In this issue of Blood, Ouedraogo et al have investigated the role of HIV and Epstein-Barr virus (EBV) replication in the persistence of monoclonal gammopathy.1 It has been known for some time that patients with HIV infection have an increased incidence of monoclonal gammopathy and plasma cell dyscrasias.2,3 The exact mechanism of monoclonal gammopathy in patients with HIV infection is unknown, but in many patients the monoclonal gammopathy and other B-cell abnormalities can be reversed with antiretroviral therapy. However, a proportion of patients will have persistent monoclonal gammopathy.
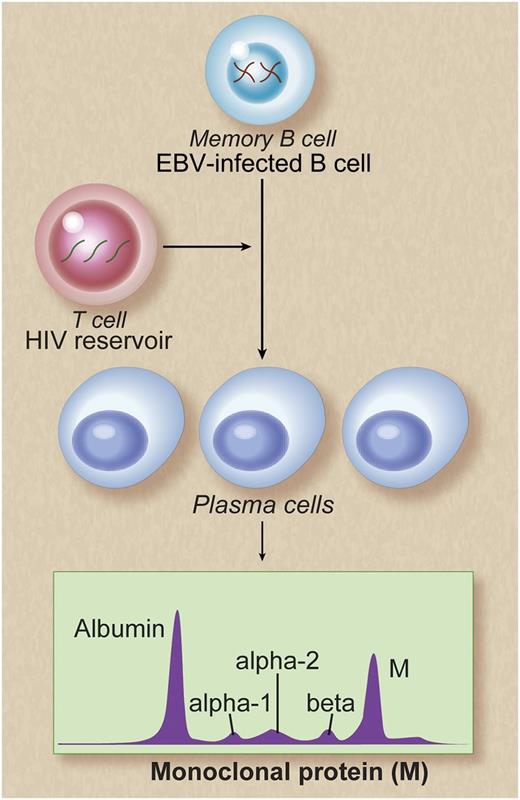
Potential mechanisms of persistent monoclonal gammopathy in patients with chronic HIV infection. Memory B cells are reservoirs of EBV infection. Poor control of HIV viral replication may result in polyclonal B-cell activation, possibly as a result of T-cell deficiency caused by HIV infection. This in turn may lead to persistent EBV replication and terminal differentiation of EBV-infected memory B cells to plasma cells. This is reflected as persistent monoclonal gammopathy, detectable in the peripheral blood. Alternative explanations may be that the EBV-infected memory B cells may act as stimulators of plasma cells or that the observed increased numbers of EBV-infected memory B cells and increased numbers of plasma cells may be independent of one another. Future functional studies are needed to better characterize mechanisms leading to persistent monoclonal gammopathy in HIV-infected patients. Professional illustration by Debra T. Dartez.
Potential mechanisms of persistent monoclonal gammopathy in patients with chronic HIV infection. Memory B cells are reservoirs of EBV infection. Poor control of HIV viral replication may result in polyclonal B-cell activation, possibly as a result of T-cell deficiency caused by HIV infection. This in turn may lead to persistent EBV replication and terminal differentiation of EBV-infected memory B cells to plasma cells. This is reflected as persistent monoclonal gammopathy, detectable in the peripheral blood. Alternative explanations may be that the EBV-infected memory B cells may act as stimulators of plasma cells or that the observed increased numbers of EBV-infected memory B cells and increased numbers of plasma cells may be independent of one another. Future functional studies are needed to better characterize mechanisms leading to persistent monoclonal gammopathy in HIV-infected patients. Professional illustration by Debra T. Dartez.
Ouedraogo et al identified 21 patients with HIV infection in whom a monoclonal protein (M-protein) was identified by serum electrophoresis and immunofixation. The M-protein was unquantifiable (ie, had a very low concentration) in the majority (15/21; 71%) of HIV patients, with a median of 0.31 g/dL. The age of patients with monoclonal gammopathy was lower in this small cohort of HIV-infected patients (range, 20-58 y) than in previous reports based on the general population (typically detected above the age of 50).4 All HIV-infected patients were treated with antiretroviral therapy, and the monoclonal gammopathy resolved in 12 of 21 (58%) patients; the remaining 9 (42%) patients had a persistent monoclonal gammopathy after at least 5 years of antiretroviral therapy. These 9 patients also had higher serum immunoglobulin levels as well as circulating plasmablasts and plasma cells in peripheral blood. Interestingly, 6 of 9 (66%) and 1 of 12 (8%) patients with persistent and transient monoclonal gammopathy had quantifiable EBV DNA in plasma, respectively. In addition, the EBV DNA B-cell reservoir and the EBV DNA produced by infected B cells in culture were significantly higher in patients with persistent monoclonal gammopathy when compared with patients with transient gammopathy and normal controls. These observations based on small numbers are supportive of a biological role for EBV in persistent monoclonal gammopathy in HIV infection.
Furthermore, these results are analogous to the monoclonal gammopathy seen in patients with solid organ transplantation.5,6 The increased risk of posttransplant persistent monoclonal gammopathy has been associated with increased frequency of EBV-infected cells and EBV reactivation.7,8 Indeed, recent population-based data provide evidence to support a role for EBV infection in the pathogenesis of plasma cell dyscrasias in solid organ transplant recipients.9 In addition, previous studies have found serum-free light-chain abnormalities (a marker of polyclonal B-cell activation) to be associated with HIV infection and lymphoproliferative malignancies.10
Taken together, the results from Ouedraogo et al advance our understanding of immune dysregulation and plasma cell dyscrasias in patients with chronic HIV infection. The observed M-protein patterns (ie, earlier age of onset, transient monoclonal gammopathy associated with antiretroviral therapy, smaller M-protein concentrations, and a possible role for EBV infection) provide further evidence that the pathogenesis of HIV-associated monoclonal gammopathy is likely distinct from the monoclonal gammopathy observed in the general population. On the basis of small numbers, this study suggests that ongoing HIV replication may fuel reactivation of EBV infection, leading to persistent monoclonal gammopathy in patients with chronic HIV infection (see figure). At this time, the long-term clinical implications of persistent monoclonal gammopathy in patients with HIV infection are unclear; however, previous studies have indicated an increased risk of plasma cell neoplasms in patients with HIV infection.3 In this context, future studies should aim at understanding the mechanisms of B-cell dysregulation in patients with persistent HIV and EBV infection and the risk of plasma cell dyscrasias in these patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.