKL1 activity is regulated posttranslationally by its subcellular localization, which is dependent on the actin cytoskeleton.9-11 When MKL1 is bound to monomeric (G)–actin via its N-terminal RPEL domains, it is predominantly localized in the cytoplasm. In unstimulated fibroblasts, MKL1 protein cycles between the cytoplasm and the nucleus but is primarily found in the cytoplasm because of a higher efficiency of nuclear export compared with nuclear import. MKL1 binding to G-actin promotes its nuclear export and prevents the necessary nuclear import molecules from having access to its nuclear localization signals.12 Upon actin polymerization to form filamentous actin (F-actin), available stores of G-actin are depleted, promoting MKL1 nuclear accumulation. Once in the nucleus, MKL1 binds and activates SRF, which is localized to serum response elements on the promoters of genes, many of which are highly expressed in muscle cells.
Serum stimulation of NIH3T3 cells results in RhoA activation and subsequent actin polymerization, leading to MKL1 nuclear accumulation.11 Other environmental stimuli that promote activation of the Rho-GTPase superfamily have also been shown to affect MKL1 subcellular localization. However, the subcellular localization of MKL1 is not always regulated by RhoA-driven actin polymerization. In some cell types, MKL1 resides primarily in the nucleus, similar to the constitutive nuclear localization of Myocardin in cardiac muscle cells.13 In primary rat aortic smooth muscle cells, MKL1 is nuclear even under serum-starved and RhoA-inhibitory conditions, but disruption of the actin cytoskeleton prevents MKL1 nuclear localization.7 MKL1 is also constitutively nuclear in rat cortical and hippocampal neurons.14
In the present study, we investigated how the subcellular localization of MKL1 is regulated during megakaryocyte differentiation. We show that 12-O-tetradecanoylphorbol-13-acetate (TPA)–induced megakaryocytic differentiation of human erythroleukemia (HEL) cells results in MKL1 translocation from the cytoplasm to the nucleus that is dependent on both RhoA activation and actin polymerization. In addition, we show that MKL1 nuclear localization in primary murine megakaryocytes is induced by murine thrombopoietin (mTPO) and demonstrate that the nuclear localization of MKL1 induced by TPA and mTPO in HEL cells and primary murine megakaryocytes, respectively, is correlated with target gene activation.
Methods
Cell line culture
HEL cells were grown in RPMI 1640 medium (Life Technologies) supplemented with 10% heat-inactivated FBS (Gemini), l-glutamine (Life Technologies), and penicillin/streptomycin (Life Technologies). Serum-starved conditions had a final concentration of 0.2% FBS. HEL cl.5 (HEL-iMKL1) cells have doxycycline-inducible expression of murine MKL1, as described previously.3 These cells were maintained in 5 μg/mL of blasticidin (Life Technologies) and 375 μg/mL of hygromycin B (Life Technologies). For actin- and Rho-manipulation assays, HEL-iMKL1 and primary murine megakaryocytes were treated with 0.5μM latrunculin B, 2 μM cytochalasin D, 0.5 μM jasplakinolide (Millipore), 1 unit/mL of Rho activator (Cytoskeleton), or 4 μg/mL of Rho inhibitor (Cytoskeleton). To inhibit MKL1, HEL-iMKL1 cells and primary murine megakaryocytes were treated with 10μM CCG-1423 (Cayman Chemical) as described previously.15 HEL-iMKL1 cell differentiation was induced with 15nM TPA (Sigma-Aldrich).
Primary cell sorting, culture, and transduction
All procedures were performed in compliance with relevant laws and institutional guidelines and were approved by the Yale University Institutional Animal Care and Use Committee. Cells from C57Bl6J mice were used for primary cell experiments. BM was enriched for stem/progenitor cells by lineage depletion using immunomagnetic separation (BD Biosciences) and then stained with PE-biotin lineage cell detection cocktail (Miltenyi Biotec), allophycocyanin-H7 CD117/c-kit (BD Biosciences), Alexa Fluor 647 Sca-1 (BioLegend), PE-Cy5 CD150 (BioLegend), PE-Cy7 CD105 (BioLegend), and FITC CD41 (BD Biosciences). PreMegE (Lin−CD117+CD41−CD150+CD105−) were sorted (MoFlo; Beckman-Coulter) as described previously.16 PreMegE cells were cultured overnight in growth medium Stemspan Serum Free Expansion Media (StemCell Technologies), 30% BIT 9500 (StemCell Technologies), l-glutamine, penicillin/streptomycin, Gentamicin Reagent Solution (Life Technologies), 100 ng/mL of murine SCF (mSCF), 50 ng/mL of murine Fms-like tyrosine kinase 3 (mFlt3) ligand, 20 ng/mL of murine IL-3 (mIL-3), and 50 ng/mL of mTPO (all from PeproTech). Cells in growth medium were transduced with virus by spinfection using 8 μg/mL of polybrene (American Bioanalytical). For making virus, 3 × 107 human HEK293 cells expressing the Moloney murine leukemia virus Gag and Pol (293GP cells) maintained in DMEM high-glucose medium (Life Technologies) with 10% FBS and l-glutamine were transfected using 54 μL of Lipofectamine 2000 Reagent (Life Technologies) with 40 μg of MSCV2.2- or MIGR1-based plasmid and 14 μg of VSVG packaging vector. Viral supernatant was collected every 12 hours for 2-5 days after transfection and concentrated using Amicon Ultra Centrifugal Filter Units with a 10 000 kDa molecular weight cutoff (Millipore).
Time-lapse video microscopy
PreMegE cells were sorted, cultured in growth medium overnight, transduced the next day by spinfection, and resuspended in fresh growth medium. After 1 day, cells were placed in megakaryocyte differentiation medium (Stemspan Serum Free Expansion Media, 30% BIT 9500, l-glutamine, and 20 ng/mL of mTPO). After 1 day, cells were plated onto human fibronectin (BD Biosciences)–coated 35-mm glass-bottom dishes (MatTeK) in the absence of cytokines and allowed to adhere overnight. Live-cell imaging was performed using a Vivaview system (Olympus) with a 20× objective. Differential interference contrast and green fluorescence images were taken every 5 minutes for 1 day with an Orca-R2 camera (Hamamatsu). Imaging data were analyzed and compiled using Metamorph for Vivaview Version 7.7.5.0 software (Olympus).
Immunofluorescence
HEL-iMKL1 cells were grown on fibronectin-coated coverslips, fixed with formaldehyde, permeabilized with 0.1% Triton X-100 (United States Biochemical), blocked in 5% donkey serum, and incubated with anti–murine MKL1 (Abcam) followed by FITC-conjugated anti–rabbit Ab (Life Technologies). Texas-Red phalloidin (Life Technologies) and Vectashield mounting medium containing DAPI (Vector Laboratories) were used to visualize F-actin and nuclei, respectively. Slides were examined with an Olympus BX51 microscope equipped with a SensicamQE CCD camera (Cooke) using IPLab software (BD Biosciences).
Cell fractionation, immunoprecipitation, and immunoblotting
For cell fractionation, HEL-iMKL1 cells were resuspended in lysis buffer (150mM NaCl, 0.1% Triton X-100, 50mM HEPES, pH 7.4, 2mM MgAc2, 10% glycerol, 1mM DTT, and 0.1% NP40) and centrifuged at 1300g for 10 minutes at 6°C. Supernatant was saved as the cytoplasmic fraction. The pellet (nuclear fraction) was resuspended in fresh lysis buffer and sonicated. Lysate was incubated with anti-SRF (Santa Cruz Biotechnology), anti-MKL1 (Abcam), or rabbit IgG (Santa Cruz Biotechnology) conjugated to protein A beads (Santa Cruz Biotechnology). All samples were diluted with Laemmli sample buffer (Bio-Rad). Anti-TATA Binding Protein (Abcam) and anti-GAPDH (Sigma-Aldrich) were used to determine nuclear and cytoplasmic purity, respectively.
GTPase-activation assays
HEL-iMKL1 cells (1 × 106/mL) were cultured overnight under serum-starved conditions, TPA was added for the indicated times, and the culture plates were placed on ice. Nonadherent cells were collected and pelleted at 300g for 10 minutes at 6°C. MLB lysis buffer (Millipore) was added directly to the remaining adherent cells, the adherent cell lysate was added to the pellet of nonadherent cells, and complete lysis was confirmed by repeat pipetting. Lysate was cleared by centrifugation at 14 000g for 5 minutes at 6°C. GTPase activity was assayed according to Millipore RhoA, Rac1, and Cdc42 Activation Assay Kit protocols. RhoA activity over time was visualized by fluorescence resonance energy transfer (FRET), as described previously.17 Briefly, a RhoA biosensor FRET probe18 was transduced into HEL-iMKL1 cells and transduced cells were sorted based on yellow fluorescent protein expression. After cells were serum starved overnight on a 35-mm glass-bottom plate, RhoA activity was visualized using a Leica SP5 confocal microscope at 37°C with 5% O2. Image analysis was performed using ImageJ Version 1.46 software, as described previously.18 After shading was corrected and the background was subtracted, each CFP and FRET image was multiplied with a binary threshold–based mask to eliminate noise outside of the cell. The FRET ratio image was generated by dividing the raw FRET image by the CFP image.
Results
Megakaryocyte differentiation of HEL cells induces MKL1 nuclear accumulation
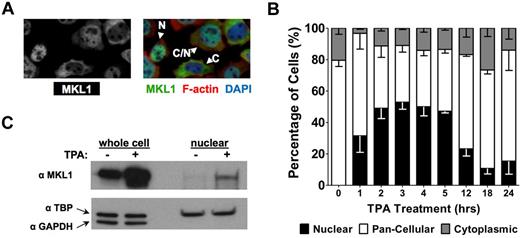
HEL cells, originally derived from an acute myeloid leukemia of the M6 subtype, differentiate down the megakaryocytic lineage when cultured with TPA. The MKL1/SRF pathway plays a critical role in the megakaryocyte maturation of HEL cells.3 To assess the subcellular location of MKL1 during megakaryocytopoiesis, the HEL-iMKL1 subclone, which expresses murine MKL1 in response to doxycycline, was used.3 Cells were treated with TPA and then stained for MKL1 and polymerized actin (Figure 1A). In the absence of TPA and under low-serum conditions, there were no cells with MKL1 exclusively in the nucleus; MKL1 was found in both the cytoplasm and the nucleus (pan-cellular), with approximately 20% of cells showing a strong cytoplasmic predominance (Figure 1B). Treatment of HEL-iMKL1 cells with TPA promoted MKL1 nuclear accumulation as quickly as 1 hour after treatment, peaking at 3 hours. Subcellular fractionation of HEL-iMKL1 cells revealed an increase in MKL1 in the nuclear fraction after 3 hours of TPA treatment, which supported the immunofluorescence observations (Figure 1C). Therefore, MKL1 nuclear localization in HEL-iMKL1 cells is initiated early in TPA-induced megakaryocyte differentiation.
Megakaryocyte differentiation of HEL cells promotes MKL1 nuclear accumulation. (A) Immunofluorescence images of MKL1 localization in HEL-iMKL1 cells. On the left is MKL staining alone; right, overlay with DAPI and phalloidin staining. N indicates nuclear; C/N, pancellular; and C, cytoplasmic. (B) Doxycycline-treated HEL-iMKL1 cells were serum starved overnight and treated with TPA for the indicated times. The percentage of cells with exclusively nuclear localization (black bars) increases during the first 3 hours after TPA treatment and then declines. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. (C) Western blot analysis of HEL-iMKL1 cells after treatment with TPA for 3 hours. Cells were fractionated into nuclear and cytoplasmic compartments and cell lysates were probed for MKL1, TATA-binding protein (TPB; nuclear), and GAPDH (cytoplasmic) expression. Note that the nuclear fraction lacks cytoplasmic contamination.
Megakaryocyte differentiation of HEL cells promotes MKL1 nuclear accumulation. (A) Immunofluorescence images of MKL1 localization in HEL-iMKL1 cells. On the left is MKL staining alone; right, overlay with DAPI and phalloidin staining. N indicates nuclear; C/N, pancellular; and C, cytoplasmic. (B) Doxycycline-treated HEL-iMKL1 cells were serum starved overnight and treated with TPA for the indicated times. The percentage of cells with exclusively nuclear localization (black bars) increases during the first 3 hours after TPA treatment and then declines. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. (C) Western blot analysis of HEL-iMKL1 cells after treatment with TPA for 3 hours. Cells were fractionated into nuclear and cytoplasmic compartments and cell lysates were probed for MKL1, TATA-binding protein (TPB; nuclear), and GAPDH (cytoplasmic) expression. Note that the nuclear fraction lacks cytoplasmic contamination.
MKL1 nuclear accumulation in HEL cells depends on actin dynamics and RhoA activity
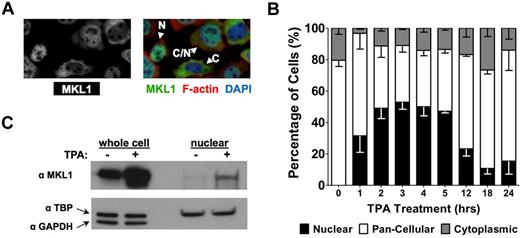
The location of MKL1 in myocytes and fibroblasts has been attributed to cytoskeleton organization and Rho-GTPase activity. To determine whether TPA promotes changes in the actin cytoskeleton, untreated and TPA-treated HEL-iMKL1 cells were stained with phalloidin, which binds to F-actin but not to G-actin. HEL-iMKL1 cells treated with TPA showed a significant increase in F-actin (Figure 2A), with long actin filaments visibly stretching across the cell. To determine whether manipulation of the actin cytoskeleton affects MKL1 localization in megakaryocytic cells, the ratios of G-actin to F-actin were altered using actin-manipulating drugs. The addition of latrunculin B, cytochalasin D, and jasplakinolide to HEL-iMKL1 cells for 1 hour after a 2-hour TPA treatment strongly affected MKL1 localization (Figure 2B-C). In the absence of drugs, 3 hours of TPA treatment resulted in 55% of cells compartmentalizing MKL1 solely in the nucleus (Figure 2Bi). Latrunculin B, which destabilizes F-actin, prevented TPA-induced MKL1 nuclear localization (Figure 2Bii). In contrast, treatment with the F-actin stabilizer jasplakinolide resulted in complete nuclear localization (Figure 2Biv). Although it blocks the addition of G-actin to actin polymers, cytochalasin D induced MKL1 nuclear localization in HEL-iMKL1 cells (Figure 2Biii), as was seen previously in fibroblast models and may be because of depletion of G actin by stabilization of actin dimers.11
Actin polymerization induces MKL1 nuclear accumulation. (A) Untreated (left) and 1-hour TPA-treated (right) HEL-iMKL1 cells were stained for filamentous actin using phalloidin (red) and for DNA using DAPI (blue). Filamentous actin was significantly increased in the large, flattened TPA-treated cells. (B) HEL-iMKL1 cells were treated with TPA for 2 hours and then incubated with the indicated compounds for an additional hour. Left images indicate MKL1 staining; right images overlay MKL1 (green) staining with DAPI (blue) counterstaining. MKL1 accumulated in the nucleus of control (DMSO) cells and cells that had a reduction in monomeric actin (jasplakinolide [iv] and cytochalasin D [iii]), but remained cytoplasmic in cells with increased monomeric actin (latrunculin B [ii]). (C) Graph quantifying the subcellular localization of MKL1 in HEL-iMKL1 cells shown in panel B. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. All images were taken using a 20× objective.
Actin polymerization induces MKL1 nuclear accumulation. (A) Untreated (left) and 1-hour TPA-treated (right) HEL-iMKL1 cells were stained for filamentous actin using phalloidin (red) and for DNA using DAPI (blue). Filamentous actin was significantly increased in the large, flattened TPA-treated cells. (B) HEL-iMKL1 cells were treated with TPA for 2 hours and then incubated with the indicated compounds for an additional hour. Left images indicate MKL1 staining; right images overlay MKL1 (green) staining with DAPI (blue) counterstaining. MKL1 accumulated in the nucleus of control (DMSO) cells and cells that had a reduction in monomeric actin (jasplakinolide [iv] and cytochalasin D [iii]), but remained cytoplasmic in cells with increased monomeric actin (latrunculin B [ii]). (C) Graph quantifying the subcellular localization of MKL1 in HEL-iMKL1 cells shown in panel B. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. All images were taken using a 20× objective.
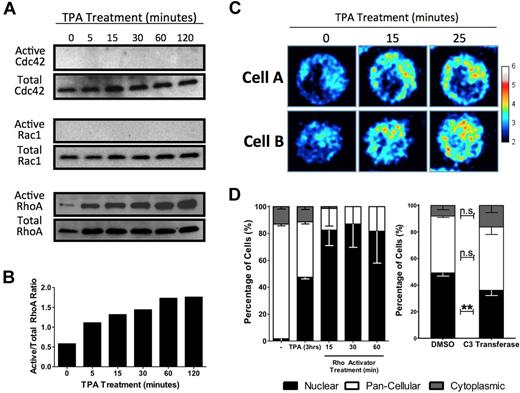
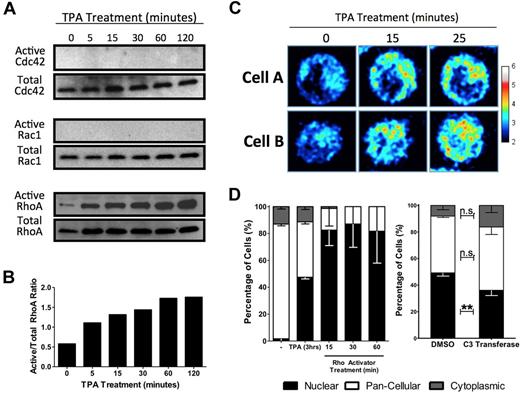
Polymerization of actin is often driven by GTPase activation. The fine, striated actin filaments formed in response to TPA are characteristic of stress fibers, which are associated with activated Rho rather than the Rac or Cdc42 GTPase families. To determine the specific GTPase involved in HEL cell MKL1 nuclear accumulation, activated GTPase pull-down assays were performed. Although RhoA, Rac1, and Cdc42 are all expressed in serum starved HEL cells, only RhoA was activated by TPA. TPA (Figure 3A-B) induced doubling of active RhoA levels within 5 minutes. A RhoA single-chain biosensor was used to confirm RhoA activation by TPA.18 This biosensor reports RhoA activity by emitting a FRET signal when the C-terminal Rho-binding domain binds to GTP-bound RhoA at its N-terminal domain. HEL-iMKL1 cells showed an increase in FRET, indicating elevated RhoA activity, within 15 minutes after treatment with TPA, which is consistent with data from the active RhoA pull-down assay (Figure 3C).
MKL1 localization is dependent on RhoA activation. (A) HEL cells were serum starved overnight and treated with TPA for the indicated times. Cell lysates were assayed for active Cdc42 (top), Rac1 (middle), and RhoA (bottom), as indicated. (B) Densitometric analysis of RhoA activation as a ratio of active RhoA to total RhoA confirmed activation of RhoA in HEL cells by TPA (representative of 3 independent experiments). (C) Time-lapse FRET images of 2 cells expressing a RhoA biosensor revealed an increase in RhoA activity after TPA treatment over the indicated time intervals. The relative RhoA activity scale is shown at right. (D) Serum-starved HEL-iMKL1 cells treated with TPA or calpeptin (a Rho activator) in the absence of TPA for the indicated time periods show that Rho stimulation is sufficient to drive nuclear accumulation of MKL1 (left). Serum-starved HEL-iMKL1 cells were pretreated with DMSO or cell-permeable C3 transferase (a Rho inhibitor) for 4 hours and then treated with TPA for 3 hours (right). Inhibition of RhoA decreased TPA-induced nuclear localization of MKL1. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate the SEM. n.s. indicates not significant. **P < .01.
MKL1 localization is dependent on RhoA activation. (A) HEL cells were serum starved overnight and treated with TPA for the indicated times. Cell lysates were assayed for active Cdc42 (top), Rac1 (middle), and RhoA (bottom), as indicated. (B) Densitometric analysis of RhoA activation as a ratio of active RhoA to total RhoA confirmed activation of RhoA in HEL cells by TPA (representative of 3 independent experiments). (C) Time-lapse FRET images of 2 cells expressing a RhoA biosensor revealed an increase in RhoA activity after TPA treatment over the indicated time intervals. The relative RhoA activity scale is shown at right. (D) Serum-starved HEL-iMKL1 cells treated with TPA or calpeptin (a Rho activator) in the absence of TPA for the indicated time periods show that Rho stimulation is sufficient to drive nuclear accumulation of MKL1 (left). Serum-starved HEL-iMKL1 cells were pretreated with DMSO or cell-permeable C3 transferase (a Rho inhibitor) for 4 hours and then treated with TPA for 3 hours (right). Inhibition of RhoA decreased TPA-induced nuclear localization of MKL1. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate the SEM. n.s. indicates not significant. **P < .01.
To test directly the role of RhoA in accumulation of MKL1 in the nucleus, HEL-iMKL1 cells were treated with drugs that activate or inhibit Rho. Treatment of HEL-iMKL1 cells with calpeptin, a Rho activator, resulted in nuclear accumulation comparable to a 3-hour exposure to TPA (Figure 3D left). HEL-iMKL1 cells pretreated with the cell-permeable Rho inhibitor C3 transferase and then treated with TPA for 3 hours showed a statistically significant (P < .01) decrease in MKL1 nuclear accumulation in response to TPA (Figure 3D right). These results confirm the involvement of RhoA signaling and actin polymerization in MKL1 nuclear localization in response to TPA treatment in HEL-iMKL1 cells.
MKL1 nuclear localization in HEL cells results in activation of MKL1-dependent genes
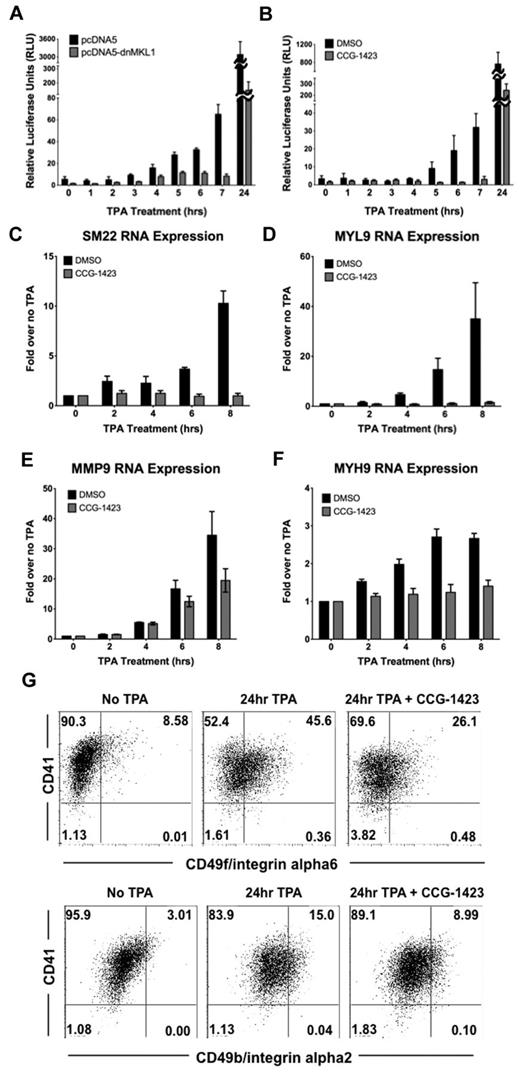
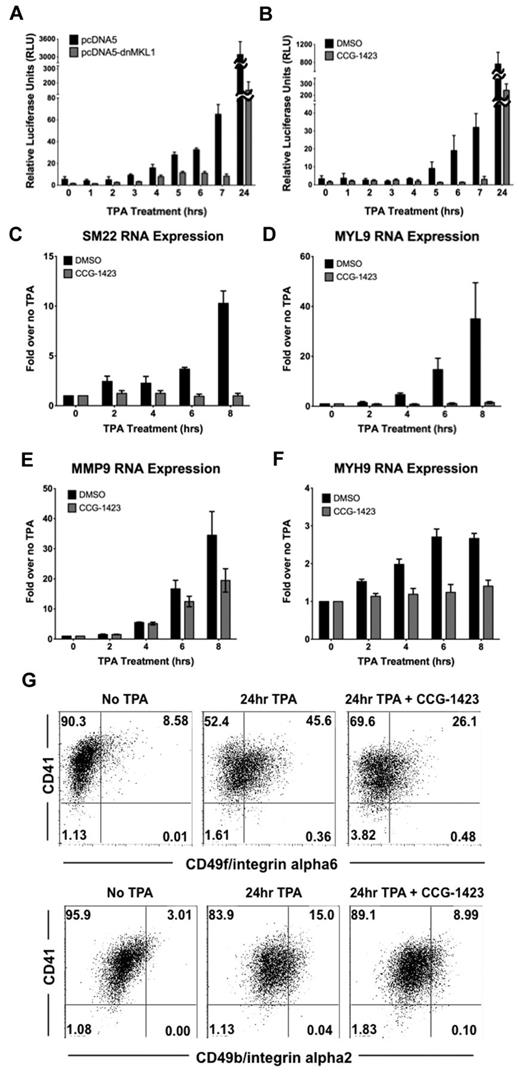
To assess the physiologic relevance of nuclear-localized MKL1, we investigated whether nuclear MKL1 binds to SRF and promotes transcriptional activation of an SRF-dependent promoter. Immunoprecipitation of SRF in untreated and TPA-treated samples showed that MKL1 complexes with SRF in the nucleus; however, there was no detectable increase in MKL1 binding to SRF after 3 hours of exposure to TPA (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similarly, immunoprecipitation of MKL1 did not show an increase in SRF binding after TPA treatment, but association between the 2 proteins was detected. In contrast, movement of MKL1 to the nucleus was correlated with an increase in MKL1 transcription–dependent activity as determined by luciferase assays and endogenous target gene up-regulation. Human smooth muscle 22 (SM22 or human transgelin) is one of the most highly increased genes in response to MKL1 overexpression in TPA-treated HEL cells.3 HEL-iMKL1 cells were transfected with a SM22 promoter firefly luciferase (SM22-luc) construct along with a Renilla control plasmid. Cells were then treated with TPA for different times and luciferase activity was measured. TPA treatment induced activation of the SM22 promoter in a pattern similar to MKL1 nuclear localization with an expected delay (approximately 4 hours) to account for luciferase transcription and translation; luciferase activity began to increase approximately 4 hours after TPA administration and increased over time (Figure 4A). This TPA-driven activation was inhibited when cells were cotransfected with a dominant-negative MKL1 (dnMKL1) construct that specifically blocks the transcriptional activity of MKL1, thus validating the idea that TPA-induced luciferase activation requires nuclear MKL1. Treatment of HEL-iMKL1 cells with CCG-1423,15 a small-molecule inhibitor of MKL1 that prevents MKL1 nuclear localization,19 similarly reduced TPA-induced SM22-luc activation (Figure 4B). Analysis of expression of endogenous SM22 by quantitative PCR revealed a TPA-induced increase within 6 hours. This transcription was also dependent on MKL1; administration of the MKL1 inhibitor CCG-1423 prevented TPA-induced transcriptional activation of SM22 (Figure 4C). The TPA-induced increase in mRNA for MYL9, MYH9, and MMP9, all of which function in megakaryocyte differentiation and maturation,2,20 was also found to be MKL1 dependent (Figure 4D-F). Activation of these genes by TPA also requires actin polymerization (supplemental Figure 2A). Cells treated with CCG-1423 for 24 hours were more than 85% viable (data not shown), but did not adhere to the culture plate in response to TPA, suggesting that MKL1 target genes play a role in promoting TPA-induced HEL cell adhesion (supplemental Figure 2B). When induced to differentiate, HEL-iMKL1 cells normally undergo up-regulation of megakaryocyte surface markers, including CD49b (integrin alpha2) and CD49f (integrin alpha6). Cells treated with CCG-1423 had impaired up-regulation of these integrins (Figure 4G). These data using inhibitors of MKL1 transcriptional activity (both dnMKL1 and CCG-1423) strongly suggest that nuclear localization of MKL1 results in activation of key megakaryocyte target genes.
MKL1 nuclear localization induces activation of SM22 promoter expression. (A) Luciferase activity from SM22 promoter-luc was increased when HEL-iMKL1 cells were treated with TPA (data are derived from 3 independent experiments). This activation was blocked by introduction of a dn-MKL1 construct, but was unaffected by the control plasmid (pcDNA5). (B) Activation of the SM22 promoter by TPA treatment is also inhibited by CCG-1423, a small-molecule inhibitor of MKL1 (data are derived from 3 independent experiments). (C-F) Relative endogenous mRNA levels after the indicated times of TPA administration with or without CCG-1423 treatment for SM22 (C), MYL9 (D), MMP9 (E), and MYH9 (F). Error bars indicate the SEM. Data are derived from 3 independent experiments. (G) CD41 and CD49f (top) or CD49b (bottom) expression on HEL-iMKL1 cells with and without CCG-1423 incubation and subsequent TPA treatment for 24 hours.
MKL1 nuclear localization induces activation of SM22 promoter expression. (A) Luciferase activity from SM22 promoter-luc was increased when HEL-iMKL1 cells were treated with TPA (data are derived from 3 independent experiments). This activation was blocked by introduction of a dn-MKL1 construct, but was unaffected by the control plasmid (pcDNA5). (B) Activation of the SM22 promoter by TPA treatment is also inhibited by CCG-1423, a small-molecule inhibitor of MKL1 (data are derived from 3 independent experiments). (C-F) Relative endogenous mRNA levels after the indicated times of TPA administration with or without CCG-1423 treatment for SM22 (C), MYL9 (D), MMP9 (E), and MYH9 (F). Error bars indicate the SEM. Data are derived from 3 independent experiments. (G) CD41 and CD49f (top) or CD49b (bottom) expression on HEL-iMKL1 cells with and without CCG-1423 incubation and subsequent TPA treatment for 24 hours.
mTPO induces MKL1 nuclear localization in primary megakaryocytes
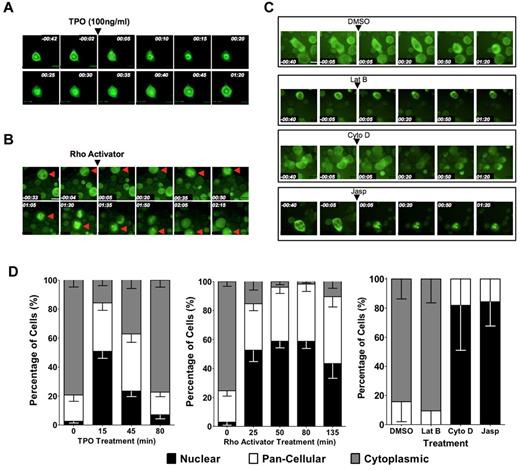
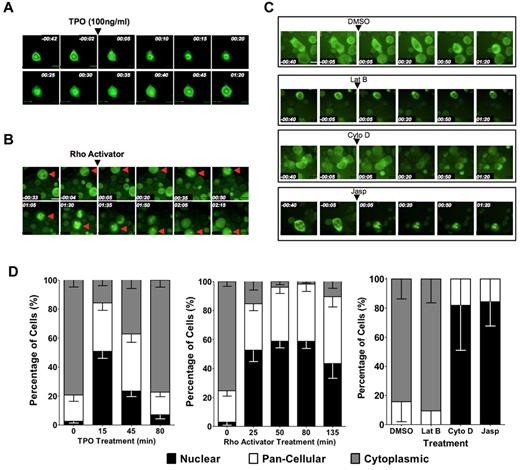
We next investigated whether subcellular localization of MKL1 is also regulated by induction of megakaryocytic maturation in primary cells by mTPO, the primary physiologic inducer of megakaryocyte differentiation. mTPO is known to activate RhoA in human megakaryocytes.21 To assess the subcellular localization of MKL1 over time in primary differentiating megakaryocytes, biphenotypic murine PreMegEs16 were transduced with virus encoding the MKL1-GFP fusion protein and differentiated to megakaryocyte progenitors by administration of mTPO. The cells were then plated on fibronectin-coated plates and mTPO starved overnight. The next day, cells were visualized for 40 minutes in this cytokine-starved state, during which time MKL1-GFP was predominantly cytoplasmic. Within 5 minutes of administration of mTPO to a final concentration of 100 ng/mL, visualization revealed redistribution of MKL1 to the nucleus, and within 15 minutes, MKL1 was predominantly nuclear (Figure 5A,D). Treatment of mTPO-starved cells with IL-11, a cytokine that promotes, but is not essential for, megakaryocyte differentiation, did not elicit a similar response (data not shown). The addition of the RhoA activator calpeptin to cytokine-starved primary megakaryocytes also resulted in MKL1 nuclear accumulation, although the kinetics were slower than those observed with mTPO (Figure 5B,D). Treatment of primary megakaryocytes with actin-manipulating drugs also resulted in MKL1 localization patterns similar to those observed in HEL-iMKL1 cells (Figure 5C-D); latrunculin B maintained cytoplasmic localization whereas cytochalasin D and jasplakinolide promoted MKL1 nuclear accumulation. To determine whether mTPO-induced nuclear accumulation of MKL1 is needed for SRF target gene activation, we assessed the effects of CCG-1423 on gene expression associated with mTPO-induced differentiation in primary cells. Similar to HEL-iMKL1, pretreatment of primary PreMegEs with CCG-1423 prevented the expression of the SRF-activated megakaryocyte-associated genes MYH9 and MYL9 (supplemental Figure 3). The similarities between primary cells and the HEL-iMKL1 cell line suggest shared mechanisms driving MKL1 nuclear localization via actin dynamics during megakaryocyte differentiation.
MKL1 localization in primary megakaryocytes is regulated by TPO stimulation, Rho signaling, and actin dynamics. PreMegE cells were sorted from the BM of wild-type mice, transduced with a MIGR1-MKL1GFP fusion construct, and differentiated down the megakaryocyte lineage. (A) Time-lapse microscopy captured the rapid relocation (within 15 minutes) of MKL1 to the nucleus in cells treated with 100 ng/mL of mTPO. MKL1 transitions out of the nucleus 45 minutes after the initial nuclear accumulation. (B) MKL1 nuclear accumulation is driven by calpeptin-induced Rho activation. (C) MKL1 subcellular localization in primary cells is affected by organization of the actin cytoskeleton. MKL1 accumulates in the nucleus in response to jasplakinolide and cytochalasin D, but not in control cells exposed to DMSO alone or to latrunculin B. Time elapsed is indicated in hours and minutes (hh:mm) and a 30μM scale bar is indicated. (D) Quantification of the percentage of cells with indicated MKL1 subcellular localization in the experiments shown in panels A through C. Data were quantified from 2 independent experiments, with 7 fields per condition and at least 3 cells per field. Error bars indicate the SEM.
MKL1 localization in primary megakaryocytes is regulated by TPO stimulation, Rho signaling, and actin dynamics. PreMegE cells were sorted from the BM of wild-type mice, transduced with a MIGR1-MKL1GFP fusion construct, and differentiated down the megakaryocyte lineage. (A) Time-lapse microscopy captured the rapid relocation (within 15 minutes) of MKL1 to the nucleus in cells treated with 100 ng/mL of mTPO. MKL1 transitions out of the nucleus 45 minutes after the initial nuclear accumulation. (B) MKL1 nuclear accumulation is driven by calpeptin-induced Rho activation. (C) MKL1 subcellular localization in primary cells is affected by organization of the actin cytoskeleton. MKL1 accumulates in the nucleus in response to jasplakinolide and cytochalasin D, but not in control cells exposed to DMSO alone or to latrunculin B. Time elapsed is indicated in hours and minutes (hh:mm) and a 30μM scale bar is indicated. (D) Quantification of the percentage of cells with indicated MKL1 subcellular localization in the experiments shown in panels A through C. Data were quantified from 2 independent experiments, with 7 fields per condition and at least 3 cells per field. Error bars indicate the SEM.
Discussion
The present study focused on the regulation of MKL1 subcellular localization during megakaryocyte differentiation using both the HEL cell line and primary megakaryocytes. As has been reported for smooth muscle cells and fibroblasts, MKL1 resides in the cytoplasm of unstimulated HEL-iMKL1 cells and quickly accumulates in the nucleus in response to TPA stimulation. Our data provide evidence that MKL1 nuclear accumulation requires a cascade of events wherein TPA activates RhoA, which promotes actin polymerization and allows for subsequent MKL1 nuclear accumulation. Our results not only show that the induction of megakaryocyte differentiation in HEL cells promotes MKL1 nuclear localization, but also that this nuclear localization promotes the transcription of MKL1-dependent genes. In addition, we show that mTPO, an essential cytokine in megakaryocytopoiesis, drives MKL1 nuclear localization and subsequent gene expression in primary megakaryocytes.
HEL cells, although derived from an erythroid leukemia, retain the properties of megakaryocyte erythroid progenitors because they can differentiate down both the megakaryocytic and erythroid lineages. Although the induction of megakaryocytopoiesis drives MKL1 nuclear accumulation, there are likely other pathways that can also promote megakaryocyte differentiation in the absence of MKL1 because Mkl1-KO mice do form megakaryocytes. Although a majority of MKL1-deficient megakaryocytes are low ploidy, there is a population of cells that can achieve high ploidy. This supports the role of MKL1 as a promoter of megakaryocyte differentiation but not the exclusive inducer of this process. We know from recent studies that other transcription factors such as Erythroid Krüppel-like Factor (EKLF/KLF1) and miRNAs such as miR-150 contribute to fate determination of the megakaryocyte erythroid progenitor.18,22,23 In addition, data from our laboratory show that MKL2 performs functions redundant with MKL1 in Mkl1-KO mice.24
As a model, HEL cells and in vitro culture of primary cells were used in the present study to elucidate the pathway by which MKL1 accumulates in the nucleus of megakaryocytes. However, data from cell lines and primary cells grown in vitro do not necessarily reflect accurately the behavior of primary cells in vivo. TPO is the primary physiologic cytokine that drives megakaryocytopoiesis, but is not the only stimulant, so other factors may similarly promote MKL1 nuclear localization in megakaryocytes in vivo. Mice lacking either Tpo or Mpl gene expression are viable. Although they have a nearly 90% decrease in platelet production, they do have mature megakaryocytes.25 Within the BM microenvironment, collagen I, one of the components of the extracellular matrix, regulates the early stages of megakaryocyte differentiation by activation of the Rho-ROCK-Myosin Light Chain-Myosin IIA pathway.26-28 In a 2009 study, Gilles et al demonstrated nuclear localization of MKL1 in human megakaryocytes on adhesion to either collagen I or convulxin, both of which are Rho activators.2 Other physiologic Rho activators include G protein–coupled receptors, receptor tyrosine kinases, integrins, and cadherins. One of the major G protein-coupled receptor ligands found in the serum is lysophosphatidic acid, which has been clearly implicated in the activation of the MKL/SRF pathway.29 Contact with the extracellular matrix and other cells can also catalyze RhoA and Rac1 activation through integrin and E-cadherin signaling, respectively.30-33 Most recently, the rigidity and tension created by the surrounding collagen matrix was reported to affect the ability of MKL1 to accumulate in the nucleus of fibroblasts.34 MKL1 nuclear accumulation was also stimulated by individually stretching cells, which elicits changes in the cytoskeleton.
The HEL cell model suggests that MKL1 may localize to the nucleus at the time of lineage commitment. However, our primary cell data do not address the role of MKL1 in cell-fate determination. Our assays show that in already committed megakaryocytes (PreMegE cells treated with TPO for 1 day), the readdition of TPO after overnight starvation is sufficient to promote MKL1 nuclear localization. Our early studies suggest that inhibition of MKL1 nuclear localization in primary megakaryocytes via CCG-1423 prevents the induction of gene expression programs associated with TPO-mediated megakaryocyte differentiation. Determining the changes in actin dynamics in response to CCG-1423 and the effect of Rho inhibition on gene expression will further elucidate the mechanism by which MKL1 participates in megakaryocytopoiesis.
Our transient transfection studies in HEL cells support the hypothesis that MKL1 nuclear localization results in transcription of MKL1 target genes. The early time points strongly suggest that TPA treatment induces activation of genes dependent on the nuclear localization of MKL1. However, it is not yet clear why SM22-luc activity continued to increase over 24 hours, whereas the bulk of MKL1 visualized by immunofluorescence appeared to become cytoplasmic 12-18 hours after TPA administration. The continued luciferase activation appeared to still be dependent on MKL1, because the dnMKL1- and CCG-1423–treated samples showed 20- and 4-fold less expression, respectively. These data, along with the immunoprecipitation results, suggest that there may be active MKL1 retained on the chromatin 24 hours after TPA treatment, whereas the bulk of MKL1 (as detected by immunofluorescence) has returned to the cytoplasm by this time. We hypothesize that when MKL1 floods the nucleus after TPA stimulation, it occupies and becomes stabilized at chromatin target sites and that only MKL1 left unbound relocates back to the cytoplasm after attenuation of the RhoA signal. Another possibility is that 24 hours after TPA treatment, activation of SM22 may have been initiated by MKL1 but may be maintained by another mechanism.
MKL1 was named for its association with acute megakaryoblastic leukemia (AMKL), a pediatric leukemia characterized by infiltration of the BM with blasts having an immature megakaryocyte surface phenotype and morphology. In AMKL with the chromosomal translocation t(1;22), there is a fusion gene of OTT and MKL1 from chromosomes 1 and 22, respectively.35,36 Unlike MKL1, OTT-MKL1 is constitutively nuclear. OTT-MKL1 is therefore insensitive to extracellular triggers, including TPO, that we propose are designed to control the transcriptional activity of MKL1. Aberrant MKL1 signaling could result from the expression of OTT-MKL1 in AMKL. A clear understanding of the physiologic regulation of MKL1 in normal megakaryocyte maturation provides relevant information for future studies of OTT-MKL1. For example, MKL1 is generally kept in an inactive state in the cytoplasm and activated to promote differentiation on accumulation in the nucleus. Because OTT-MKL1 is constitutively nuclear, it will be present in the nucleus to bind to and potentially interfere with the ability of nuclear MKL1 to promote megakaryocyte maturation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Richard Treisman for the MKL1-GFP plasmid and Stephanie Donaldson for excellent mouse husbandry.
This study was supported by the National Institutes of Health (grant F31 HL094118 to E.C.S. and grants DK086267 and DK072442 (the Yale Center of Excellence in Molecular Hematology) and the CT stem cell fund to D.S.K.; the Yale flow cytometry facility is supported by grant CA016359).
National Institutes of Health
Authorship
Contribution: E.C.S. designed and performed the experiments and wrote the manuscript; A.M.T. performed the experiments and wrote the manuscript; R.C.C, L.W., and Y.G. performed the experiments and provided technical expertise; and K.L.H. contributed scientific knowledge and was instrumental in the research design; and D.S.K. provided mentorship and intellectual input and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Diane S. Krause, Yale University School of Medicine, Department of Laboratory Medicine, P O Box 208073, 333 Cedar St, New Haven, CT 06520-8073; e-mail: diane.krause@yale.edu.

![Figure 2. Actin polymerization induces MKL1 nuclear accumulation. (A) Untreated (left) and 1-hour TPA-treated (right) HEL-iMKL1 cells were stained for filamentous actin using phalloidin (red) and for DNA using DAPI (blue). Filamentous actin was significantly increased in the large, flattened TPA-treated cells. (B) HEL-iMKL1 cells were treated with TPA for 2 hours and then incubated with the indicated compounds for an additional hour. Left images indicate MKL1 staining; right images overlay MKL1 (green) staining with DAPI (blue) counterstaining. MKL1 accumulated in the nucleus of control (DMSO) cells and cells that had a reduction in monomeric actin (jasplakinolide [iv] and cytochalasin D [iii]), but remained cytoplasmic in cells with increased monomeric actin (latrunculin B [ii]). (C) Graph quantifying the subcellular localization of MKL1 in HEL-iMKL1 cells shown in panel B. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. All images were taken using a 20× objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/7/10.1182_blood-2012-05-429993/4/m_zh89991301820002.jpeg?Expires=1767729145&Signature=5EegpHE5JGeKAxjJTWF1HbgW8zkutc8BoFUvCZVHtJBqoOQogX0VWqgwaSdZb184rxygnANwsRvO8Vr9m3WLLtPd9~wER~SwKAf0qioSgrfXUU5O7mYPuxWvIqO7FOsfRMZm03Zxt3XRDInTh92Pup7tj0yYavGGJga~QO7p86y10rfutAqLR8Vximv0lev~DP~x-fROdybXC6pZFBDf-2DAAxqY1qTYYxuEWhOfRuwuBDFKsA60Ks07Ae39ggmpAvMDmqkf6zmR2r4C32UKil5d6QfwLw1kOZK8SVcMKYeF0NNLF-nN2P5RXWRFYUGBgk12mReqj~jJTa8rbUvY9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Actin polymerization induces MKL1 nuclear accumulation. (A) Untreated (left) and 1-hour TPA-treated (right) HEL-iMKL1 cells were stained for filamentous actin using phalloidin (red) and for DNA using DAPI (blue). Filamentous actin was significantly increased in the large, flattened TPA-treated cells. (B) HEL-iMKL1 cells were treated with TPA for 2 hours and then incubated with the indicated compounds for an additional hour. Left images indicate MKL1 staining; right images overlay MKL1 (green) staining with DAPI (blue) counterstaining. MKL1 accumulated in the nucleus of control (DMSO) cells and cells that had a reduction in monomeric actin (jasplakinolide [iv] and cytochalasin D [iii]), but remained cytoplasmic in cells with increased monomeric actin (latrunculin B [ii]). (C) Graph quantifying the subcellular localization of MKL1 in HEL-iMKL1 cells shown in panel B. No fewer than 75 cells per time point were analyzed from 5 random fields of view in 3 independent experiments. Error bars indicate SEM. All images were taken using a 20× objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/7/10.1182_blood-2012-05-429993/4/m_zh89991301820002.jpeg?Expires=1767823976&Signature=UDNN0gbmANZvJX37ppIVRp3ZxEC6~FMrbRrJ2FgJVa9wgvTgXQd04Srwwxovsq96KErPm~uM7qpJ97GgpWYLbW3pF9gqcnfhvCiZ9cMhiCAJdnKhYQG9YsCuOgZn4vRFsenL5fMNJuB6uTbL20Ni6EpeUF3IhWe-tI9rxCFw1ljWytUCh0mIAEbSFg~MyR9tOOH6zyjkpLqMnUCAa0ilqecCccI~0KV~znwah3qAN5QfyZS2D8LQVpNkCiOBBhpXzBFmRImW-RgJZ39oW7sksLZwVkqeFhWy-2XL0qt6F23ht-eHO92UgH94kzweqEsbyg5qNdsHNJGPCtBUZQT~hg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)