Key Points
TKIs impair B-cell immune responses in CML through off-target inhibition of kinases important for B-cell signaling.
Our results call for close monitoring of patients on TKI to assess the long-term impact of impaired B-cell function.
Abstract
Tyrosine kinase inhibitors (TKIs) have significant off-target multikinase inhibitory effects. We aimed to study the impact of TKIs on the in vivo B-cell response to vaccination. Cellular and humoral responses to influenza and pneumococcal vaccines were evaluated in 51 chronic phase chronic myeloid leukemia (CML) patients on imatinib, or second-line dasatinib and nilotinib, and 24 controls. Following vaccination, CML patients on TKI had significant impairment of IgM humoral response to pneumococcus compared with controls (IgM titer 79.0 vs 200 U/mL, P = .0006), associated with significantly lower frequencies of peripheral blood IgM memory B cells. To elucidate whether CML itself or treatment with TKI was responsible for the impaired humoral response, we assessed memory B-cell subsets in paired samples collected before and after imatinib therapy. Treatment with imatinib was associated with significant reductions in IgM memory B cells. In vitro coincubation of B cells with plasma from CML patients on TKI or with imatinib, dasatinib, or nilotinib induced significant and dose-dependent inhibition of Bruton’s tyrosine kinase and indirectly its downstream substrate, phospholipase-C-γ2, both important in B-cell signaling and survival. These data indicate that TKIs, through off-target inhibition of kinases important in B-cell signaling, reduce memory B-cell frequencies and induce significant impairment of B-cell responses in CML.
Introduction
The tyrosine kinase inhibitors (TKIs) imatinib, nilotinib, and dasatinib are remarkably effective as single-agent therapy for chronic myeloid leukemia (CML) in chronic phase (CP).1-3 To date, very few in vivo human studies have addressed the long-term impact of these molecular-targeted drugs on the immune function. Data from in vitro and animal studies have documented seemingly contradictory effects of imatinib on the immune response, ranging from impaired antigen-specific T-cell responses4-6 to reversal of T-cell tolerance7 and potentiation of antitumor immune responses.8,9 The limited in vitro data available with second-generation TKIs nilotinib (Novartis) and dasatinib (Bristol-Myers Squibb) show impaired antigen-specific T-cell responses10-15 ; however, recent studies report rapid mobilization and expansion of BCR-ABL–negative lymphoid cells in dasatinib-treated patients.16-18 Few studies have examined the impact of TKIs on B-cell responses to antigen in vivo,19 although hypogammaglobulinemia has been reported in CML patients treated with imatinib.20 A recent murine study reported that imatinib may directly impair class-switch recombination following B-cell activation through downregulation of activation-induced cytidine deaminase.21 To date, no studies have examined the impact of first- and second-generation TKIs on B-cell responses to vaccination in patients with CML.
We hypothesized that TKI may interfere with vaccine-induced cellular and humoral immune responses in CML patients on TKI through their off-target multikinase inhibitory effects.11,22,23 We characterized T- and B-cell responses to vaccination against influenza and pneumococcus in CP-CML patients receiving imatinib, dasatinib, and nilotinib and healthy controls. We found that the B-cell response to pneumococcal vaccine is significantly impaired in CML patients, associated with loss of memory B-cell subsets. Furthermore, we showed that all 3 TKIs suppress an important kinase in B-cell receptor (BCR) signaling and survival, namely, Bruton’s tyrosine kinase (Btk), and indirectly its downstream substrate phospholipase C (PLC)–γ2 in a dose-dependent manner. Our findings suggest that TKIs may interfere with B-cell activation and induction of humoral immune responses in vivo through their off-target multikinase inhibitory effects.
Design and methods
Patients and controls
Fifty-one CP-CML patients in complete cytogenetic response (CCyR) on standard-dose imatinib (n = 26), dasatinib (n = 13), or nilotinib (n = 12) and 24 adult controls were recruited in this study during 2 influenza seasons (2008 and 2009). Patient characteristics are summarized in Tables 1 and 2. All patients were on second-line therapy with dasatinib or nilotinib and had failed previous therapy with imatinib (supplemental Table 1; see the Blood Web site). Healthy controls were recruited among hospital staff. The median age for CML patients was 52 years and for controls 41 years (P = .10). All patients and controls were vaccinated against influenza (Influenza vaccine Ph. Eur. 2008/2009 or 2009/2010; CSL Biotherapies, Germany) and the pandemic influenza A (H1N1) in 2009.24 Forty-five of 51 CML patients and 12 of 24 healthy controls were concomitantly immunized with the 23-valent polysaccharide pneumococcal (PPS) vaccine (Pneumovax-II; Sanofi Pasteur MSD, United Kingdom). Only patients and controls who had not received a pneumococcal vaccine within the previous 5 years were reimmunized.
Peripheral blood mononuclear cells (PBMCs) and serum samples were collected from all patients and donors prior to vaccination, and responses were assessed at 4 weeks and at 2 to 3 months postimmunization. All patients and adult controls gave informed written consent, and the local institutional ethics board approved the study protocol.
Determination of influenza-specific CD8+ and CD4+ T-cell responses by flow cytometry
Multiparameter flow cytometry was employed to assess immunologic T-cell responses to influenza virus both quantitatively by GILGFVFTL/HLA-201 (FluMP) pentamer staining and qualitatively using intracellular cytokine (IC) assay for tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), interleukin 2 (IL-2), and the cytotoxicity marker CD107a as described previously.25
Cells were acquired on FACSCalibur (BD Biosciences, Oxford, United Kingdom), and data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Determination of pneumococcal serum titers for immunoglobulins IgM and IgG
Serum titers of pneumococcal IgM and IgG antibodies were determined using enzyme-linked immunosorbent assay as previously described.26
Determination of plasma levels of imatinib, dasatinib, and nilotinib
TKI and metabolite plasma detection was based on liquid chromatography–tandem mass spectrometry using the TurboFlow sample preparation as previously described.27
B-cell phenotyping
PBMCs were stained with phycoerythrin (PE)-cyanin7–conjugated anti-CD19 (Coulter Immunotech High Wycombe, United Kingdom), PE-conjugated anti-human IgD (Southern Biotechnology Associates, Birmingham, AL), antigen-presenting cell (APC)–conjugated anti-human IgM (The Jackson Laboratory, Bar Harbor, ME), and fluorescein isothiocyanate (FITC)–conjugated anti-CD27 (DakoCytomation, Glostrup, Denmark).
Intracellular phospho-specific flow cytometric assay
We assessed the impact of therapeutic doses of imatinib, dasatinib, and nilotinib on Btk phosphorylation using intracellular phospho-specific flow cytometric assay, following coincubation of B cells from CML patients on TKI therapy (imatinib, n = 3; nilotinib, n = 4; and dasatinib, n = 3) with autologous serum. Briefly, following stimulation with H2O2 for 15 minutes at 37°C, PBMCs were fixed and stained with PE-conjugated antiphosphorylated Btk (pBtk-PE) and APC-conjugated anti-CD19 (both BD Biosciences, San Jose, CA). Data were acquired on the FACSCalibur, and FlowJo was used for analysis.
To assess the impact of TKI on normal B cells, PBMCs from healthy controls were cultured in the presence or absence of increasing concentrations of TKIs, namely, 1 to 50 μM of imatinib, 1 to 25 μM of nilotinib, and 1 to 100 nM of dasatinib (all LC Laboratories, Woburn, MA) for 2 hours. PBMCs were then stimulated with goat anti-human IgG and IgM antigen-binding fragment [F(ab')2] (10 μg/mL) for 20 minutes at 37°C. Cells were stained with pBtk-PE or PE-conjugated antiphosphorylated PLC-γ2 (pPLC-γ2-PE), APC-conjugated anti-CD19 (BD Biosciences), PerCP-conjugated anti-human IgM (BD Biosciences, Oxford, United Kingdom), and FITC-conjugated anti-CD27 (DakoCytomation).
Statistical analysis
Fisher’s exact test was used to compare proportions. Continuous variables were compared using the Mann-Whitney test or the Kruskal-Wallis test. Paired samples were compared using the Wilcoxon signed rank. Multivariate analysis was performed using a logistic regression model. All reported P values are 2 sided. Analyses were performed using SPSS version 17 (IBM, Armonk, NY).
Results
Vaccination with influenza A induces CD8+ and CD4+ T-cell responses in patients on TKI and healthy controls
The induction of T-cell responses to flu vaccination was assessed directly ex vivo by flow-cytometric enumeration of antigen-specific CD8+ and CD4+ T lymphocytes using IC assay for IFN-γ and TNF-α. A T-cell response was defined to be flu-specific if at least 1 cytokine was detected following in vitro antigen stimulation. Before vaccination, T-cell responses against flu could be detected in 21 of 51 (41.2%) patients on TKI and 12 of 24 (50%) controls (P = .15), indicating the presence of preexisting memory T cells to flu in patients with CML on TKI and in healthy controls (Figure 1A).
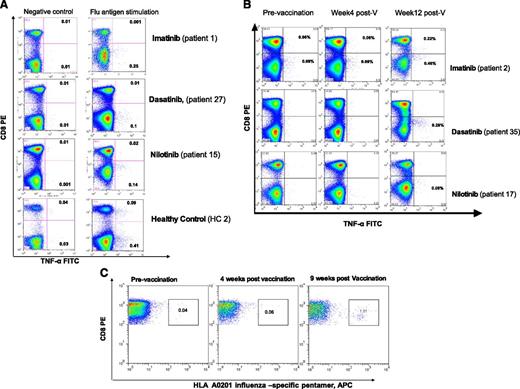
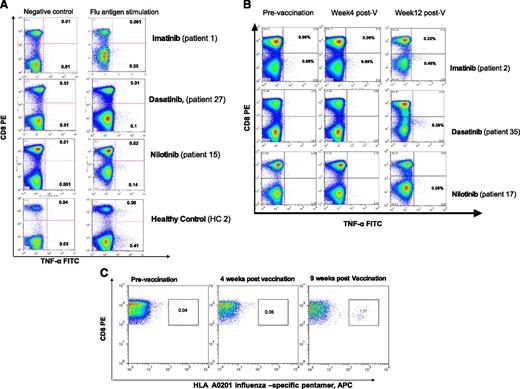
T-cell responses to influenza A vaccination in patients with CML on TKI and healthy controls. PBMCs collected before and 2 to 3 months postvaccination were thawed and stimulated for 24 hours with or without seasonal influenza vaccine at a final concentration of 1.5 µg/mL of hemagglutinin antigens or with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (2 μg/mL; Sigma Aldrich, Gillingham, United Kingdom) (positive control) for 19 hours at 37°C. Brefeldin A (10 µg/mL) (Sigma Aldrich) was added alone or with monensin (0.7 µL/mL) (BD/Pharmingen, San Diego, CA) and the degranulation marker CD107a-FITC (BD/Pharmingen). PBMCs were washed and stained with anti-CD3 and anti-CD8 antibodies, fixed/permeabilized (all BD Biosciences, Oxford, United Kingdom), and stained with anti–IFN-γ, anti–TNF-α, and anti–IL-2 antibodies (all BD/Pharmingen). Data acquisition was performed using FACSCalibur (BD Biosciences, Oxford, United Kingdom), and a minimum of 300 000 events were acquired. The threshold of positivity for cytokines and CD107a was set in order to minimize nonspecific staining in nonstimulated cells (negative control). Following vaccination, a response was considered positive if there was a minimum of 0.10% flu-specific T cells producing TNF-α or INF-γ and the percentage of antigen-specific T cells producing TNF-α or INF-γ was twofold or higher compared with prevaccination level. (A) Examples of preexisting CD8+ and CD4+ T-cell responses to influenza before vaccination in patients on TKI and a healthy control. (B) Examples of T-cell responses to influenza A vaccination in patients on TKI using IC assay. (C) Detection of influenza-specific CD8+ T cells using an HLA-A2–restricted GILGFVFTL (FluMP) pentamer: the fluorescence-activated cell sorter plot from a CML patient on dasatinib showing a robust CD8+ T-cell response to influenza vaccination is presented. Unstimulated PBMCs from HLA-A0201 patients and healthy controls were stained with HLA-A0201/GILGFVFTL (FluMP) Pro5 MHC I Pentamer (ProImmune, Oxford, United Kingdom) conjugated to APC and costained with anti–CD8-FITC (ProImmune) and anti–CD3-PerCP (BD Biosciences).
T-cell responses to influenza A vaccination in patients with CML on TKI and healthy controls. PBMCs collected before and 2 to 3 months postvaccination were thawed and stimulated for 24 hours with or without seasonal influenza vaccine at a final concentration of 1.5 µg/mL of hemagglutinin antigens or with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (2 μg/mL; Sigma Aldrich, Gillingham, United Kingdom) (positive control) for 19 hours at 37°C. Brefeldin A (10 µg/mL) (Sigma Aldrich) was added alone or with monensin (0.7 µL/mL) (BD/Pharmingen, San Diego, CA) and the degranulation marker CD107a-FITC (BD/Pharmingen). PBMCs were washed and stained with anti-CD3 and anti-CD8 antibodies, fixed/permeabilized (all BD Biosciences, Oxford, United Kingdom), and stained with anti–IFN-γ, anti–TNF-α, and anti–IL-2 antibodies (all BD/Pharmingen). Data acquisition was performed using FACSCalibur (BD Biosciences, Oxford, United Kingdom), and a minimum of 300 000 events were acquired. The threshold of positivity for cytokines and CD107a was set in order to minimize nonspecific staining in nonstimulated cells (negative control). Following vaccination, a response was considered positive if there was a minimum of 0.10% flu-specific T cells producing TNF-α or INF-γ and the percentage of antigen-specific T cells producing TNF-α or INF-γ was twofold or higher compared with prevaccination level. (A) Examples of preexisting CD8+ and CD4+ T-cell responses to influenza before vaccination in patients on TKI and a healthy control. (B) Examples of T-cell responses to influenza A vaccination in patients on TKI using IC assay. (C) Detection of influenza-specific CD8+ T cells using an HLA-A2–restricted GILGFVFTL (FluMP) pentamer: the fluorescence-activated cell sorter plot from a CML patient on dasatinib showing a robust CD8+ T-cell response to influenza vaccination is presented. Unstimulated PBMCs from HLA-A0201 patients and healthy controls were stained with HLA-A0201/GILGFVFTL (FluMP) Pro5 MHC I Pentamer (ProImmune, Oxford, United Kingdom) conjugated to APC and costained with anti–CD8-FITC (ProImmune) and anti–CD3-PerCP (BD Biosciences).
Following vaccination, flu-specific T-cells were induced in 24 of 51 (47.0%) patients on TKI (median 0.15% TNF-α+CD3+ T cells, range 0.05% to 0.64%) and 15 of 24 (62.5%) healthy controls (median 0.40% TNF-α+CD3+ T cells, range 0.12% to 2.0%), P = .16 (supplemental Table 2A-B; Figure 1B).
In 12 HLA-A*0201+ CML patients (including 3 imatinib-, 6 dasatinib-, and 3 nilotinib-treated patients) and 4 HLA-A*0201+ controls, we also confirmed the presence of circulating flu-specific memory CD8+ T cells by HLA-A2/FluMP pentamer staining. An increase of at least twofold flu-specific CD8+ T cells was detected in 2 of 4 controls and 5 of 12 patients (median 0.44% of total CD8+ T cells, range 0.1% to 1.51%). All patients and donors with detectable flu-specific CD8+ T cells by pentamer analysis also had detectable responses by IC staining, indicating that HLA-A2/FluMP CD8+ T cells are functional (data not shown). An example of a patient on dasatinib with a robust CD8+ T-cell response (patient 33) to influenza vaccination is presented in Figure 1C.
We next evaluated the functional quality of the influenza vaccine–induced T-cell response by flow-cytometric analysis of markers related to T-helper cell 1 (TNF-α, IFN-γ, and IL-2) and degranulation/cytotoxicity (CD107a) in individuals with a positive vaccine-induced T-cell response (responders) and in whom sufficient numbers of cells were available for analysis (ie, 9 of 24 “responders” in the CML and 7 of 15 “responders” in the healthy control groups). We found no significant differences in the quality of the T-cell response to influenza vaccine in the 2 groups as summarized in supplemental Table 2C.
CML patients on TKI achieve lower pneumococcal IgM titers after vaccination
Forty-five CML patients on TKI and 12 healthy controls were vaccinated against the PPS vaccine (Pneumovax II), and all could be evaluated for response. Prior to vaccination, the median pneumococcal IgG levels were 123 U/mL in CML patients compared with 71.5 U/mL in controls (P = .3); 16 of 45 patients and 3 of 12 controls had prevaccination pneumococcal IgG levels >200 U/mL (P = .7). In contrast, the prevaccine pneumococcal IgM levels were significantly lower in CML patients on TKI (median 15, range 3-72 U/mL) compared with healthy controls (median 38, range 13-78 U/mL), P = .002.
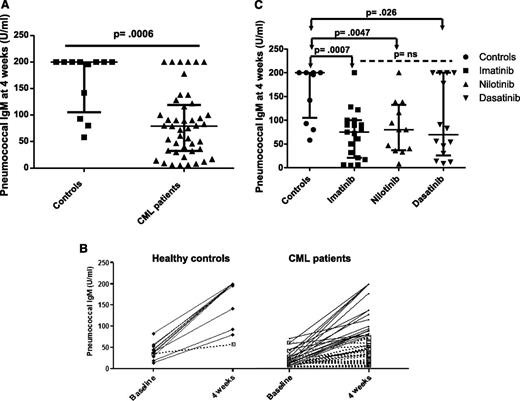
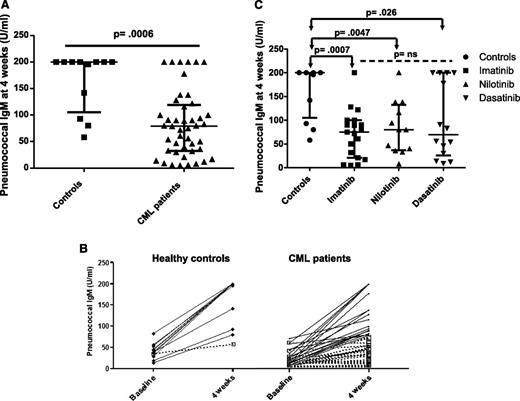
We assessed the humoral response to PPS vaccine by measuring pneumococcal IgM levels 4 weeks following vaccination. Eleven of 12 (92%) controls had a positive IgM humoral response (defined as a fourfold rise in serum IgM titer or IgM >200 U/mL postvaccination) compared with only 18 of 45 (40%) CML patients on TKI (P = .002). Moreover, pneumococcal IgM titers achieved at 4 weeks were significantly lower in CML patients on TKI compared with controls (median 79 U/mL, range 5-200 vs 200 U/mL, range 58-200, P = .0006; Figure 2A-B), supporting the notion that CML patients on TKI have an impaired IgM response to vaccination. Of note, we only found a weak correlation between the pre- and postvaccination IgM levels in CML patients on TKI (R2 = 0.17).
Pneumococcal IgM response following vaccination. (A) Pneumococcal IgM titers are presented at 4 weeks following vaccination in healthy controls and CML patients on TKI. A positive IgM pneumococcal response was defined as a fourfold rise in serum IgM titers or an IgM titer >200 U/mL 4 weeks postimmunization irrespective of the preimmunization titer. (B) The pneumococcal IgM response is presented before and 4 weeks after vaccination in responders (black lines) and nonresponders (dashed lines) for healthy controls and CML patients on TKI. (C) The postimmunization pneumococcal IgM titers are presented for CML patients on imatinib, nilotinib, and dasatinib. Bars represent medians with interquartile range.
Pneumococcal IgM response following vaccination. (A) Pneumococcal IgM titers are presented at 4 weeks following vaccination in healthy controls and CML patients on TKI. A positive IgM pneumococcal response was defined as a fourfold rise in serum IgM titers or an IgM titer >200 U/mL 4 weeks postimmunization irrespective of the preimmunization titer. (B) The pneumococcal IgM response is presented before and 4 weeks after vaccination in responders (black lines) and nonresponders (dashed lines) for healthy controls and CML patients on TKI. (C) The postimmunization pneumococcal IgM titers are presented for CML patients on imatinib, nilotinib, and dasatinib. Bars represent medians with interquartile range.
We also assessed humoral responses to the vaccine in patients and controls without prior evidence of pneumococcal infection or immunization (defined as IgG <200 U/mL prior to vaccination). In 24 of 45 evaluable patients, 6 of 24 (25%) failed to mount both an IgM and IgG response compared with 0 of 9 healthy controls (P = .15), suggesting the presence of global B-cell memory impairment in a proportion of CML patients on TKI. The characteristics of the 6 CML patients who failed to mount an IgM and IgG response to PPS vaccine are presented in Table 2. We found no significant differences in the underlying characteristics (including age, Sokal score, spleen size, duration, and response to TKI therapy) between the 6 of 24 patients who failed to mount a humoral response compared with the 18 of 24 responders.
We did not find significant differences in the postvaccine humoral response rates (7/19, 4/12, and 7/14; P = .87) or pneumococcal IgM serum titers (median 75, 80, and 69 U/mL; P = .7) in patients treated with imatinib, nilotinib, or dasatinib, respectively (Figure 2C), although our study was not specifically designed to look at differences in the 3 treatment groups.
IgM memory B cells are markedly reduced in CML patients who do not mount a pneumococcal IgM response after vaccination
To further elucidate the mechanisms underlying the impaired humoral immune response to PPS in patients with CML on TKI, we determined the percentages of IgM memory B cells (CD19+ CD27+ IgMhigh IgD+/lo) and switched memory B cells (CD19+ CD27+ IgM– IgD–) before and 4 weeks following immunization and correlated these with pneumococcal IgM and IgG levels, respectively. We had sufficient samples to perform this analysis in 39 patients and 10 healthy controls. Of note, the percentage of IgM memory and switched memory B cells did not differ significantly before and after immunization (data not shown).
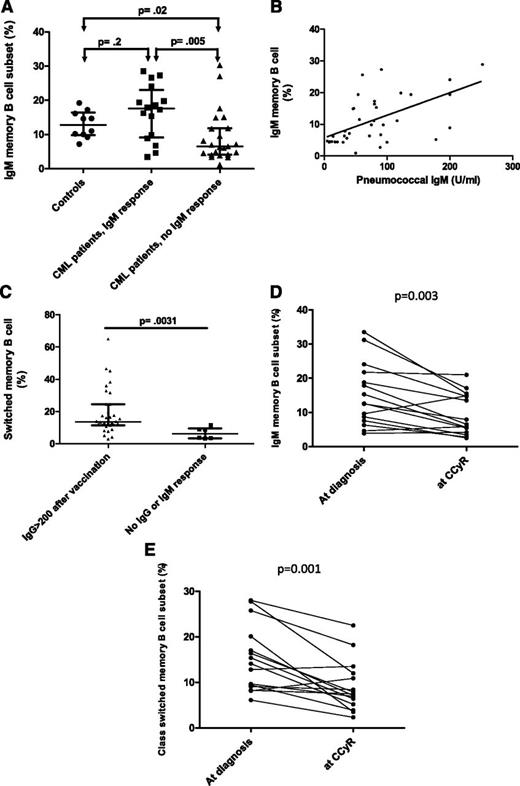
CML patients were stratified based on their 4-week pneumococcal IgM response into 2 groups of “vaccine responders” and “vaccine nonresponders” (Figure 2B). We found no significant difference in fold increase in antipneumococcal IgM response in CML responders and healthy controls (P = .5). In contrast, CML patients who failed to mount a pneumococcal IgM response had significantly lower IgM memory B-cell frequencies at vaccination compared with patients who mounted a positive pneumococcal IgM response (median 6.5% vs 17.6%, P = .005) and compared with healthy controls (median 6.5% vs 12.8%, P = .02) (Figure 3A).28 Furthermore, in patients with CML, we found a significant correlation between IgM memory B-cell frequencies at vaccination and the postvaccine pneumococcal IgM titer (R2 = 0.36, P < .0001) (Figure 3B). Interestingly, the IgM memory B-cell frequency for the 1 healthy donor who failed to mount a positive IgM pneumococcal vaccine response was within the normal range (14.6%).
Relationship between memory B-cell subsets and pneumococcal humoral response. PBMCs were incubated with PE-cyanin7–conjugated anti-CD19 (Coulter Immunotech High Wycombe), PE-conjugated anti-human IgD (Southern Biotechnology Associates), APC-conjugated anti-human IgM (The Jackson Laboratory), and FITC-conjugated anti-CD27 (DakoCytomation). Cells were then washed and acquired on FACSCalibur (BD Biosciences, Oxford, United Kingdom). A minimum of 5000 events were acquired on the B-cell gate, and the results are expressed as a percentage of CD19 events. FlowJo software (TreeStar) was used for data analysis. Because IgM memory B cells express both IgD and IgM, coexpression of either IgD or IgM together with CD27 was used to define this subset. IgM memory B cells (CD19+ CD27+ IgMhigh IgD+/lo) and switched memory B cells (CD19+ CD27+ IgM– IgD–) were calculated using a modified Piqueras classification.28 (A) Patients who fail to mount a pneumococcal IgM response have significantly lower frequencies of IgM memory B cells compared with responders and healthy controls. (B) Scatter plot evaluating the association between pneumococcal IgM titers and IgM memory B-cell frequencies in CML patients. Samples were correlated using the Spearman rank correlation test. (C) Frequencies of class-switched memory B cells in the 33 patients who achieved a postimmunization IgG >200 U/mL compared with the 6 patients who failed to mount a positive pneumococcal IgM and IgG response; bars represent medians with interquartile range. A positive IgM Pneumovax II response was defined as a fourfold rise in serum IgM titers or an IgM titer >200 U/mL 4 weeks postimmunization irrespective of the preimmunization titer. A positive IgG response was defined as a twofold rise in serum IgG titer or an IgG titer >200 U/mL at 1 or 3 months.26 (D) IgM memory B-cell frequencies at diagnosis (prior to initiation of imatinib) and once CCyR was achieved on imatinib. (E) Class-switched memory B-cell frequencies at diagnosis (prior to initiation of imatinib) and once CCyR was achieved on imatinib. (F) B-cell phenotype of a CML patient who developed a positive pneumococcal IgM response (patient A) compared with a nonresponder (patient B).
Relationship between memory B-cell subsets and pneumococcal humoral response. PBMCs were incubated with PE-cyanin7–conjugated anti-CD19 (Coulter Immunotech High Wycombe), PE-conjugated anti-human IgD (Southern Biotechnology Associates), APC-conjugated anti-human IgM (The Jackson Laboratory), and FITC-conjugated anti-CD27 (DakoCytomation). Cells were then washed and acquired on FACSCalibur (BD Biosciences, Oxford, United Kingdom). A minimum of 5000 events were acquired on the B-cell gate, and the results are expressed as a percentage of CD19 events. FlowJo software (TreeStar) was used for data analysis. Because IgM memory B cells express both IgD and IgM, coexpression of either IgD or IgM together with CD27 was used to define this subset. IgM memory B cells (CD19+ CD27+ IgMhigh IgD+/lo) and switched memory B cells (CD19+ CD27+ IgM– IgD–) were calculated using a modified Piqueras classification.28 (A) Patients who fail to mount a pneumococcal IgM response have significantly lower frequencies of IgM memory B cells compared with responders and healthy controls. (B) Scatter plot evaluating the association between pneumococcal IgM titers and IgM memory B-cell frequencies in CML patients. Samples were correlated using the Spearman rank correlation test. (C) Frequencies of class-switched memory B cells in the 33 patients who achieved a postimmunization IgG >200 U/mL compared with the 6 patients who failed to mount a positive pneumococcal IgM and IgG response; bars represent medians with interquartile range. A positive IgM Pneumovax II response was defined as a fourfold rise in serum IgM titers or an IgM titer >200 U/mL 4 weeks postimmunization irrespective of the preimmunization titer. A positive IgG response was defined as a twofold rise in serum IgG titer or an IgG titer >200 U/mL at 1 or 3 months.26 (D) IgM memory B-cell frequencies at diagnosis (prior to initiation of imatinib) and once CCyR was achieved on imatinib. (E) Class-switched memory B-cell frequencies at diagnosis (prior to initiation of imatinib) and once CCyR was achieved on imatinib. (F) B-cell phenotype of a CML patient who developed a positive pneumococcal IgM response (patient A) compared with a nonresponder (patient B).
Impaired IgM responses to vaccination with PPS vaccines have been reported in the elderly.29 To exclude an impact of age on the pneumococcal humoral response in our slightly older CML patient population compared with controls, we performed univariate and multivariate analyses including age, Sokal score, spleen size, and IgM memory B-cell frequencies. In univariate and multivariate analyses, the IgM memory B-cell frequency at vaccination was the only independent predictor for a positive IgM humoral response (P = .006).
The gating strategy employed for the analysis of the B-cell phenotype in vaccine responders and nonresponders is shown in supplemental Figure 1. Of note, we found no significant difference in CD19+ B-cell frequencies between responders and nonresponders (P = .92, data not shown).
In line with the normal prevaccine pneumococcal IgG titers in CML, we found no significant differences in the frequencies of switched memory B cells between CML patients on TKI and controls before vaccination (median 13.2% vs 8.9%, P = .30; data not shown); however, the frequencies of switched memory B cells were significantly lower in the 6 patients who failed to mount an appropriate IgM and IgG response compared with the 33 patients who had an appropriate IgM or IgG response to the pneumococcal vaccine (median 6.3% vs 13.7%, P = .0031; Figure 3C and Table 2).
Treatment with imatinib is associated with a significant decrease in the frequencies of IgM memory and class-switched memory B cells
To investigate whether the loss of the IgM memory B-cell subset in CML patients is related to CML itself or to treatment with TKIs, we studied B-cell subsets in paired samples collected from 15 CP-CML patients at diagnosis and once CCyR was achieved on imatinib. The patient characteristics are summarized in Table 3. Only patients on imatinib were studied because paired samples from diagnosis and following therapy were not available for patients on dasatinib and nilotinib.
No significant differences were found in the frequencies of IgM memory and switched memory B cells in CML patients at diagnosis (ie, prior to initiation of imatinib) (n = 15) compared with healthy controls (n = 10) (median 12.6% vs 12.8%, P = .85, and 14.1% vs 8.9%, P = .21, respectively). However, we found a significant reduction in IgM memory B-cell frequencies in CP-CML patients following treatment with imatinib compared with diagnosis (median 6.5%, range 2.5% to 21.0% at CCyR vs 12.6%, range 3.9% to 33.5% at diagnosis; P = .003) (Figure 3D). Similarly, there was a significant reduction in the frequencies of class-switched memory B cells following treatment with imatinib compared with diagnosis (median 7.48%, range 2.3% to 22.3% at CCyR vs 14.1%, range 6.1% to 28.0% at diagnosis; P = .001) (Figure 3E), indicating that TKIs are responsible for the lower frequencies of memory B cells in CML. Fluorescence-activated cell sorter plots from 2 representative patients are presented in Figure 3F.
Plasma from CML patients on TKI coincubated with autologous B cells inhibits Btk phosphorylation
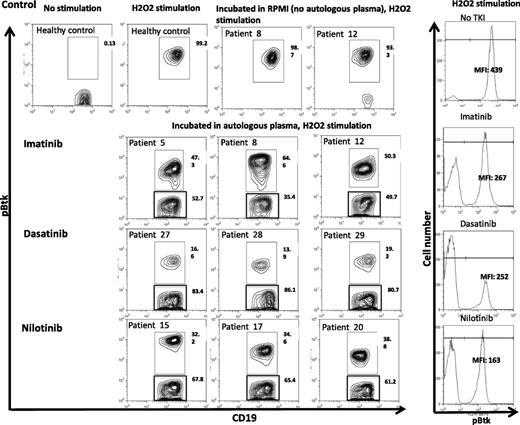
To understand the molecular basis through which TKIs inhibit B-cell activation, we coincubated plasma from 3 vaccinated CML patients on imatinib (patients 5, 8, and 12), 4 on nilotinib (patients 15, 17, and 20), and 3 on dasatinib (patients 27, 28, and 29) with autologous B cells and assessed their impact on Btk phosphorylation by phospho-flow analysis on gated CD19+ B cells. Imatinib, dasatinib, and nilotinib levels in CML plasma samples used for these studies were within therapeutic range (supplemental Table 3).
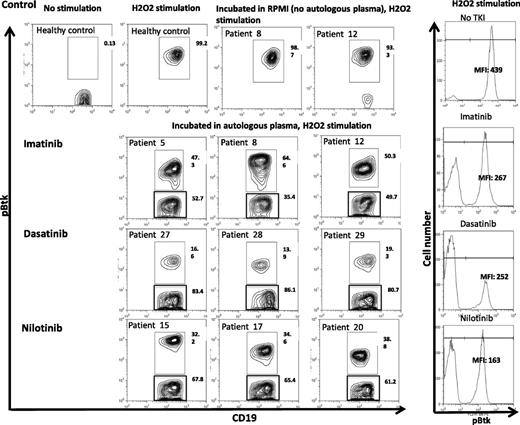
We noted significant inhibition in the mean fluorescence intensity (MFI) and percentage Btk phosphorylation in B cells of patients treated with imatinib (median inhibition 50%, range 35% to 53%), nilotinib (median inhibition 65%, range 61% to 68%), and dasatinib (median inhibition 83%, range 81% to 86%) (Figure 4).
Inhibition of Btk phosphorylation in CD19+ B cells from CML patients on TKI coincubated with autologous plasma. Cryopreserved PBMCs from CML patients on TKI (imatinib, n = 3; nilotinib, n = 3; and dasatinib, n = 3) were thawed, washed, and cocultured with autologous plasma or RPMI/10% fetal calf serum overnight. PBMCs were then stimulated with 5 mL of 50 mM of H2O2 for 15 minutes at 37°C. The stimulation was terminated by the addition of 5 mL prewarmed Cytofix Buffer (BD Biosciences, San Jose, CA) at 37°C for 12 minutes. Cells were fixed/permeabilized and stained with pBtk-PE (BD Biosciences) and APC-conjugated anti-CD19 (BD Biosciences). Data acquisition was performed on the FACSCalibur, and FlowJo software was used for analysis. MFI of Btk phosphorylation following incubation with autologous plasma in gated CD19+ B cells from representative CML patients on imatinib, dasatinib, or nilotinib is presented (right panel).
Inhibition of Btk phosphorylation in CD19+ B cells from CML patients on TKI coincubated with autologous plasma. Cryopreserved PBMCs from CML patients on TKI (imatinib, n = 3; nilotinib, n = 3; and dasatinib, n = 3) were thawed, washed, and cocultured with autologous plasma or RPMI/10% fetal calf serum overnight. PBMCs were then stimulated with 5 mL of 50 mM of H2O2 for 15 minutes at 37°C. The stimulation was terminated by the addition of 5 mL prewarmed Cytofix Buffer (BD Biosciences, San Jose, CA) at 37°C for 12 minutes. Cells were fixed/permeabilized and stained with pBtk-PE (BD Biosciences) and APC-conjugated anti-CD19 (BD Biosciences). Data acquisition was performed on the FACSCalibur, and FlowJo software was used for analysis. MFI of Btk phosphorylation following incubation with autologous plasma in gated CD19+ B cells from representative CML patients on imatinib, dasatinib, or nilotinib is presented (right panel).
Imatinib, dasatinib, and nilotinib inhibit Btk and PLC-γ2 phosphorylation in a dose-dependent manner
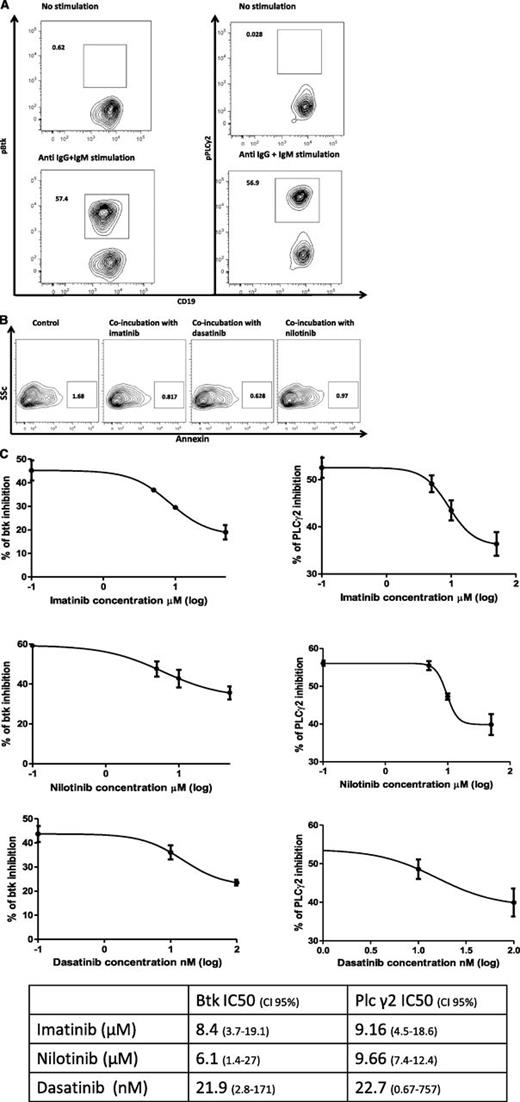
We also assessed the impact of increasing doses of imatinib, dasatinib, and nilotinib on the phosphorylation of Btk and its downstream signaling molecule PLC-γ2 in healthy donor B cells. Untreated normal B cells showed little evidence of phosphorylated PLC-γ2 (0.21%, range 0.03% to 0.84%) and pBtk (0.74%, range 0.62% to 0.81%) (Figure 5A, right and left top panel, respectively). Upon stimulation with goat anti-human IgG and IgM F(ab')2, CD19+ B cells responded by expressing increased levels of pBtk (57.4%, range 57.2% to 57.9%) and pPLC-γ2 (57.0%, range 56.9% to 58.8%) (Figure 5A, lower panel). We then investigated the impact of TKIs on Btk and PLC-γ2 phosphorylation in gated CD19+ B cells derived from healthy donors. Coincubation with TKIs for 2 to 14 hours did not impair B-cell viability (Figure 5B). Imatinib inhibited phosphorylation of Btk (50% inhibition concentration [IC50] = 8.4 µM) and PLC-γ2 (IC50 = 9.16 µM) in gated CD19+ B cells in a dose-dependent manner (Figure 5C). We next determined the impact of nilotinib and dasatinib on pBtk and pPLC-γ2. Similarly, nilotinib and dasatinib dose-dependently inhibited pBtk (IC50 = 6.1 µM and IC50 = 21.9 nM, respectively) and pPLC-γ2 (IC50 = 9.66 µM and IC50 = 22.7 nM, respectively) (Figure 5C, left panel). Each experiment was performed a minimum of 3 times.
Btk and PLC-γ2 phosphorylation inhibition by imatinib, dasatinib, and nilotinib. (A) To assess the impact of TKI on normal B cells, PBMCs from healthy controls were isolated and cultured in the presence or absence of increasing concentrations of TKIs, namely, 1 to 50 μM of imatinib (LC Laboratories), 1 to 50 μM of nilotinib (LC Laboratories), or 1 to 100 nM of dasatinib (LC Laboratories) for 2 hours. PBMCs were then stimulated with goat anti-human IgG and IgM F(ab')2 (0.5 mg/mL solution) at a final concentration of 10 μg/mL for 20 minutes, and cells were stained with pBtk-PE or pPLC-γ2-PE, APC-conjugated anti-CD19 (BD Biosciences, San Jose, CA), PerCP-conjugated anti-human IgM (BD Biosciences, Oxford, United Kingdom), and FITC-conjugated anti-CD27 (DakoCytomation). Cells were gated on lymphocytes: the panels on the top depict the unstimulated negative control, and on the bottom anti-IgG and IgM-induced phosphorylation of Btk (left) and PLC-γ2 (right). (B) PBMCs were coincubated with imatinib (10 μM), nilotinib (10 μM), and dasatinib (100 nM) for 14 hours, and the viability of CD19+ B cells was assessed by staining with FITC-conjugated annexin and APC-conjugated anti-CD19 (both BD Biosciences, San Jose, CA). (C) Curve fit (linear regression) of TKI doses plotted against the percentage of Btk phosphorylation inhibition induced by each of the 3 TKIs, imatinib, nilotinib, and dasatinib (each experiment was performed a minimum of 3 times). The Y bar represents the percentage of gated population in which phosphorylated Btk or PLC-γ2 are detected.
Btk and PLC-γ2 phosphorylation inhibition by imatinib, dasatinib, and nilotinib. (A) To assess the impact of TKI on normal B cells, PBMCs from healthy controls were isolated and cultured in the presence or absence of increasing concentrations of TKIs, namely, 1 to 50 μM of imatinib (LC Laboratories), 1 to 50 μM of nilotinib (LC Laboratories), or 1 to 100 nM of dasatinib (LC Laboratories) for 2 hours. PBMCs were then stimulated with goat anti-human IgG and IgM F(ab')2 (0.5 mg/mL solution) at a final concentration of 10 μg/mL for 20 minutes, and cells were stained with pBtk-PE or pPLC-γ2-PE, APC-conjugated anti-CD19 (BD Biosciences, San Jose, CA), PerCP-conjugated anti-human IgM (BD Biosciences, Oxford, United Kingdom), and FITC-conjugated anti-CD27 (DakoCytomation). Cells were gated on lymphocytes: the panels on the top depict the unstimulated negative control, and on the bottom anti-IgG and IgM-induced phosphorylation of Btk (left) and PLC-γ2 (right). (B) PBMCs were coincubated with imatinib (10 μM), nilotinib (10 μM), and dasatinib (100 nM) for 14 hours, and the viability of CD19+ B cells was assessed by staining with FITC-conjugated annexin and APC-conjugated anti-CD19 (both BD Biosciences, San Jose, CA). (C) Curve fit (linear regression) of TKI doses plotted against the percentage of Btk phosphorylation inhibition induced by each of the 3 TKIs, imatinib, nilotinib, and dasatinib (each experiment was performed a minimum of 3 times). The Y bar represents the percentage of gated population in which phosphorylated Btk or PLC-γ2 are detected.
Collectively, our data provide clear evidence that all 3 TKIs can suppress B-cell activation through their off-target kinase inhibition.
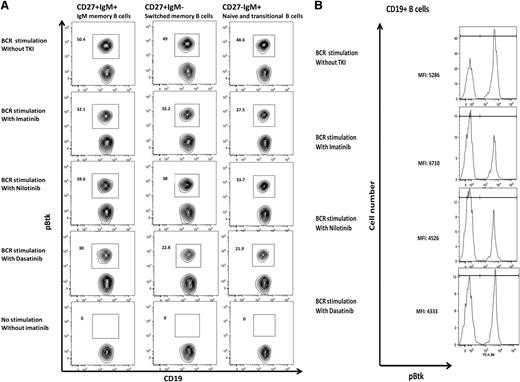
Imatinib, dasatinib, and nilotinib inhibit Btk and PLC-γ2 within the memory B-cell subset
To assess if TKIs can inhibit B-cell activation in memory B-cell subsets, we examined the impact of these drugs on phosphorylation of Btk and PLC-γ2 by phospho-flow analysis on gated naive B cells (CD19+CD27–), IgM memory B cells (CD19+ CD27+ IgMhigh IgD+/lo), and switched memory B cells (CD19+ CD27+ IgM– IgD–). With all 3 TKIs tested, we showed a reduction in levels of pBtk in gated CD19+ B cells as assessed by quantitation of MFI on phospho-flow analysis. Furthermore, inhibition of phosphorylation could be demonstrated in the memory B-cell subsets as shown in Figure 6.
Btk and PLC-γ2 phosphorylation inhibition in B-cell subsets. (A) Btk phosphorylation in B-cell subsets cultured in the presence or absence of 10 μM of imatinib, 100 nM of dasatinib, or 10 μM of nilotinib for 2 hours and stimulated with 10 μg/mL of anti-human IgG and IgM F(ab')2 for 20 minutes. Effect of the TKI on pBtk inhibition is shown in gated IgM memory B-cell, switched memory B-cell, and naive B-cell subsets. Each experiment was performed a minimum of 3 times. (B) MFI of BTK phosphorylation in gated CD19+ B cells from a representative healthy donor following incubation with 10 μM of imatinib, 100 nM of dasatinib, or 10 μM of nilotinib.
Btk and PLC-γ2 phosphorylation inhibition in B-cell subsets. (A) Btk phosphorylation in B-cell subsets cultured in the presence or absence of 10 μM of imatinib, 100 nM of dasatinib, or 10 μM of nilotinib for 2 hours and stimulated with 10 μg/mL of anti-human IgG and IgM F(ab')2 for 20 minutes. Effect of the TKI on pBtk inhibition is shown in gated IgM memory B-cell, switched memory B-cell, and naive B-cell subsets. Each experiment was performed a minimum of 3 times. (B) MFI of BTK phosphorylation in gated CD19+ B cells from a representative healthy donor following incubation with 10 μM of imatinib, 100 nM of dasatinib, or 10 μM of nilotinib.
Discussion
In this study, we show that CP-CML patients treated with imatinib, dasatinib, or nilotinib have significant impairment in their B-cell response to PPS vaccine. The impaired humoral response to the vaccine was associated with loss of memory B-cell subsets following treatment with TKIs. Although in patients on second-line dasatinib and nilotinib a prior effect of imatinib on B-cell function cannot be excluded, our in vitro data confirmed that all 3 TKIs are capable of dose-dependently suppressing 1 or more kinases important in BCR signaling, survival, and memory formation, as demonstrated by reduced phosphorylation of Btk and indirectly its substrate PLC-γ2, providing a possible mechanism for TKI-induced B-cell impairment.
In contrast, we did not find a significant difference in the memory T-cell response to influenza vaccine in patients with CML on TKI and healthy controls. Although a number of studies have shown that TKIs inhibit T-cell proliferation in vitro,4-6 our data are in keeping with previous reports of successful induction of T-cell responses to viral and tumor antigen vaccines in patients with CML on TKIs.24,30-33 The discrepancy in the results of in vitro and in vivo studies may be partly related to the differential impact of TKIs on regulatory T cells in vivo,8 which in turn may facilitate the induction of a successful effector T-cell response. Our study is, however, limited by the small sample size, and it is possible that with a larger cohort of patients and controls, small differences in the T-cell response to vaccination could be detected. It is also possible that TKI may interfere with the induction of naive T-cell responses, not tested here, or may impact on T-cell responses to a weaker cognate antigen.
We found a strong correlation between pneumococcal IgM vaccine response and IgM memory B-cell frequencies; 60% of patients with CP-CML on TKI had significantly impaired pneumococcal IgM antibody responses to vaccination, associated in almost all cases with a significant loss of IgM memory B cells. In comparison, effective pneumococcal IgM responses following vaccination were seen in nearly all controls and in 18 of 45 CML patients on TKI, associated with normal IgM memory B-cell frequencies. Impaired IgM responses to vaccination in association with significant reduction in IgM memory B cells has been reported in a number of conditions, including common variable immunodeficiency, HIV, and congenital asplenia, as well as in the elderly and children under the age of 2 years. The importance of IgM memory B cells in host protection against pneumococcal infection has been studied most extensively in common variable immunodeficiency patients; in these patients, a strong correlation was shown between IgM memory B-cell frequencies and the incidence of encapsulated bacterial infection.34,35 Similarly in HIV-positive individuals, loss of memory B cells correlated with a decrease in the pneumococcal IgM response.26 It is, however, not clear whether CML patients on TKI have more pneumococcal infection compared with the normal population. Whereas earlier studies suggested an increased infection rate in dasatinib-treated CML patients,36,37 larger prospective studies have failed to confirm these results.2 Patients with CP-CML with low IgM memory B cells may derive some protection against infections from prior immune memory or cross-protection from other immune subsets. It is possible that patients with CP-CML on TKI who do not mount adequate responses to PPS vaccine may respond to vaccination with the conjugated pneumococcal vaccine, which obviously would need testing in similar settings.38 In our study, we also investigated the impact of other factors including age, gender, spleen size, and Sokal score on the pneumococcal humoral response. On univariate and multivariate analyses, IgM memory B-cell frequency remained the only significant predictive factor for a vaccine-induced pneumococcal IgM response.
IgM memory B cells recognize T-independent antigens such as PPS by virtue of a prediversified surface IgM and can respond immediately to antigen without T-cell help.39,40 Although an IgM response is believed to be the hallmark for PPS vaccine, switched memory B lymphocytes may also be involved in the anti-PPS antibody response. Studies in SCID mice transplanted with human B lymphocytes showed that both IgM memory and switched memory B lymphocytes are involved in the antipolysaccharide immune response.41 In our study, a small number of CML patients (6/24 evaluable) failed to mount both an IgM and IgG response to the vaccine; the poor humoral response was associated with significantly lower switched and IgM memory B-cell frequencies, supporting a role for switched memory B cells in the anti-PPS humoral response. These data suggest that TKIs may affect both T-dependent and T-independent B-cell activation signals in vivo, resulting in global B-cell dysfunction.
The impaired humoral response and loss of B-cell subsets seen in our study could be a consequence of CML itself or a direct effect of TKI treatment. B-cell progenitors are part of the leukemic clone in a subset of CML patients,42 and it is therefore possible that TKIs may block BCR-ABL in Ph+ B-cell lymphoid cells, thereby inducing B-cell immune deficiency. Conversely, Ph+ B cells may be hypo- or dysfunctional and as a result fail to mount an effective humoral response to a pathogenic antigen. However, our data do not support these scenarios as we observed a significant decrease in the frequencies of memory B-cell subsets at CCyR, when a state of minimal residual disease was achieved, compared with diagnosis. Instead, our results favor a direct quantitative and qualitative effect of TKI on B cells. We found a significant reduction in IgM memory and switched memory B cells following treatment with imatinib, suggesting that TKIs might interfere with the production and maintenance of B-cell memory. These data may provide a possible explanation for recent reports of graft-versus-host disease (GVHD) response to imatinib, despite a lack of correlation with PDGF receptor phosphorylation,43 and further support the use of TKI in B-cell–mediated immune disorders such as rheumatoid arthritis or chronic GVHD.19,43
Polysaccharides stimulate B cells via cross-linking of multiple antigen receptors, resulting in activation of Btk, a critical enzyme in the T-cell–independent type 2 signaling cascade.40 Btk and its downstream substrate PLC-γ2 are involved in BCR signaling, survival, and memory formation.23,44-47 We hypothesized that through their off-target kinase inhibition, TKIs may impair the intracellular phosphorylation of Btk and indirectly inhibit its downstream substrate PLC-γ2, resulting in impaired IgM responses to vaccination and a decrease in the memory B-cell compartment. We found that coincubation of plasma from CML patients on TKI with autologous B cells resulted in significant inhibition of Btk phosphorylation. Plasma from CML patients on dasatinib induced more profound suppression of Btk kinase activity compared with plasma from patients on imatinib or nilotinib, suggesting that dasatinib may have more potent off-target Btk inhibitory activity compared with the more specific BCR-ABL inhibitors such as imatinib or nilotinib. These findings support previous work showing dasatinib to be a strong inhibitor of Btk phosphorylation.44 Therapeutic concentrations of imatinib, dasatinib, and nilotinib were also shown to inhibit Btk phosphorylation in activated B cells from healthy controls. Finally, we showed that all 3 TKIs suppress Btk activity in memory B-cell subsets, known to be critical to B-cell memory development and class switching.48 Our study was not designed to address the underlying reasons for differential responses to pneumococcal vaccine in CML patients on TKI. A possible explanation could be variations in serum TKI levels due to interindividual differences in drug metabolism49 or adherence that may in turn impact the in vivo B-cell response to the vaccine.50
In conclusion, treatment with TKIs is associated with loss of memory B-cell subsets and impaired humoral immune responses to PPS vaccine, likely driven by the off-target kinase inhibitory activity of these drugs. Our results call for close monitoring of patients on TKI to assess the long-term impact of impaired B-cell function on immune surveillance and susceptibility to infection and cancer. The inhibitory effect of imatinib, dasatinib, and nilotinib on memory B-cell expansion and antibody production provides further rationale for studies of selective TKIs in the treatment of autoimmune diseases and cGVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Kay Kendall Leukemia Fund (KKL 314), the Marie Curie award FP7-PEOPLE-2007-4-3-IRG 224980, and the Institut National du Cancer (INCa, Paris, France).
Authorship
Contribution: K.R., H.d.L., P.K., and D. Marin designed the study and research; H.d.L., K.R., D. Marin, P.K., and A.K. analyzed data; H.d.L., K.R., and D. Marin wrote the manuscript; H.d.L., M.H., A.K., A.S., S.B., R.J.F., and T.S. performed the experiments; D. Marin, J.A., D. Milojkovic, and H.d.L. recruited patients; H.d.L., A.B., I.G., K.S., and S.A. collected the clinical samples; and M.H., A.K., A.A., K.S., D. Milojkovic, N.C., S.M., A.C., L.F., J.G., K.S., and E.J.S. commented on the manuscript.
Conflict-of-interest disclosure: H.d.L. received lecture fees from Bristol-Myers Squibb. K.R. received a grant from Novartis. D. Marin received honoraria and lecture fees from Novartis and Bristol-Myers Squibb. J.A. received honoraria and lecture fees from Novartis and Bristol-Myers Squibb. J.G. received lecture fees from Novartis and Bristol-Myers Squibb. D. Milojkovic received lecture fees from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Katayoun Rezvani, Department of Stem Cell Transplantation and Cellular Therapy, 1515 Holcombe Blvd, Box 448, Houston, TX 77030-4009; e-mail: krezvani@mdanderson.org.
References
Author notes
H.d.L. and A.K. contributed equally to this study.