Key Points
XPO1/CRM1 is upregulated in a BCR-ABL1 kinase-dependent and -independent manner and negatively controls PP2A tumor suppressor activity.
KPT-330 antagonizes survival of TKI-resistant Ph+ acute leukemias in vitro, in CML-BC animals, and in a CML-AP patient.
Abstract
As tyrosine kinase inhibitors (TKIs) fail to induce long-term response in blast crisis chronic myelogenous leukemia (CML-BC) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL), novel therapies targeting leukemia-dysregulated pathways are necessary. Exportin-1 (XPO1), also known as chromosome maintenance protein 1, regulates cell growth and differentiation by controlling the nucleocytoplasmic trafficking of proteins and RNAs, some of which are aberrantly modulated in BCR-ABL1+ leukemias. Using CD34+ progenitors from CML, B-ALL, and healthy individuals, we found that XPO1 expression was markedly increased, mostly in a TKI-sensitive manner, in CML-BC and Ph+ B-ALL. Notably, XPO1 was also elevated in Ph− B-ALL. Moreover, the clinically relevant XPO1 inhibitor KPT-330 strongly triggered apoptosis and impaired the clonogenic potential of leukemic, but not normal, CD34+ progenitors, and increased survival of BCR-ABL1+ mice, 50% of which remained alive and, mostly, became BCR-ABL1 negative. Moreover, KPT-330 compassionate use in a patient with TKI-resistant CML undergoing disease progression significantly reduced white blood cell count, blast cells, splenomegaly, lactate dehydrogenase levels, and bone pain. Mechanistically, KPT-330 altered the subcellular localization of leukemia-regulated factors including RNA-binding heterogeneous nuclear ribonucleoprotein A1 and the oncogene SET, thereby inducing reactivation of protein phosphatase 2A tumor suppressor and inhibition of BCR-ABL1 in CML-BC cells. Because XPO1 is important for leukemic cell survival, KPT-330 may represent an alternative therapy for TKI-refractory Ph+ leukemias.
Introduction
Although the success of tyrosine kinase inhibitors (TKIs) as first-line therapy for chronic myelogenous leukemia (CML) in the chronic phase (CML-CP) is fully justified by the BCR-ABL1 kinase dependence of leukemic progenitors, the etiopathogenesis of Philadelphia-positive (Ph+) acute leukemias is still unclear.1-3 In fact, the presence of BCR-ABL1 mutations and nonrandom secondary genetic abnormalities can only partially explain the lack of long-term response and/or development of resistance to TKIs (including ponatinib) and other therapeutic options.1,4-8 Thus, the biological processes underlying emergence and maintenance of CML-blast crisis (BC) and Ph+ B-cell acute lymphoblastic leukemia (ALL) likely involve different combinations of BCR-ABL1–independent genetic or epigenetic (cell-autonomous and microenvironment-induced) molecular events, in addition to BCR-ABL1 oncogene-driven mechanisms occurring in a kinase-dependent and kinase-independent manner.1,9,10 Posttranscriptional control of gene expression (messenger RNA [mRNA] processing, stability, export, and translation) plays an essential role in the emergence, maintenance, and/or progression of different types of cancer including Ph+ acute leukemias.1,11-15 In these hematologic malignancies, altered expression and activity of the nucleocytoplasmic shuttling heterogeneous ribonuclear proteins (hnRNPs) results in aberrant metabolism of their mRNA cargo that, in most cases, encompasses oncogenes, tumor suppressor proteins, and growth/survival–regulating or differentiation-regulating factors.11,15 Karyopherins also function to mediate the nucleocytoplasmic exchange of proteins and RNA through nuclear pore complexes.14,16-18 Specifically, the karyopherin β family member XPO1 (exportin-1, also called chromosome maintenance protein 1 [CRM1]) is a critical regulator of cell proliferation and survival19-22 that is overexpressed in several hematologic and nonhematologic malignancies in some of which it was described as a poor prognostic factor.22-30 Different inhibitors of XPO1-mediated export through the nuclear pore complex have been developed31 ; among these, the selective inhibitors of nuclear export (SINE, Karyopharm Therapeutics Inc) are small molecules based on leptomycin B (LMB) that irreversibly bind to Cys528 in the cargo-binding groove of XPO1 to prevent XPO1-cargo interaction.22,24-26,32 Preclinical in vitro and/or in vivo studies have shown that the closely related SINE compounds KPT-251, KPT-276, and KPT-330 have strong antileukemic activity in acute myelogenous leukemia, T-cell ALL, mantle-cell lymphoma, and chronic lymphocytic leukemia, likely through signals mediated by altered subcellular localization of p53, IκBα, and/or FoxO3a.22,24-26,32 Notably, the SINE KPT-330 is currently in clinical trials for advanced hematologic malignancies and solid tumors (NCT01607892 and NCT01607905).
Here, we report that XPO1 is also overexpressed in Ph+ acute leukemias, and that SINE-mediated XPO1 inhibition decreases survival of leukemic, but not normal, CD34+ progenitors, thereby impairing leukemogenesis both in vitro and in an animal model of Ph+ acute leukemia. Mechanistically, KPT-330–induced inhibition of XPO1-mediated nuclear export not only altered subcellular localization of p53, IκBα, and FoxO3a but, importantly, directly subverted the BCR-ABL1-heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1)-SET network,33 thereby restoring the activity of the protein phosphatase 2A (PP2A) tumor suppressor, an event sufficient to selectively kill CML-BC and Ph+ ALL blasts.34
Materials and methods
Cell cultures and primary cells
Parental, BCR-ABL1–expressing 32Dcl3 and BaF3 cells and primary CD34+ bone marrow (BM) progenitors were maintained and used in clonogenic and apoptosis assays, as reported in supplemental Methods. Frozen samples of BM hematopoietic cells from the BM of unidentifiable CML and ALL patients were obtained from The Ohio State University (OSU) Leukemia Tissue Bank, Columbus, OH; the Division of Hematology; Maisonneuve-Rosemont Hospital, Montréal, QC; the Hammersmith Hospital, Imperial College, London, UK; and from the Department of Hematology, Aarhus University Hospital, Aarhus, Denmark. BM cells from different healthy donors (NBM) were purchased from Cincinnati Children’s Hospital or The OSU. All experiments with human specimens were carried out with approval from The OSU Institutional Review Board. All experiments were conducted in accordance with the Declaration of Helsinki. Infections with the SV40 small-T antigen (small-t) and BCR-ABL1–expressing retroviruses in 32Dcl3 and/or Ba/F3 cells were performed as described.34 32D-BCR/ABL cells expressing the shuttling-deficient hnRNP A1 mutants have already been described.35 Where indicated, cells were treated with the BCR-ABL1 kinase inhibitor imatinib (Novartis); src inhibitor PP2 (Calbiochem); mTORC inhibitor rapamycin, protein kinase C (PKC) inhibitor PKC-412; phosphatidylinositol-3 kinase (PI-3K) inhibitor LY294002 (Sigma-Aldrich); Jak2 inhibitor TG101348 (SAR302503, TargeGen/Aventis); mitogen-activated protein kinase kinase 1 inhibitor U0126 (Promega); or the SINE compounds KPT-330 (MW: 443.31, chemical formula: C17H11F6N7O), KPT-185, and KPT-207 (Karyopharm Therapeutics, Inc) used at the time and concentrations indicated in “Results.”
Western blot analysis, subcellular fractionation, and PP2A assay
Whole-cell lysates and subcellular fractions were subjected to sodium dodecyl sulfate- polyacrylamide gel electrophoresis followed by immunoblot with the following antibodies: anti-XPO1/CRM1, anti-SET, anti-p53, and anti-p21 (Santa Cruz Biotechnology); anti-GRB2, anti-phospho signal transducer and activator of transcription 5 (STAT5)Y694, anti-pAktS473, and anti-p42/44 mitogen-activated protein kinase (MAPK)T202/Y204 (Cell Signaling Technology); anti-ABL (BD Translab); anti-hemagglutination activity (HA) (Covance); and anti-hnRNP A1 (Sigma-Aldrich). Densitometric analysis was performed using Image J software (National Institutes of Health). PP2A phosphatase activity was quantitated as described previously.34
Confocal microscopy
Cells were cytospun onto glass slides, Triton X-100 (0.1% volume/volume) permeabilized, and incubated for 10 minutes with 4% normal goat serum. The cells were then immunostained with the anti-hnRNP A1 (Sigma-Aldrich); anti-SET, anti-cancerous inhibitor of PP2A (CIP2A), and anti-FoxO3a (Santa Cruz Biotechnology); anti-HA (Covance); anti-PP2Ac (Millipore); or anti-IκBα (Cell Signaling Technology). They were then incubated with AF647-conjugated rabbit or mouse secondary antibodies (Invitrogen). Confocal micrographs were taken using the FV1000 Confocal Laser Scanning Microscope (Olympus) with a PLAPONSC 60×/1.4 oil lens. Microscope settings remained unchanged between treated and untreated slides, and images were processed using FluoView software (Olympus).
CML-BC mouse model
Six-week-old immunocompromised ICR-SCID mice (n = 30) were intravenously injected with 3 × 105 32D-BCR/ABL cells (n = 20) or used as controls (n = 10). After 1 week, engraftment was confirmed by BCR-ABL1–nested reverse transcription polymerase chain reaction (RT-PCR) performed on total RNA from the peripheral blood (PB) of cell-injected animals as described.34 Thereafter, KPT-330 (15 mg/kg in 0.6% Pluronic F-68 and 0.6% Plasdone K-29/32)25 was administered by oral gavage to leukemic mice (n = 12) and age-matched mice (n = 7) twice per week. As controls, 8 leukemic mice were left untreated, and 3 age-matched SCID mice received neither cells nor treatment. At 5 weeks after transplant, 2 mice per group were euthanized, and spleens were subjected to macroscopic and microscopic (hematoxylin and eosin [H&E] staining) evaluation of leukemic-cell infiltration. The remaining mice were used for Kaplan-Meier survival analysis. At 16 weeks posttransplant, surviving KPT-330–treated animals were euthanized, and the spleen, liver, BM, and PB were isolated and subjected to histopathologic examinations, together with the organs collected from untreated leukemic mice immediately postmortem. PB samples were used for RNA isolation and PCR-mediated detection of BCR-ABL1 transcripts (at 10 and 16 weeks posttransplant), and were cytospun on glass slides for morphologic evaluation after Wright-Giemsa staining. Cytospins and tissue sections were visualized with a Zeiss Axioskope 2 Plus; Achroplan (Zeiss) lenses used were 40×/0.65 (PB and BM), 10×/0.25 (liver), and 4×/0.10 (spleen). Images were taken with a QICLICK-F-M-12 CCD camera (QImaging) equipped with a red, green, and blue liquid crystal color-filter module for capturing color images.
Case patient
A 37-year-old man with hyperleukocytosis and severe bone pain was diagnosed at the Gabrail Cancer Center (Canton, OH) with accelerated-phase CML (CML-AP). The patient's condition was refractory to 9 prior therapies, including different TKIs and several investigational agents. After refusing BM transplant, the patient received KPT-330 for compassionate use. The regimen scheme was a run-in dose (12 mg/m2 orally) on days 1, 3, and 5, followed by 3 administrations of KPT-330 at the therapeutic dose of 16.5 mg/m2 on days 1, 3, and 5 of the second week. White blood cell (WBC) count, the presence of immature cells in PB smears, serum levels of lactate dehydrogenase (LDH), and splenomegaly were monitored. At 1 week after KPT-330 administration, the patient declined dose escalation.
Statistical analysis
The 1-tailed unpaired Student t test was used for the in vitro studies except for colony-forming assays, which were analyzed by the 1-tailed paired Student t test. EC50 values were calculated by nonlinear regression curves fit using the Prism software (GraphPad). The log-rank (Mantel-Cox) test was used to evaluate differences in survival duration of treated vs untreated mice. P < .05 was considered statistically significant.
Results
XPO1 expression is enhanced in Ph+ acute leukemia (CML-BC and B-ALL) progenitors
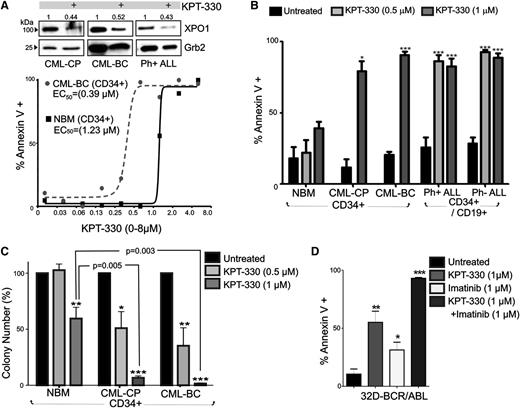
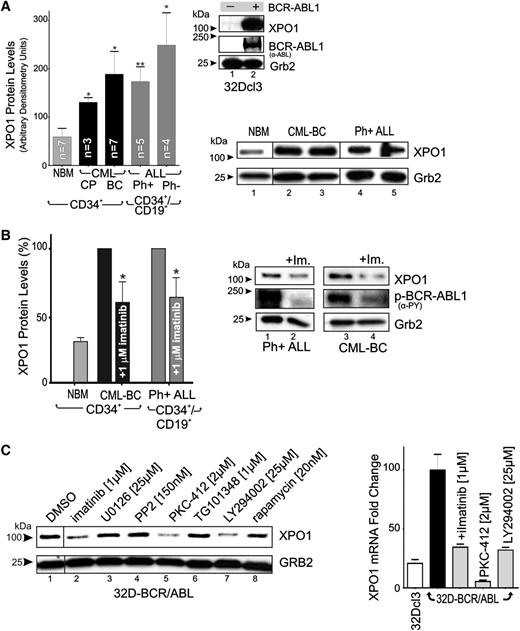
Leukemic transformation of 32Dcl3 myeloid precursors by forced p210 BCR-ABL1 expression results in a dramatic induction of XPO1 protein levels (Figure 1A). Similarly, western blot analysis showed that XPO1 expression was markedly higher in CML-CP (n = 3) than in NBM (n = 7) CD34+ BM progenitors (Figure 1A), suggesting that BCR-ABL1, which drives CML-CP emergence and maintenance,1 is responsible for increased XPO1 expression. Accordingly, CD34+ CML-BC (n = 7) and CD34+/CD19+ t(9;22)(q34;q11) B-ALL (n = 5) progenitors, both expressing BCR-ABL1 at levels higher than CML-CP,1 also present significantly higher XPO1 expression (Figure 1A). However, XPO1 levels were further increased in Ph− B-ALL (n = 4), suggesting that BCR-ABL1 kinase-dependent and kinase-independent molecular mechanisms may cooperate to increase XPO1 expression also in Ph+ acute leukemias. Indeed, suppression of BCR-ABL1 kinase activity by imatinib (1 µM, 72 hours) (Figure 1B, right) only partially decreased XPO1 expression, which remained at levels significantly higher in CD34+ CML-BC (n = 3) and CD34+/CD19+ Ph+ ALL cells (n = 3) than in CD34+ NBM cells (n = 3) (Figure 1B, left). XPO1 protein expression was also significantly downmodulated on LY294002 and PKC412 but not U0126, PP2, and TG101348 treatment (Figure 1C, left). Similarly, XPO1 mRNA levels were decreased on BCR-ABL1 and PI-3K inhibition and were further reduced on inhibition of PKC and nonrelated kinases (eg, c-Kit) by PKC-412 treatment (Figure 1C, right). Thus, BCR-ABL1 might induce XPO1 transcription through modulation of PI-3K/Akt- but not MAPK-, Src-, Jak2-, and S6K-mediated signals. Furthermore, the marked effect of PKC-412 indicates that conventional PKCs or other PKC-412–sensitive kinases might be involved in the BCR-ABL1 kinase-independent regulation of XPO1 expression.
XPO1 expression is enhanced in Ph+ acute leukemia (CML-BC and B-ALL) progenitors. (A) Top right panel: XPO1 and BCR-ABL1 protein levels in BCR-ABL1- or empty vector–transduced 32Dcl3 myeloid cells were determined by immunoblot. Left panel: Protein levels of XPO1 expressed as mean ± standard error of the mean (SEM) of densitometric units after normalization with Grb2 levels, were determined by immunoblot of NBM (n = 7), CML-CP (n = 3), and CML-BC (n = 7) CD34+ progenitors, and Ph+ B-ALL (n = 5) and Ph− B-ALL (n = 4) CD34+/CD19+ progenitors. Bottom right panel: Sample of immunoblots used to determine XPO1 protein levels used for quantification. (B) Left panel: XPO1 protein levels expressed as mean ± SEM in vehicle- or imatinib-treated (1 µM, 12 hours) CML-BC CD34+ cells and Ph+ B-ALL CD34+/CD19+ cells. Right panel: Representative immunoblot of XPO1 protein levels and BCR-ABL1 activity (anti-PY) in vehicle- or imatinib-treated (1 µM, 12 hours) Ph+ B-ALL CD34+/CD19+ (lanes 1 and 2) and CML-BC CD34+ (lanes 3 and 4) cells. (C) Left panel: XPO1 protein levels in 32D-BCR/ABL cells treated (24 hours) with the indicated kinase inhibitors. Right panel: XPO1 mRNA levels assessed by quantitative reverse-transcription PCR in 32Dcl3 and untreated and kinase inhibitor–treated (24 hours) 32D-BCR/ABL cells. Asterisks indicate P values vs NBM; *P < .05, **P < .01.
XPO1 expression is enhanced in Ph+ acute leukemia (CML-BC and B-ALL) progenitors. (A) Top right panel: XPO1 and BCR-ABL1 protein levels in BCR-ABL1- or empty vector–transduced 32Dcl3 myeloid cells were determined by immunoblot. Left panel: Protein levels of XPO1 expressed as mean ± standard error of the mean (SEM) of densitometric units after normalization with Grb2 levels, were determined by immunoblot of NBM (n = 7), CML-CP (n = 3), and CML-BC (n = 7) CD34+ progenitors, and Ph+ B-ALL (n = 5) and Ph− B-ALL (n = 4) CD34+/CD19+ progenitors. Bottom right panel: Sample of immunoblots used to determine XPO1 protein levels used for quantification. (B) Left panel: XPO1 protein levels expressed as mean ± SEM in vehicle- or imatinib-treated (1 µM, 12 hours) CML-BC CD34+ cells and Ph+ B-ALL CD34+/CD19+ cells. Right panel: Representative immunoblot of XPO1 protein levels and BCR-ABL1 activity (anti-PY) in vehicle- or imatinib-treated (1 µM, 12 hours) Ph+ B-ALL CD34+/CD19+ (lanes 1 and 2) and CML-BC CD34+ (lanes 3 and 4) cells. (C) Left panel: XPO1 protein levels in 32D-BCR/ABL cells treated (24 hours) with the indicated kinase inhibitors. Right panel: XPO1 mRNA levels assessed by quantitative reverse-transcription PCR in 32Dcl3 and untreated and kinase inhibitor–treated (24 hours) 32D-BCR/ABL cells. Asterisks indicate P values vs NBM; *P < .05, **P < .01.
XPO1 is important for Ph+ leukemia cell survival: in vitro KPT-330 antileukemic activity
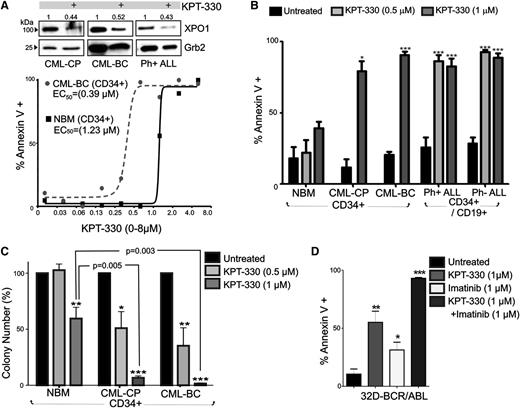
To determine the importance of XPO1 in BCR-ABL1 leukemogenesis, the clinically relevant SINE, KPT-330, was used to specifically inhibit XPO1 activity. CD34+ BM progenitors from patients with CML-CP (n = 3) and CML-BC (n = 4), CD34+/CD19+ progenitors from patients with Ph+ ALL (n = 3) and Ph− ALL (n = 3), and NBM CD34+ progenitors (n = 4) were treated with KPT-330 (0-8 µM, 72 hours) and were assayed for drug-induced apoptosis. Annexin-V/7-amino-actinomycin D staining revealed that KPT-330 triggered apoptosis in CD34+ leukemic (CML-BC) and normal (NBM) hematopoietic progenitors with an EC50 equal to 0.39 µM and 1.23 µM, respectively (Figure 2A). Notably, KPT-330 (1 µM) treatment induced apoptosis in ∼75% CML-CP and ∼95% CML-BC CD34+ BM cells, with no significant induction of apoptosis in KPT-330–treated CD34+ NBM samples (Figure 2B). Similarly, KPT-330 treatment (0.5-1 µM) of CD34+/CD19+ leukemic blasts isolated from patients with B-ALL also induced apoptosis in ∼85% of Ph+ and nearly 95% of Ph− leukemic progenitors (Figure 2B).
KPT-330 decreased survival and clonogenic potential in CML-BC and Ph+ B-ALL cells. (A) Top panel: Representative (n = 3) western blot showing XPO1 protein levels in vehicle- or KPT-330–treated (1 µM, 72 hours) CD34+ CML-CP and CML-BC, and CD34+/CD19+ Ph+ ALL progenitor cells. Numbers above the blots indicate relative densitometric units. Bottom panel: Graph shows percentage of apoptosis (annexin V+) in vehicle- or KPT-330–treated (0-8 µM, 72 hours) NBM and CML-BC CD34+ cells. EC50 was calculated as described in “Methods.” (B) Graph shows percentage of annexin V+ cells (mean ± SEM) in vehicle- and KPT-330 (0.5-1 µM, 72 hours)–treated NBM (n = 3), CML-CP (n = 3) and CML-BC (n = 3) CD34+ BM cells, and CD34+/CD19+ Ph+ B-ALL (n = 3) and Ph− B-ALL (n = 3) BM cells. (C) Colony-forming ability of vehicle- or KPT-330–treated (0.5-1 µM, 72 hours) NBM (n = 3), CML-CP (n = 3), and CML-BC (n = 3) CD34+ BM cells. Clonogenic potential, shown as mean ± SEM, was normalized to the respective untreated sample. (D) Annexin V+ cells (mean ± SEM) in 32D-BCR/ABL cells treated with KPT-330 (1 µM, 24 hours) and imatinib (1 µM, 24 hours) used alone or in combination. Significance was determined using the Student t test of 3 identical experiments. Asterisks indicate P values vs NBM; *P < .05, **P < .01, ***P < .001.
KPT-330 decreased survival and clonogenic potential in CML-BC and Ph+ B-ALL cells. (A) Top panel: Representative (n = 3) western blot showing XPO1 protein levels in vehicle- or KPT-330–treated (1 µM, 72 hours) CD34+ CML-CP and CML-BC, and CD34+/CD19+ Ph+ ALL progenitor cells. Numbers above the blots indicate relative densitometric units. Bottom panel: Graph shows percentage of apoptosis (annexin V+) in vehicle- or KPT-330–treated (0-8 µM, 72 hours) NBM and CML-BC CD34+ cells. EC50 was calculated as described in “Methods.” (B) Graph shows percentage of annexin V+ cells (mean ± SEM) in vehicle- and KPT-330 (0.5-1 µM, 72 hours)–treated NBM (n = 3), CML-CP (n = 3) and CML-BC (n = 3) CD34+ BM cells, and CD34+/CD19+ Ph+ B-ALL (n = 3) and Ph− B-ALL (n = 3) BM cells. (C) Colony-forming ability of vehicle- or KPT-330–treated (0.5-1 µM, 72 hours) NBM (n = 3), CML-CP (n = 3), and CML-BC (n = 3) CD34+ BM cells. Clonogenic potential, shown as mean ± SEM, was normalized to the respective untreated sample. (D) Annexin V+ cells (mean ± SEM) in 32D-BCR/ABL cells treated with KPT-330 (1 µM, 24 hours) and imatinib (1 µM, 24 hours) used alone or in combination. Significance was determined using the Student t test of 3 identical experiments. Asterisks indicate P values vs NBM; *P < .05, **P < .01, ***P < .001.
Accordingly, the cytokine-dependent colony-forming ability of CML (CP and BC) CD34+ progenitors was severely impaired in a dose-dependent manner by exposure to KPT-330. In fact, KPT-330 treatment (0.5-1 µM) significantly reduced the clonogenic potential of CML-CP by ∼45% (0.5 µM) and ∼95% (1 µM), and that of CML-BC by ∼65% (0.5 µM) and ∼100% (1 µM), with less substantial effects on the clonogenic potential of progenitors isolated from healthy donors, which was reduced 3% (0.5 µM) and 35% to 40% (1 µM) by KPT-330 treatment (Figure 2C). Notably, detrimental effects on survival of BCR-ABL1+ cells were also observed on treatment of 32D-BCR/ABL (p210) and Ba/F3-BCR/ABL (p190) with 2 other SINE compounds, KPT-207 (150 nM) and KPT-185 (150 nM) (supplemental Figure 1A), which are structurally similar to KPT-330, but with poor pharmacokinetic properties making them unsuitable for in vivo use.22,32 This impaired survival induced by SINEs may not only result from inhibition of XPO1 activity but also from their reported negative effect on XPO1 expression.36,37 Indeed, XPO1 levels were found decreased in KPT-330–treated CML (CP and BC) and Ph+ ALL CD34+ progenitors (Figure 2A). Interestingly, 24-hour treatment with KPT-330 and imatinib (1 µM each) markedly potentiated apoptosis of IL-3–cultured BCR-ABL1+ cells, which was nearly 95% compared with the ∼30% and ∼50% observed in imatinib-treated and KPT-330–treated IL-3–cultured cells, respectively (Figure 2D), suggesting that KPT-330 inhibits both XPO1-mediated BCR-ABL1–dependent and –independent leukemogenic signals. This finding is also supported by the evidence that KPT-330 specifically binds only XPO1 and interacts, but not inhibits, Aurora A and p70S6 kinases at 10-μM concentration in functional LANCE TR-FRET assays (data not shown).
Molecular mechanisms of KPT-330 antileukemic activity in BCR-ABL1+ cells
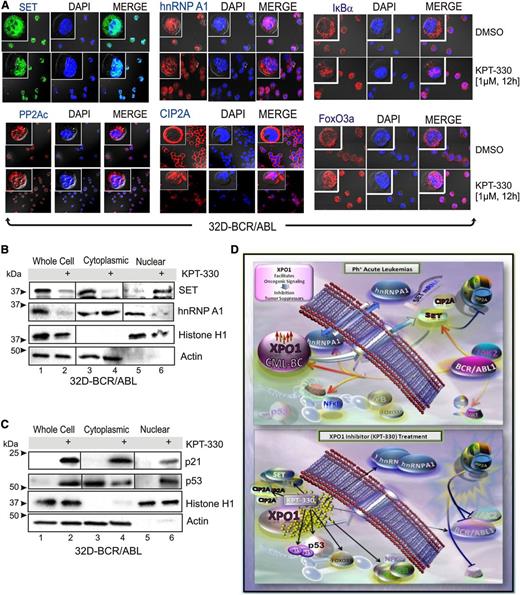
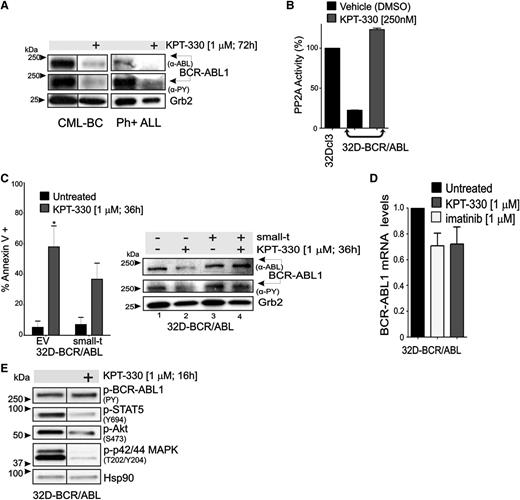
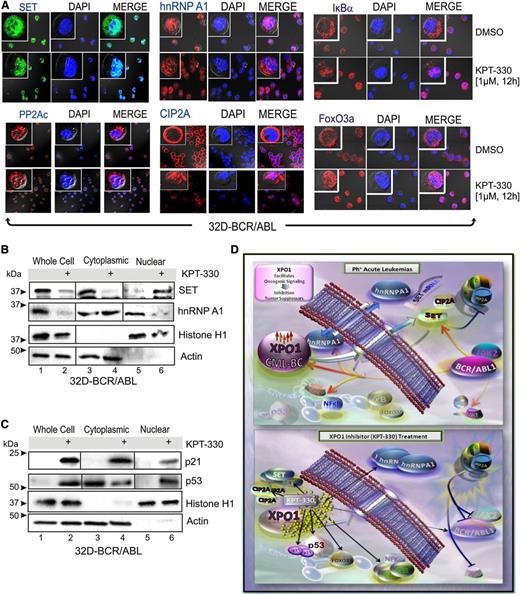
Because XPO1 is predicted to target hnRNP A138 and has been shown to directly interact with both BCR-ABL139 and the PP2A inhibitors SET40 and CIP2A,41 we sought to investigate whether KPT-330–induced apoptosis of Ph+ leukemic blasts depends, at least in part, on interference with the BCR-ABL1/Jak2–induced hnRNP A1/SET pathway responsible for inhibition of PP2A tumor suppressor activity in Ph+ acute leukemias15,33,34 (Figure 3). Confocal microscopy and western blot of subcellular fractionated lysates revealed that treatment of 32D-BCR/ABL cells with KPT-330 (1 µM, 12 hours) sequestered the SET and CIP2A proteins in the nucleus, without altering the subcellular localization of PP2Ac (Figure 3A-B). Similarly, KPT-330 treatment also resulted in altered subcellular localization of hnRNP A1 that, surprisingly, accumulated in the cytoplasm rather than in the nucleus of 32D-BCR/ABL cells (Figure 3A-B). Interestingly, localization of HA-tagged shuttling-deficient hnRNP A1 mutants35 carrying either the G274A mutation in the nuclear import/export M9 domain (hnRNP A1-G274A-HA), which makes hnRNP A1 protein cytoplasmic, or a bipartite-basic-type nuclear localization signal together with the G274A mutation, which has a defined nuclear localization, was not altered on inhibition of XPO1 activity by treatment with KPT-330 (supplemental Figure 2). This finding suggests that the integrity of the M9 domain is important for the effect of XPO1 on both hnRNP A1 localization and nuclear export of hnRNP A1 mRNA cargo. Moreover, consistent with the effect of KPT-SINE compounds in other hematologic malignancies,22,24-30,32 confocal microscopy (Figure 3A) and subcellular fractionation (Figure 3C) analyses revealed that KPT-330 treatment (1 µM, 72 hours) induced a nuclear accumulation of IkBα, FoxO3a, and p53, and enhanced levels of the p53-target p21. Conversely, we did not observe a nuclear accumulation of either p210 or p190 BCR-ABL1 (not shown); however, both p190 and p210 BCR-ABL1 decreased in amount and activity in KPT-330–treated (1 µM, 72 hours) CD34+ CML-BC (n = 3) and CD34+/CD19+ B-ALL (n = 3) progenitors (Figure 4A). This was likely the result of the nuclear accumulation of SET and CIP2A, and subsequent restoration of PP2A activity as was observed in KPT-330–treated (250 nM, 48 hours) 32D-BCR/ABL cells, which presented levels of active PP2A similar to parental 32Dcl3 cells (Figure 4B).
KPT-330 treatment alters the subcellular localization of tumor suppressors and negative regulators of PP2A. (A) Single-channel and merged confocal micrographs of 32D-BCR/ABL cells treated with vehicle or KPT-330 (1 µM, 12 hours) and stained with anti-SET, anti-PP2Ac, anti-hnRNP A1, anti-CIP2A, anti-IkBα, or anti-FoxO3a antibody (left panels; green or red), 4,6 diamidino-2-phenylindole (middle; blue), and merged (right). SET and hnRNP A1 (B), and p21 and p53 (C) protein levels in nuclear and cytoplasmic subcellular fractionated extracts from vehicle- and KPT-330–treated (1 µM, 12 hours) 32D-BCR/ABL cells. Histone H1 and Grb2 levels were used as a control for purity of nuclear and cytoplasmic fractions, respectively. (D) KPT-330–mediated XPO1 inhibition abrogates leukemogenesis by altering nuclear/cytoplasmic shuttling. In Ph+ acute leukemia progenitors, XPO1 expression is increased at least in part through a BCR-ABL1 kinase-dependent mechanism, and is responsible for nuclear export of the SET oncogene and CIP2A, and for the nucleocytoplasmic shuttling activity of hnRNP A1, a regulator of SET mRNA metabolism. SET and CIP2A are BCR-ABL1/Jak2- and BCR-ABL1-regulated inhibitors of the PP2A tumor suppressor, respectively. In these cells, XPO1 activity also controls the subcellular localization of important regulators of cell survival as p53, p21, IκBα, and FoxO3a. Bottom panel: On inhibition of XPO1 activity with the SINE KPT-330, the SET and CIP2A proteins are sequestered in the nucleus, which leads to activation of PP2A that, in turn, triggers inhibition/degradation of BCR-ABL1 contributing to cell death. Cytoplasmic accumulation of hnRNP A1 also contributes to the decreased SET levels. In addition, nuclear accumulation of p53, p21, IκBα, and FoxO3a also likely contribute to impair leukemogenesis of Ph+ acute leukemia progenitors.
KPT-330 treatment alters the subcellular localization of tumor suppressors and negative regulators of PP2A. (A) Single-channel and merged confocal micrographs of 32D-BCR/ABL cells treated with vehicle or KPT-330 (1 µM, 12 hours) and stained with anti-SET, anti-PP2Ac, anti-hnRNP A1, anti-CIP2A, anti-IkBα, or anti-FoxO3a antibody (left panels; green or red), 4,6 diamidino-2-phenylindole (middle; blue), and merged (right). SET and hnRNP A1 (B), and p21 and p53 (C) protein levels in nuclear and cytoplasmic subcellular fractionated extracts from vehicle- and KPT-330–treated (1 µM, 12 hours) 32D-BCR/ABL cells. Histone H1 and Grb2 levels were used as a control for purity of nuclear and cytoplasmic fractions, respectively. (D) KPT-330–mediated XPO1 inhibition abrogates leukemogenesis by altering nuclear/cytoplasmic shuttling. In Ph+ acute leukemia progenitors, XPO1 expression is increased at least in part through a BCR-ABL1 kinase-dependent mechanism, and is responsible for nuclear export of the SET oncogene and CIP2A, and for the nucleocytoplasmic shuttling activity of hnRNP A1, a regulator of SET mRNA metabolism. SET and CIP2A are BCR-ABL1/Jak2- and BCR-ABL1-regulated inhibitors of the PP2A tumor suppressor, respectively. In these cells, XPO1 activity also controls the subcellular localization of important regulators of cell survival as p53, p21, IκBα, and FoxO3a. Bottom panel: On inhibition of XPO1 activity with the SINE KPT-330, the SET and CIP2A proteins are sequestered in the nucleus, which leads to activation of PP2A that, in turn, triggers inhibition/degradation of BCR-ABL1 contributing to cell death. Cytoplasmic accumulation of hnRNP A1 also contributes to the decreased SET levels. In addition, nuclear accumulation of p53, p21, IκBα, and FoxO3a also likely contribute to impair leukemogenesis of Ph+ acute leukemia progenitors.
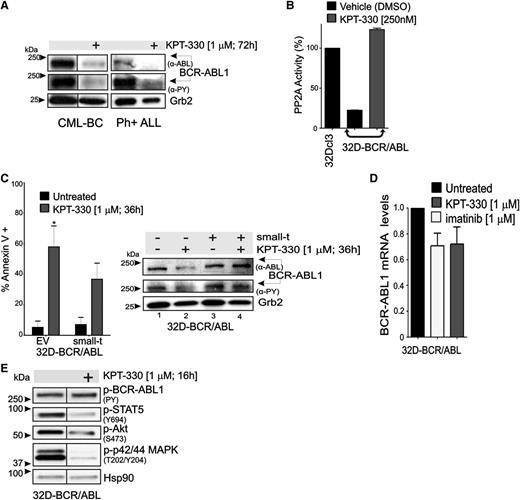
KPT-330 treatment increases PP2A activity and downregulates BCR-ABL1 expression and activity. (A) Representative (n = 3) western blot showing BCR-ABL1 activity (anti-PY) and expression (anti-ABL) in vehicle- and KPT-330–treated CD34+ CML-BC cells and CD34+/CD19+ Ph+ ALL cells. (B) PP2A activity in 32Dcl3 (positive control), vehicle- and KPT-330–treated (250 nM; 48 hours) 32D-BCR/ABL cells. PP2A activity was normalized to 32Dcl3 cells. (C) Left panel: Graph shows percentage of apoptotic cells (annexin V+) in vehicle- or KPT-330–treated (1 µM, 36 hours) parental and small-t–expressing 32D-BCR/ABL cells. Significance was determined using the Student t test of 3 identical experiments. Asterisks indicate P values vs untreated; *P < .05. Right panel: BCR-ABL1 activity (anti-PY) and expression (anti-ABL) in vehicle- and KPT-330 (1 µM, 24 hours)–treated parental and small-t–expressing 32D-BCR/ABL cells. (D) Quantitative reverse-transcription PCR shows BCR-ABL1 mRNA levels in untreated, imatinib (1 µM, 24 hours)–treated, and KPT-330 (1 µM, 24 hours)–treated 32D-BCR/ABL1 cells. (E) Western blot shows effect of KPT-330 (1 µM, 16 hours) on the activity of BCR-ABL1 (anti-PY), STAT5 (anti-pSTAT5Y694), Akt (anti-pAktS473), and p42/44 MAPK (anti-pMAPKT202/Y204). Heat shock protein 90 was used as a control for equal loading.
KPT-330 treatment increases PP2A activity and downregulates BCR-ABL1 expression and activity. (A) Representative (n = 3) western blot showing BCR-ABL1 activity (anti-PY) and expression (anti-ABL) in vehicle- and KPT-330–treated CD34+ CML-BC cells and CD34+/CD19+ Ph+ ALL cells. (B) PP2A activity in 32Dcl3 (positive control), vehicle- and KPT-330–treated (250 nM; 48 hours) 32D-BCR/ABL cells. PP2A activity was normalized to 32Dcl3 cells. (C) Left panel: Graph shows percentage of apoptotic cells (annexin V+) in vehicle- or KPT-330–treated (1 µM, 36 hours) parental and small-t–expressing 32D-BCR/ABL cells. Significance was determined using the Student t test of 3 identical experiments. Asterisks indicate P values vs untreated; *P < .05. Right panel: BCR-ABL1 activity (anti-PY) and expression (anti-ABL) in vehicle- and KPT-330 (1 µM, 24 hours)–treated parental and small-t–expressing 32D-BCR/ABL cells. (D) Quantitative reverse-transcription PCR shows BCR-ABL1 mRNA levels in untreated, imatinib (1 µM, 24 hours)–treated, and KPT-330 (1 µM, 24 hours)–treated 32D-BCR/ABL1 cells. (E) Western blot shows effect of KPT-330 (1 µM, 16 hours) on the activity of BCR-ABL1 (anti-PY), STAT5 (anti-pSTAT5Y694), Akt (anti-pAktS473), and p42/44 MAPK (anti-pMAPKT202/Y204). Heat shock protein 90 was used as a control for equal loading.
To determine the relative contribution of PP2A in mediating the effects of KPT-330 on BCR-ABL1 activity/expression and survival of CML-BC progenitors, PP2A activity was constitutively suppressed in 32D-BCR/ABL cells on expression of the SV40 small-t.34 Annexin V/7-amino-actinomycin D staining revealed that KPT-330 (1 µM, 36 hours) induced apoptosis in ∼60% and ∼35% of parental and small-t–expressing 32D-BCR/ABL cells, respectively (Figure 4C, left), indicating that ∼50% of KPT-330–induced apoptosis is mediated by activation of PP2A. Accordingly, small-t expression prevented the KPT-330–induced downregulation of BCR-ABL1 expression and activity (Figure 4C, right). Consistent with the existence of a KPT-330–induced PP2A-dependent posttranslational control of BCR-ABL1 activity/expression, BCR-ABL1 mRNA levels were not significantly changed in KPT-330–treated cells (1 µM, 36 hours) compared with untreated and imatinib (1 µM, 36 hours)–treated 32D-BCR/ABL cells (Figure 4D). Nonetheless, short exposure to KPT-330 (1 µM, 16 hours) did not reduce BCR-ABL1 activity, but it impaired that of STAT5, Akt, and p42/44 MAPKs (Figure 4E), suggesting that the proapoptotic activity of KPT-330 does not totally rely on inhibition of BCR-ABL1 kinase activity.
Similar to KPT-330, treatment with KPT-185 and/or KPT-207 also resulted in altered expression of the BCR-ABL1 downstream effectors hnRNP A1, hnRNP E2, and hnRNP K; inactivation and downregulation of BCR-ABL1 itself; activation of PP2A; and downregulation of the BCR-ABL1–target and hnRNP K-target c-Myc, likely as a result of both hnRNP K downregulation and PP2A activation (supplemental Figure 1). Thus, KPT-330 triggers apoptosis of BCR-ABL1–expressing cells by activating tumor suppressor proteins such as PP2A and p53, and inhibiting the activity of oncogenes and proliferation/survival factors (eg, BCR-ABL1, Akt, STAT5, MAPK, c-Myc, and nuclear factor κB) (Figure 3D).
KPT-330 exerts strong antileukemic activity in a CML-BC mouse model
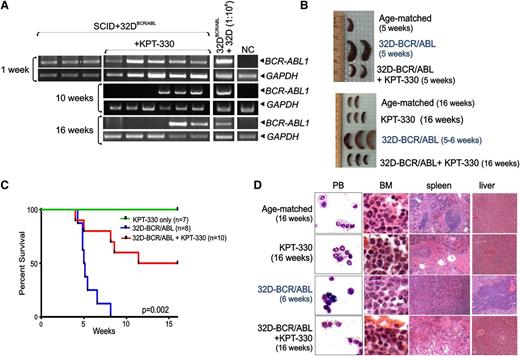
To evaluate the therapeutic relevance of KPT-330 in Ph+ acute leukemias in vivo, we used an allograft model of CML-BC in which ICR-SCID mice were intravenously injected with 32D/BCR-ABL cells. After 1 week, nested RT-PCR–mediated detection of BCR-ABL1 transcripts in PB of cell-injected animals (n = 20) confirmed engraftment of BCR-ABL1–expressing cells (Figure 5A). RNA extracted from a 1:106 mixture of BCR-ABL1+ vs parental 32Dcl3 cells, and from PB of age-matched mice was used as positive and negative controls, respectively (Figure 5A). Treatment with KPT-330 (15 mg/kg; twice per week by oral gavage) was thereafter started in leukemic mice (n = 12). As controls, a group of leukemic mice (n = 8) received vehicle only, whereas a group of healthy animals (n = 7) received KPT-330 at the same regimen. When half of the vehicle-treated mice died (5 weeks after cell injection), 2 moribund untreated animals, 2 KPT-330–treated animals, and 1 age-matched healthy animal were euthanized and evaluated for signs of leukemia. Although vehicle-treated animals displayed massive splenomegaly (Figure 5B) due to marked infiltration of immature myeloid cells (see also Figure 5D), gross examination (Figure 5B), and histopathologic examination (not shown) of spleens from KPT-330–treated mice appeared similar to that of the age-matched animal (Figure 5B). Untreated leukemic mice all died with a median survival time of 5 weeks, whereas 50% of KPT-330–treated animals died of leukemia mostly between week 8 and 11 after cell injection (Figure 5C). At weeks 10 and 16 after cell injection, nested RT-PCR on PB revealed that 3 of the living KPT-330–treated leukemic mice tested negative for BCR-ABL1 (Figure 5A). Because after 15 weeks of drug treatment (16 weeks after cell injection), 50% of the treated mice were still alive (Figure 5C) with no external signs of disease, all surviving KPT-330–treated animals (cell-injected and control drug–treated mice) and 2 age-matched healthy mice were euthanized, and hematopoietic and nonhematopoietic organs were macroscopically and microscopically inspected for signs of leukemia. Gross examination revealed no differences between the 2 KPT-330–treated groups and age-matched mice, including absence of splenomegaly in the drug-treated cell-injected mice (Figure 5B, bottom panel). Furthermore, Wright-Giemsa–stained PB cytospins and H&E-stained sections of BM, spleen, and liver from KPT-330–treated (16 weeks) leukemic animals showed organ architecture and cellularity similar to those of drug-treated or age-matched control animals with no infiltration of leukemic blasts, which were readily detectable in PB and organs of untreated leukemic animals (Figure 5D). Overall, KPT-330 treatment significantly increased the survival time (P = .002) of leukemic mice by antagonizing disease evolution and, as expected without inducing either toxicity or abnormal behavior in nonleukemic KPT-330–treated animals, which were all alive at the end of the study (Figure 5C).
KPT-330 treatment increases survival time of leukemic mice. (A) Nested RT-PCR for BCR-ABL1 mRNA in the PB measured 1, 10, and 16 weeks after injection. PB of age-matched mice (NC) and a 1:106 dilution of 32D-BCR/ABL cells with 32Dcl3 cells were used as negative and positive controls, respectively. GAPDH mRNA levels were used as a control. (B) Gross anatomy of spleens isolated from vehicle- and KPT-330–treated (15 mg/kg, 2 times weekly) mice injected with 32D-BCR/ABL cells, age-matched controls, and/or KPT-330 only–treated mice at 5 weeks (top panel) and 16 weeks (bottom panel) after cell-injection. (C) Kaplan-Meier curve shows effect of KPT-330 treatment (15 mg/kg, 2 times weekly) on survival of SCID mice injected with 32D-BCR/ABL cells (n = 10, red line). Untreated mice injected with cells (n = 8, blue line) or KPT-330–treated mice that did not receive cells (n = 7, green line) were used as controls. Survival was calculated by the Kaplan-Meier method, and the log-rank test evaluated the differences among survival distributions: P = .002 (32D-BCR/ABL–untreated vs 32D-BCR/ABL KPT-330–treated mice). (D) Wright/Giemsa staining of PB and H&E staining of sections from the BM, spleen, and liver of untreated and KPT-330–treated control and cell-injected mice.
KPT-330 treatment increases survival time of leukemic mice. (A) Nested RT-PCR for BCR-ABL1 mRNA in the PB measured 1, 10, and 16 weeks after injection. PB of age-matched mice (NC) and a 1:106 dilution of 32D-BCR/ABL cells with 32Dcl3 cells were used as negative and positive controls, respectively. GAPDH mRNA levels were used as a control. (B) Gross anatomy of spleens isolated from vehicle- and KPT-330–treated (15 mg/kg, 2 times weekly) mice injected with 32D-BCR/ABL cells, age-matched controls, and/or KPT-330 only–treated mice at 5 weeks (top panel) and 16 weeks (bottom panel) after cell-injection. (C) Kaplan-Meier curve shows effect of KPT-330 treatment (15 mg/kg, 2 times weekly) on survival of SCID mice injected with 32D-BCR/ABL cells (n = 10, red line). Untreated mice injected with cells (n = 8, blue line) or KPT-330–treated mice that did not receive cells (n = 7, green line) were used as controls. Survival was calculated by the Kaplan-Meier method, and the log-rank test evaluated the differences among survival distributions: P = .002 (32D-BCR/ABL–untreated vs 32D-BCR/ABL KPT-330–treated mice). (D) Wright/Giemsa staining of PB and H&E staining of sections from the BM, spleen, and liver of untreated and KPT-330–treated control and cell-injected mice.
Antileukemic activity of KPT-330 in a patient with TKI-resistant CML-AP
A 37-year-old man with CML-AP, hyperleukocytosis (WBC count >300 × 103/µL), splenomegaly (13 cm below the costal margin), and bone pain, whose disease was refractory to all available TKIs (including ponatinib) and to interferon, omacetaxine, and azacitidine, received a run-in dose of KPT-330 (12 mg/m2) for 1 week and, thereafter, 3 oral therapeutic doses of KPT-330 (16.5 mg/m2) on a compassionate-use protocol after refusing BM transplant. After the first therapeutically effective dose (Table 1), the patient experienced a significant reduction in bone pain, splenomegaly (from 13 cm to 4 cm below the costal margin), WBC count (from 3 × 105/µL to 7 × 103/µL), and serum LDH levels (from 513 IU/L to 264 IU/L). Blood smears revealed a dramatic reduction, albeit not a complete disappearance, of immature myeloid blasts. After 1 week from the third KPT-330 dose, WBC count (37 × 103/µL) and LDH levels (640 IU/L) increased. At this time, the patient declined to undergo KPT-330 dose escalation. Thus, the therapeutic efficacy of higher KPT-330 doses in this patient remains unknown, although the effect of KPT-330 dose escalation is currently being evaluated in other patients.
Discussion
Despite BCR-ABL1 playing a pivotal role in the emergence and maintenance of CML-BC and Ph+ B-ALL,1,10 the genetic heterogeneity of these malignancies may account for the lack of long-term response to TKI-based therapies.1,4,7,9 In fact, high levels of BCR-ABL1 expression/activity in Ph+ acute leukemias aberrantly enhances genomic instability that, together with unfaithful DNA repair machinery, leads to an accumulation of molecular and chromosomal abnormalities, some of which result in the inactivation of tumor suppressors and/or activation of oncogenic factors acting in a BCR-ABL1–independent fashion.1,42,43 Thus, successful therapy for Ph+ acute leukemias has to rely on the simultaneous targeting of BCR-ABL1 and other pathways playing an important role in the survival of leukemic stem/progenitor cells.
Indeed, we showed that BCR-ABL1 kinase-dependent and kinase-independent signals, likely involving PI-3K and PKCs, contribute to enhance transcription of the nuclear export regulator XPO1 that, in turn, controls the survival of Ph+ acute leukemia progenitors. Although this mechanism is not surprising given the involvement of XPO1 in several malignancies22,24-30,32 and its pleiotropic activity on many cell functions,31,44 the availability of specific inhibitors of XPO145 (eg, KPT-330) with an acceptable safety profile make XPO1 an attractive target for therapy for these unmanageable leukemias. In fact, KPT-330, a small molecule already in phase 1 clinical trials for solid tumors and hematologic malignancies, not only efficiently and selectively impaired the leukemogenic potential of CML-BC and Ph+ B-ALL BM progenitors and/or BCR-ABL1+ cell lines in both ex vivo and in an animal model, but also markedly reduced disease burden (eg, WBC counts and splenomegaly) in a patient with CML-AP. Notably, the KPT-330 concentrations used in all the ex vivo and in vivo studies were achieved in the current phase 1 trials with a dose of 30 mg/m2, which is below the maximal tolerated dose.46
This, together with the evidence showing that the increased survival time of long-term (15 weeks) KPT-330–treated leukemic animals, which was associated with the absence of BCR-ABL1+ circulating cells in 60% of treated mice, suggests that KPT-330 may safely be used in patients with Ph+ acute leukemia whose disease becomes refractory to TKI-based therapies.4 Moreover, we also provide evidence that KPT-330 potentiates the effect of imatinib, suggesting that KPT-330 might be used in combination with TKIs, especially in those cases in which TKI resistance is due to BCR-ABL1 amplification and blastic transformation.39,47 In fact, the first XPO1 inhibitor discovery LMB, which shares the same XPO1-interacting surface with the SINEs but because of its lack of specificity is severely toxic when used in patients with cancer,31,48 was elegantly shown to induce apoptosis of BCR-ABL1+ cells when used in combination with imatinib through a mechanism involving the LMB-induced nuclear entrapment and inactivation/degradation of BCR-ABL1.39 In KPT-330–treated BCR-ABL1+ cells, although we did not observe a BCR-ABL1 nuclear relocation (not shown), BCR-ABL1 expression/activity was markedly reduced, likely through posttranslational and not transcriptional mechanisms, in primary CD34+ CML-BC and Ph+ B-ALL BM cells, suggesting that other XPO1 targets might account for BCR-ABL1 downregulation. Similar effects on other oncogenic tyrosine kinases including Flt324 and BTK25 have been reported in other hematologic malignancies.
Among the numerous XPO1 targets44 there are the endogenous negative regulators of PP2A, SET,40 and CIP2A,44 which were described as BCR-ABL1–regulated factors active in Ph+ leukemias33,34,41,49 (Figure 3D). Here, we reported that KPT-330 treatment led to nuclear accumulation of SET and CIP2A proteins and, unexpectedly, of cytoplasmic relocation of the nucleocytoplasmic shuttling RNA-binding protein hnRNP A1. Notably, hnRNP A1 is an important regulator of BCR-ABL1 leukemogenesis and controls SET mRNA nuclear export11,15,33 (Figure 3). Specifically, hnRNP A1 expression and nuclear export activity is enhanced in a dose- and kinase-dependent manner by BCR-ABL1 that, in turn, positively regulates the nuclear export of hnRNP A1 target mRNAs (eg, SET, E2F3, and Bcl-XL.) and subsequently their protein levels.11,15,33,35 Because KPT-330 counteracts the RanGTP-mediated XPO1 transport of its cargo out of the nucleus and not vice versa,19,31,32 it is unlikely that hnRNP A1 is directly exported by XPO1 even if in silico analysis38 predicts hnRNP A1 to be an XPO1 target. Nonetheless, we cannot exclude the possibility that KPT-330 may interfere with the hnRNP A1 M9–mediated nuclear export/import that is facilitated by transportin 1 (importin β2), a protein similar to the XPO1 targets importin α1, α3, and α7.19,44,50 Indeed, we provided evidence that shuttling-deficient M9 mutant hnRNP A1 proteins35 are insensitive to KPT-330–mediated XPO1 inhibition.
Consistent with the effect of SET short hairpin RNA and with that of PP2A-activating drugs that prevent SET-PP2A interaction,33,51 the reduction of cytoplasmic SET and, likely, CIP2A41 levels induced not only by KPT-330 but also by the other structurally related SINEs (KPT-185 and KPT-207) resulted in reactivation of the PP2A tumor suppressor in BCR-ABL1+ myeloid precursors. Supporting the possibility that PP2A may play an important role as a mediator of KPT-330 antileukemic activity in Ph+ acute malignancies stands also the evidence gained by using BCR-ABL1+ cells engineered to express the PP2A inhibitor small-t. In fact, it appears that PP2A accounts for ∼50% of KPT-330–induced apoptosis. Importantly, rescue of PP2A activity in primary CML-BC and Ph+ B-ALL progenitors as well as in animal models of TKI-sensitive and TKI-resistant Ph+ acute leukemias, is an event that, per se, is sufficient to catalyze a cascade of antimitotic and proapoptotic signals (including the Src homology phosphatase-1–mediated inactivation and proteasome-dependent degradation of BCR-ABL1), leading to killing of leukemic, but not normal, hematopoietic stem/progenitor cells.1,10,51 Thus, it is not surprising that KPT-330 and other SINEs induced a marked PP2A-dependent (small-t–sensitive) BCR-ABL1 inactivation/downregulation that, in turn, might also be responsible for the observed downregulation of hnRNP K and its target c-Myc, and of the differentiation-inhibitory factor hnRNP E2.15
Although important, KPT-330–induced PP2A reactivation is likely not the only mechanism responsible for apoptosis of drug-treated Ph+ acute leukemic progenitors. In fact, KPT-330 treatment of BCR-ABL1+ myeloid precursors induced nuclear accumulation of the p53 tumor suppressor and its transcriptional target p21, the reactive oxygen species detoxifier and antagonist of cell cycle progression FoxO3a, and the negative nuclear factor κB regulator IKBα, in agreement with the effect of SINEs on these XPO1 targets in other myeloid and lymphoid malignancies.22-26,28,32,52 Likewise, it seems that KPT-330 negatively also regulates the activity of BCR-ABL1 downstream effectors such as STAT5, Akt, and MAPK independently from downregulation of BCR-ABL1 kinase activity. Note that binding and kinase assays exclude a direct effect of KPT-330 on these molecules and other factors regulating cell proliferation and survival (not shown).
Although further data on the effectiveness of KPT-330 in metastatic solid tumors and advanced hematologic malignancies as well as its safety, tolerability, and adverse effects will soon be available through the two phase 1 clinical trials, our preclinical and preliminary clinical data strongly support the immediate evaluation of this drug in patients with CML-BC and Ph+ B-ALL whose disease is refractory to the available TKI-based therapies.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Parvathi Ranganathan, Rosa Lapalombella, and John C. Byrd for scientific discussion and reagents. The hTERT.BMS mesenchymal cell line was kindly provided by Dr. Dario Campana. We are grateful to Stephen Lee for editorial assistance.
This work has been supported in part by National Institutes of Health-National Cancer Institute grants CA095512 and CA163800 (D.P.), grant CA16058 from The OSU Comprehensive Cancer Cancer), and the Leukemia and Lymphoma Society Scholarship Program (D.P.). C.J.W. is a graduate student of the OSU Molecular, Cellular and Developmental Biology program.
Authorship
Contribution: C.J.W., J.J.O., R.S., P.N., J.G.H., G.F., J.J.E., and A.-K.E. performed experiments; M.A.C., Y.L., P.H., D.C.R., J.M.G., J.A., A.R., D.M., R.G., G.M., S.S., and M.G.K. provided essential reagents including patient specimens and discussed results; C.L.S. and N.Y.G. treated the patient; C.J.W. and D.P., analyzed the results, designed the figures, and wrote the paper; and D.P. designed the research.
Conflict-of-interest disclosure: M.G.K., Y.L., and S.S. are employees of Karyopharm Therapeutics, a clinical-stage pharmaceutical company that develops SINE-targeted therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Danilo Perrotti, University of Maryland Greenebaum Cancer Center, 655 West Baltimore St, BRB 8045, Baltimore, MD 21201; e-mail: DPerrotti@som.umaryland.edu.