Key Points
Thalidomide and prednisone maintenance after transplantation improves progression-free but not overall survival.
Abstract
We conducted a randomized, controlled trial comparing thalidomide-prednisone as maintenance therapy with observation in 332 patients who had undergone autologous stem cell transplantation with melphalan 200 mg/m2. The primary end point was overall survival (OS); secondary end points were myeloma-specific progression-free survival, progression-free survival, incidence of venous thromboembolism, and health-related quality of life (HRQoL). With a median follow-up of 4.1 years, no differences in OS between thalidomide-prednisone and observation were detected (respective 4-year estimates of 68% vs 60%, respectively; hazard ratio = 0.77; P = .18); thalidomide-prednisone was associated with superior myeloma-specific progression-free survival and progression-free survival (for both outcomes, the 4-year estimates were 32% vs 14%; hazard ratio = 0.56; P < .0001) and more frequent venous thromboembolism (7.3% vs none; P = .0004). Median survival after first disease recurrence was 27.7 months with thalidomide-prednisone and 34.1 months in the observation group. Nine second malignancies were observed with thalidomide-prednisone versus 6 in the observation group. Those allocated to thalidomide-prednisone reported worse HRQoL with respect to cognitive function, dyspnea, constipation, thirst, leg swelling, numbness, dry mouth, and balance problems. We conclude that maintenance therapy with thalidomide-prednisone after autologous stem cell transplantation improves the duration of disease control, but is associated with worsening of patient-reported HRQoL and no detectable OS benefit.
Introduction
With the use of new agents, outcomes of patients with multiple myeloma have steadily improved.1,2 For those without precluding comorbidities, high-dose melphalan with autologous stem cell transplantation (ASCT) remains the standard treatment, but this therapy is not curative and optimal use of new agents as components of induction and/or maintenance treatment continue to be evaluated.3 A series of trials have tested thalidomide as maintenance therapy for patients who have undergone ASCT.4-8 These trials have consistently demonstrated that thalidomide improves durations of myeloma control, but have not consistently shown improvements in overall survival (OS). If the inability to detect improved OS despite superior durations of disease control relates to the availability of effective therapies for recurrent myeloma, understanding patient-reported health-related quality of life (HRQoL) associated with thalidomide maintenance treatment may be helpful in informing whether this therapy should be adopted. We previously reported the results of a randomized phase 2 trial assessing the tolerability of combining prednisone 50 mg given on alternate days with thalidomide given at starting doses of 200 mg or 400 mg daily and observed acceptable tolerance with the 200-mg starting dose only.9 Therefore, we initiated the present randomized, controlled trial comparing these doses of thalidomide-prednisone maintenance therapy with observation. Although our primary objective was to determine whether this treatment improves OS, we also systematically assessed the potential negative impact of maintenance therapy on HRQoL domains and patient-reported symptoms known to be associated with thalidomide-prednisone.
Methods
Study design
The Myeloma.10 (MY.10) trial was a multicenter, nonblinded, randomized controlled trial conceived, conducted, and analyzed by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) and was designed to compare the combination of thalidomide-prednisone with observation as maintenance therapy for patients who had undergone ASCT as initial treatment for multiple myeloma. The NCIC-CTG was the trial sponsor and patients were accrued from centers in Canada and, in collaboration with the Eastern Cooperative Oncology Group (ECOG), the United States. The randomization process was concealed and performed through dynamic minimization at the NCIC-CTG central office in Kingston, ON. All participating centers received approval from their local research ethics boards and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Data were held and analyzed by the NCIC-CTG; the analysis was performed by the NCIC-CTG MY.10 senior biostatistician. The NCIC-CTG's independent data safety monitoring committee reviewed, in confidence, details of trial conduct at 6 monthly meetings and performed interim analyses according to protocol-defined cumulative events. The study chair (A.K.S.) vouches for integrity of the data, wrote the first draft of the manuscript, and collated edits from coauthors. All authors agreed to publish the manuscript. Thalidomide was provided by Celgene Corporation.
Study population
Eligible patients were those with a diagnosis of multiple myeloma based on the presence of at least 10% BM plasmacytosis or histologic evidence of a plasmacytoma determined by biopsy of an osteolytic lesion or soft tissue tumor and either a quantifiable serum IgG, IgA, IgD, or IgE monoclonal protein (MCP) of any amount or 24-hour urinary light chain excretion of at least 1000 mg. Patients with less than 10% BM plasmacytosis were eligible if the above MCP criteria were satisfied and at least 1 lytic lesion was observed on skeletal survey radiographs. Within 1 year of commencing initial treatment, patients were required to have received high-dose melphalan, 200 mg/m2, followed by ASCT. Specifics of induction therapy administered before high-dose melphalan were at each investigator's discretion, but could not include thalidomide or lenalidomide. Randomization was required within 60-100 days of the stem cell reinfusion and, at that time, patients were required to have an ECOG performance status of less than 3, adequate recovery of granulocyte and platelet counts, and acceptable hepatic and renal function. Women of childbearing age were required to have a negative pregnancy test and to consent to comply with medically approved birth control measures. Those with a prior or concurrent malignancy, diabetes with end-organ damage, uncontrolled hypertension, history of gastric ulceration or bleeding, avascular necrosis of the hips, symptomatic neuropathy, confirmed thrombophilia, deep venous thrombosis, or pulmonary embolism or those with ongoing employment that prohibited the use of sedatives were ineligible. Patients fluent in English or French were required to be willing to complete HRQoL questionnaires with the baseline assessment completed before randomization. Inability to complete HRQoL questionnaires because of illiteracy, loss of sight, or other equivalent reason did not exclude study participation. Baseline evaluations included a history and physical examination, routine chemistry and hematology, serum and urine immunoelectrophoresis or immunofixation, MCP quantitation, skeletal survey radiographs, and BM examination.
Treatment protocol
Patients were stratified by center, age (less than 60 years vs other) and response to ASCT (complete response vs other) and, within 5 working days after randomization, those assigned to the experimental arm were to begin maintenance therapy with thalidomide 200 mg daily and prednisone 50 mg on alternate days. Treatment was to be continued for 4 years or until disease progression. For treatment-related toxicity, thalidomide dose reductions in 50 mg decrements to a minimum of 100 mg and a 1-time dose reduction of prednisone to 25 mg on alternate days were permitted. A specific dose attenuation schedule according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 2.010 was used. Concurrent bisphosphonates, histamine-2 blockers, and laxatives were recommended. Anticoagulant and antiplatelet medications were not mandated. After randomization, patient reevaluations, including MCP quantitations and HRQoL questionnaire completion, were performed every 2 months for 6 months, every 3 months from months 6-48, and every 6 months thereafter.
Assessment of end points
The primary end point was OS defined as time from randomization to death from any cause; patients alive at the time of the clinical cutoff date were censored at the time of their last known status. Secondary end points included myeloma-specific progression-free survival (MS-PFS), defined as time from randomization to documented progression of myeloma, with censoring on the date of death if unrelated to myeloma or in the absence of disease progression, at the time of their last known status; PFS, defined in an identical manner to MS-PFS except that death in the absence of progressive myeloma was also included as an event; the incidence of venous thromboembolism; and HRQoL. Progression of myeloma was evaluated according to the criteria of the European Bone Marrow Transplantation Myeloma Subcommittee11 ; a diagnosis of venous thromboembolism required satisfying objective criteria (see the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). HRQoL was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 instrument12 and a trial-specific myeloma-disease module. The EORTC QLQ-C30 is a self-administered cancer-specific questionnaire with multidimensional scale consisting of 5 functional domains (physical, role, emotional, cognitive, and social); 3 symptom domains (fatigue, nausea and vomiting, and pain); 6 single-symptom items (dyspnea, sleep, appetite, constipation, and diarrhea); and a global assessment domain. In addition to the core EORTC questionnaire, we added 13 questions designed to address specific symptoms and toxicities of thalidomide-prednisone because these are anticipated to be important to overall QoL (see supplemental Appendix). To be consistent with other publications evaluating thalidomide as maintenance therapy after ASCT, we also performed a nonprotocol-prescribed analysis describing among patients with disease progression the time durations from the date of first disease progression until death. A formal statistical comparison was not performed.
Statistical analyses
All patients were included and analyzed on an intention-to-treat basis. The trial was designed assuming that 5-year survival of the control group would be 40% and an absolute increase of 20% for the experimental arm would be of interest clinically, equivalent to detecting a hazard ratio (HR) of 0.56 (maintenance-to-observation). With enrollment of 324 patients, we would have 80% power to detect this increase using a 1-sided 2.5% α level test, with 96 deaths at the time of final analysis. The method of Kaplan and Meier13 was used to calculate MS-PFS, PFS, and OS, and arms were compared using the log-rank test14 stratified by baseline age and remission status. Sensitivity analyses included using an unadjusted log-rank test, a generalized Wilcoxon test statistic15 assessing only eligible patients and those patients who maintained assigned protocol therapy. Data review before the final analysis indicated that a large number of patients classified as having achieved a complete response after ASCT were reassessed as having an incomplete response predominantly because of failure to perform immunofixation; another sensitivity analysis was added to examine the effects of the revised definition of remission status on outcomes. Because all sensitivity analyses demonstrated robust results, these analyses are not described further. Three protocol-defined interim analyses were performed after observing 25%, 50%, and 75% of the events to allow early termination of the study if extreme results were observed. The 1-sided α for the final analysis was 0.01535 to ensure the overall type I error of 0.025 by the Lan and De Mets stopping rule.16 The incidence of venous thromboembolism and the frequency of adverse events were assessed using the Fisher exact test.17
Analysis of HRQoL was based on the reported change score relative to baseline. Patients with baseline HRQoL and at least 1 evaluation after baseline were included in the analysis. A linear transformation was applied to standardize the raw scores to the range 0-100. A change from baseline by 10 or more points was defined as clinically relevant. For each functional domain, patients were considered to be improved if their reported score was 10 or more points greater than baseline at any time point; as worsened if their reported score was 10 or more points less than baseline without the prementioned improvement; and as stable if changes were within 10 points during the trial. Indications of HRQoL improvement or worsening were reversed for symptom domains and single items. A χ2 test was performed to compare the by-arm patient counts in the response categories of improved, worsened, or stable. A Mantel-Haenszel χ2 test14 was used to examine whether there was a trend that a treatment arm had a higher proportion of patients with improved or stable QoL.
Results
Recruitment, baseline characteristics, and treatment received
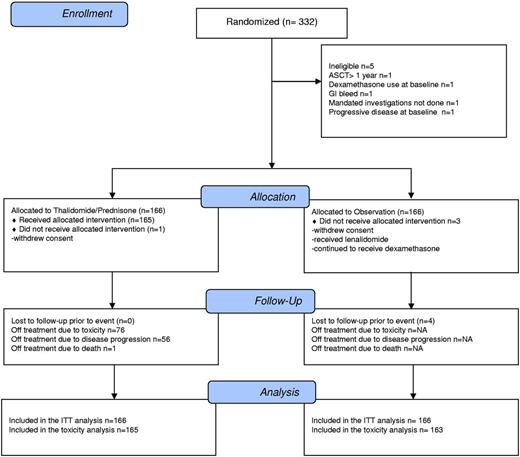
Between September 2002 and March 2009, 332 patients were accrued from 22 centers; 5 patients were ineligible (Figure 1). The study precluded the use of bortezomib or lenalidomide before ASCT to isolate the impact of maintenance with thalidomide and, therefore, accrual in later years slowed significantly. The protocol-defined event rate for the primary outcome was observed in February 2010 and a clinical cutoff date for the final analysis of February 28, 2010 was instituted. Data to this date and collected by June 15, 2010 were included in the July 7, 2010 locked database. The median follow-up time was 4.1 years. The median patient age was 58 years and the baseline characteristics of the 2 arms were well balanced (Table 1). Among those assigned to thalidomide-prednisone, 75% of patients required a dose reduction of thalidomide and 59% a dose reduction of prednisone. The median times to first dose reductions were 3.4 months for thalidomide and 5.5 months for prednisone. Median times to treatment discontinuation were 16.1 months for thalidomide and 14.9 months for prednisone. After relapse, treatment by arm (maintenance vs observation) included lenalidomide (39% vs 34%), thalidomide (13% vs 22%), or bortezomib (50% vs 46%). Therefore, 89% of patients randomized to maintenance received a bortezomib- or lenalidomide-containing regimen after disease progression.
OS and PFS
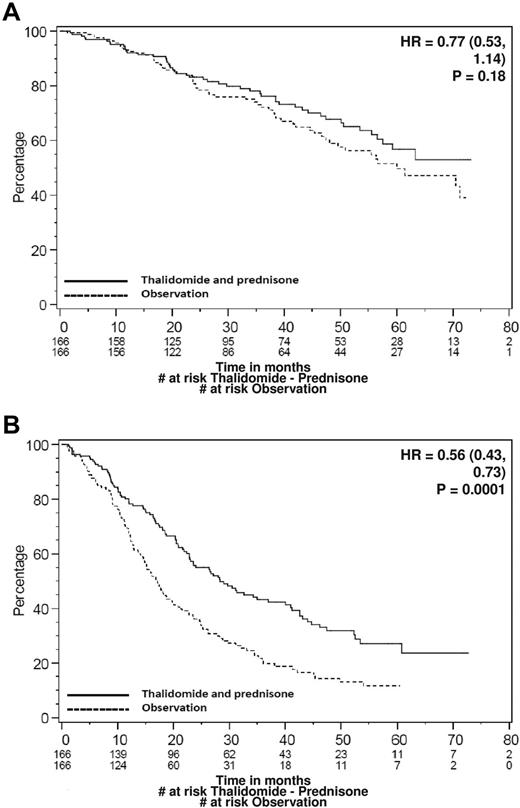
There were 111 deaths, including 50 in those allocated to thalidomide-prednisone and 61 in those allocated to observation (Table 2). A significant difference in OS was not detected, with 4-year estimates of 68% with thalidomide-prednisone and 60% with observation (HR = 0.77; P = .18; Figure 2A median survival was not yet met in either arm). The MS-PFS was superior in those allocated to thalidomide-prednisone (4-year estimates 32% vs 14%; HR = 0.56; P < .0001; Figure 2B). Similar results were observed for PFS (4-year estimates 32% vs 14%; HR = 0.55; P < .0001). Among patients experiencing disease progression and allocated to thalidomide-prednisone, the median duration of survival measured from the date of first progression was 27.7 months (95% confidence interval [CI], 17.2-35.8); the corresponding value for those allocated to observation was 34.1 months (95% CI, 27.0-43.6).
Survival curves. (A) Kaplan-Meier curve for OS. (B) Kaplan-Meier curve for PFS. Data are for treatments A (thalidomide-prednisone) and B (observation) of the study.
Survival curves. (A) Kaplan-Meier curve for OS. (B) Kaplan-Meier curve for PFS. Data are for treatments A (thalidomide-prednisone) and B (observation) of the study.
Adverse events
Twelve thromboembolic events were observed in patients receiving thalidomide-prednisone and there were no events in the observation arm (12 of 165 or 7.3% vs 0; P = .0004). Nonhematologic toxicities seen more frequently in patients receiving thalidomide-prednisone included hyperglycemia, edema, hypertension, fatigue, Cushingoid appearance, constipation, mouth dryness, dyspepsia, anxiety, memory loss, sensory neuropathy, tremor, blurred vision, depressed consciousness, cataracts, dyspnea, and bruising (Table 2). There were 9 new malignancies reported in 8 patients receiving thalidomide-prednisone, including colorectal cancer (n = 2), prostate cancer (n = 2), small-cell carcinoma of the lung (n = 1), squamous cell skin cancer (n = 2), melanoma (n = 1), and acute myeloid leukemia (n = 1). Of the patients allocated to observation, 6 developed new malignancies, including gastric cancer and small-cell carcinoma of lung (n = 1), squamous cell skin cancer (n = 2), acute myeloid leukemia (n = 1), and myelodysplasia (n = 1).
HRQoL
Baseline HRQoL scores were comparable between randomized groups for the global and 5 functional domains and the majority of symptom items; those allocated to observation had worse diarrhea (P = .04), balance problems (P = .01), and disturbing dreams (P = .06). Reporting compliance rates were comparable between the 2 arms; overall compliance was greater than 80% in the first year, but declined to 55% at 3 years. Patients assigned to thalidomide-prednisone had inferior HRQoL scores, including cognitive function (P = .01), and for the symptoms of dyspnea (P = .0007), constipation (P < .0001), thirst (P = .003), swelling in legs (P = .03), numbness (P = .02), dry mouth (P < .0001), and balance problems (P < .0001), whereas scores for appetite (P = .02) and sleep (P = .04) were improved (Table 3).
Correlative studies
Correlation between FISH-identified chromosomal changes and outcome were evaluated in this study. Genetic prognostic markers were measured on the BM received at time of study entry (ie, after ASCT) and the number of malignant cells in the tested samples was low. Therefore, only 50% of enrolled patients had an informative result and, of these, 14% demonstrated either t(4;14) or deletion 17p. Both OS (multivariate HR = 2.24; 95% CI, 1.11-4.54; P = .02) and PFS (multivariate HR = 2.23; 95% CI, 1.34-3.69; P = .002) were worse in high-genetic-risk patients. The small numbers preclude meaningful analysis by arm. Multivariately, both high-genetic-risk disease and IgA were significantly associated with worse OS compared with other isotypes.
Discussion
The concept of maintenance therapy with thalidomide for patients who have undergone ASCT has been attractive because these patients are otherwise destined to suffer progressive disease. Thalidomide is an active agent and is associated with a potentially tolerable adverse event profile. Several international groups have conducted randomized, controlled trials evaluating this treatment.4-8 Although these trials have incorporated various design specifics, each has demonstrated superior durations of disease control with thalidomide treatment. However, the trials have also been inconsistent in demonstrating improvements in OS.
Inconsistencies of OS outcomes among previous randomized trials may relate to statistical power, insufficient durations of follow-up, and effects of therapy administered after disease progression. Attal et al observed superior OS in patients assigned to maintenance therapy with thalidomide plus pamidronate compared with pamidronate alone or observation (4-year outcomes were 87% vs 75%; P = .04).4 No differences in survival after first disease progression were detected. Similarly, Spencer et al demonstrated superior OS with thalidomide-prednisone compared with prednisone (3-year outcomes were 86% vs 75%; HR = 0.41; P = .004), with no differences in survival observed after first disease progression.5 In contrast, Barlogie et al failed to detect a significant difference in survival (P = .09) in their testing of thalidomide versus observation during induction, consolidation, and maintenance phases of Total Therapy 2, but did observe that with longer follow-up, a trend toward superior survival was emerging (8-year outcomes were 57% vs 44%).7 In 2 other randomized trials, no improvements in OS were suggested. Lokhorst et al failed to detect differences (HR = 0.96; P = .77) comparing thalidomide with IFN and in a trial that included patients who did or did not undergo ASCT,6 Morgan et al observed that in the ASCT population, 3-year survival in the group receiving thalidomide was 75% compared with 80% for those allocated to observation (P = .26)8 ; this reflected no OS advantage in low-risk patients and an adverse effect of thalidomide in high-risk patients. In both of these trials, survival after first disease progression was superior in those who did not receive thalidomide maintenance therapy. The trial reported by Morgan et al was stopped early for futility by its data monitoring and ethics committee; the investigators subsequently performed complex modeling to suggest that thalidomide maintenance therapy was beneficial after correcting for optimum treatment after first disease progression.
Our present results contribute to these data by again showing that thalidomide-prednisone convincingly improves durations of disease control compared with observation (4-year PFS estimates were 32% vs 14%, respectively; HR = 0.55; P < .0001). These benefits did not translate into proportionate improvements in survival, with 4-year estimates of 68% with thalidomide-prednisone and 60% with observation (HR = 0.77; P = .18). Although our observed effect size suggests that our trial may have lacked adequate power, using our protocol-stated parameters, we would have required a sample size of 1284 patients to have 80% power of detecting statistical significance with an observed HR = 0.77. Our data are consistent with other results6,8 demonstrating that survival after first disease progression may be longer in those who have not received maintenance therapy.
Our data evaluating HRQoL demonstrate that although thalidomide was associated with superior durations of disease control, these benefits were associated with trade-offs. Patients allocated to thalidomide-prednisone experienced lower HRQoL scores for global, cognitive, and role function domains and for many symptoms. We have previously described principles associated with using HRQoL outcomes to inform decisions about adopting a new therapy, including when discordance with an efficacy end point exists.18 These principles were also applied in a review of randomized trials that evaluated HRQoL when testing therapies for patients with multiple myeloma.19 Kvam et al identified 15 trials and reported that in only 2 trials were efficacy and HRQoL outcomes discordant.19 Segeren et al compared ASCT with chemotherapy alone and observed superior response rates and time-to-progression outcomes in those undergoing ASCT,20 but did not detect improvements in event-free survival or OS; HRQoL was worse in those undergoing ASCT. Although potentially supporting a transplantation strategy, the investigators recommended against adoption of their ASCT preparative regimen, which included total body irradiation. Wisloff et al reported HRQoL outcomes for their comparison of IFN maintenance therapy with observation21 ; analyses of efficacy outcomes had shown that IFN prolonged the plateau phase of disease, but did not improve OS.22 Because HRQoL outcomes were inferior with IFN, the investigators questioned whether benefits associated with plateau-phase prolongation were meaningful to patients.
We are aware of one other randomized trial evaluating thalidomide that reports HRQoL outcomes.23 In a trial testing the addition of thalidomide to melphalan plus prednisone in patients not suitable for ASCT, Verelst et al reported that despite more frequent and severe adverse events, patients receiving thalidomide reported superior emotional function and future perspectives with no differences detected in global scores, thus supporting a decision to adopt adding thalidomide to melphalan-prednisone as standard treatment based on demonstrated improvements in PFS and survival.24 As with our trial, thalidomide was associated with poorer physical functioning, constipation, and paresthesias and less insomnia.
In conclusion, despite improving PFS, our trial failed to meet its primary end point of improving OS with thalidomide-prednisone maintenance therapy. As with 2 other trials,6,8 the failure to translate improvements in PFS to OS may be related to shorter survival of these patients after first disease progression. Our findings of inferior HRQoL outcomes in patients receiving thalidomide-prednisone call into question the benefits of a strategy to provide this agent as maintenance therapy for all patients and invite evaluations of predictive biomarkers that might better direct this treatment. Future trials should also be conducted to determine whether other agents, such as lenalidomide or bortezomib, may demonstrate survival and/or HRQoL benefits as maintenance therapy after ASCT.25-27 Finally, our results juxtaposing PFS, HRQoL, and OS support ongoing debates about which outcomes should drive treatment policies, especially when sequencing of newly available therapies is possible.28
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
NCIC-CTG grant support for this trial included NCIC-CTG grant 015469, Canadian Cancer Society Research Institute Grant 21039, and US National Institutes of Health grant CA077202. ECOG grant support included National Institutes of Health grants CA21115 and CA13650. Thalidomide was provided by Celgene Corporation. This trial was conducted by NCIC-CTG and ECOG and is registered at www.clinicaltrials.gov as NCT00049673.
National Institutes of Health
Authorship
Contribution: The study was conceived and designed by A.K.S. and members of the trial committee; J.W.C. conducted the analysis; A.K.S., L.S., and R.M.M. reviewed the data analysis and interpretation and were primarily involved in the writing of the manuscript; and all other authors contributed to the conduct of the trial, recruited the patients, and were involved in the review of results and final approval of the manuscript.
Conflict-of-interest disclosure: A.K.S. has been a consultant for Celgene, Onyx, and Millennium, has received research funding and honoraria from Millennium, and has a patent pending, filed by the Mayo Clinic, for Cereblon as a biomarker for drug response. R.M.M. has received honoraria from Celgene regarding his role on the Independent Response Committee of a clinical trial and from Lilly regarding his role as Chair of an Independent Data Safety Monitoring Committee. R.M.M. is Director of the NCIC Clinical Trials Group, which has received research funding from Amgen Canada, Ariad Pharmaceuticals, Astex Therapeutics, AstraZeneca, Boston Biomedical Inc, Bristol-Myers Squibb, Celgene, Geron Corp, GlaxoSmithKline, Janssen-Ortho, Lilly, Merck Frosst Canada, Novartis, Oncolytics Biotech, Oncothyreon, Orthobiotech, Pfizer, Roche, Sanofi-Aventis, and Schering Canada. The remaining authors declare no competing financial interests.
Correspondence: Dr A. Keith Stewart, Division of Hematology-Oncology, Mayo Clinic in Arizona, CRB1-001, 13400 East Shea Blvd, Scottsdale, AZ 85259; e-mail: stewart.keith@mayo.edu.