Key Points
Treatment with PEG-IFN-α-2a in PV and ET results in a high rate of complete hematologic and molecular responses.
Patients failing to achieve complete molecular remission tended to have higher frequencies of mutations in genes other than JAK2.
Abstract
Pegylated interferon α-2a (PEG-IFN-α-2a) has previously been shown to induce hematologic and molecular responses in patients with polycythemia vera (PV) or essential thrombocythemia (ET). Here we present a follow-up of a phase 2 trial with PEG-IFN-α-2a treatment in 43 PV and 40 ET patients with detailed molecular analysis. After a median follow-up of 42 months, complete hematologic response was achieved in 76% of patients with PV and 77% of those with ET. This was accompanied by complete molecular response (CMR) (ie, undetectable JAK2V617F) in 18% and 17%, of PV and ET patients, respectively. Serial sequencing of TET2, ASXL1, EZH2, DNMT3A, and IDH1/2 revealed that patients failing to achieve CMR had a higher frequency of mutations outside the Janus kinase–signal transducer and activator of transcription pathway and were more likely to acquire new mutations during therapy. Patients with both JAK2V617F and TET2 mutations at therapy onset had a higher JAK2V617F mutant allele burden and a less significant reduction in JAK2V617F allele burden compared with JAK2 mutant/TET2 wild-type patients. These data demonstrate that PEG-IFN-α-2a induces sustained CMR in a subset of PV or ET patients, and that genotypic context may influence clinical and molecular response to PEG-IFN-α-2a.
Introduction
Recent candidate gene and genome-wide discovery studies have identified recurrent somatic mutations in a spectrum of human malignancies, which has guided the development of targeted therapies aimed at achieving clinical and molecular remissions in different neoplastic contexts. Essential thrombocythemia (ET) and polycythemia vera (PV) are clonal myeloproliferative neoplasms (MPNs); the most common mutations identified to date in these MPNs are somatic activating mutations in the JAK2 tyrosine kinase. Approximately 95% of patients with PV and 50% to 60% of those with ET acquire the JAK2V617F somatic mutation.1-5 The earliest studies of the effect of interferon (IFN) on hematopoiesis in MPN patients observed that IFN therapy could convert MPN patients from a clonal state of hematopoiesis to a polyclonal state using X-chromosome inactivation assays.6-8 The discovery of the JAK2V617F allele in the majority of MPN patients has allowed for more precise delineation of the anticlonal effects of IFN in MPN patients.9-11 We previously reported that, after a median follow-up of 21 months, therapy with pegylated interferon α-2a (PEG-IFN-α-2a) was associated with a complete hematologic response (CHR) rate of 70% and 76% among patients with advanced PV or ET, respectively.11 PEG-IFN-α-2a treatment resulted in a significant reduction of the JAK2V617F allele burden in the majority of patients with JAK2V617F-positive PV and ET, including complete molecular responses (CMRs) (undetectable JAK2V617F mutation) in 14% of patients with PV and in 6% of those with ET. However, achievement of meaningful molecular responses required treatment with PEG-IFN-α-2a for at least 6 months, whereas a major hematologic response was achieved within 3 months of the start of therapy in most patients. In patients with PV, the JAK2V617F mutant allele burden continued to progressively decrease during the median 21-month follow-up time with no clear plateau, suggesting that longer follow-up was needed to best assess the efficacy of PEG-IFN-α-2a therapy at the genetic level.11 Importantly, an independent study of PEG-IFN-α-2a in 37 evaluable patients with newly diagnosed PV revealed similar hematologic and molecular response rates with a median follow-up of 31 months.9
Over the past 3 years, a series of new somatic mutations outside the Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling pathway have been identified in patients with PV and ET. These include mutations in TET2, DNMT3A, ASXL1, EZH2, and IDH1/2 (reviewed recently12 ). Recent studies have suggested that mutations in these epigenetic modifiers lead to alterations in hematopoietic stem cell (HSC) function,13-17 suggesting that mutations in these pathways could affect response to drugs aimed at eliminating the disease-initiating HSC compartment. Of note, Kiladjian et al10 studied a small set of patients from their phase 2 clinical trial of PEG-IFN-α-2a in PV and found that a subset of patients had persistent TET2-positive clones during PEG-IFN-α-2a therapy, despite eradication of JAK2 mutations in the setting of co-occurring JAK2 and TET2 mutations at the initiation of therapy. These data suggest that PEG-IFN-α-2a therapy can reduce or eliminate the JAK2V617F mutant clone but not the TET2 mutant clone; however, the number of informative patients with concurrent JAK2 and TET2 mutations was relatively small. Importantly, these data suggest that the clinical and molecular response to PEG-IFN-α-2a therapy in patients with mutations outside the JAK-STAT pathway might be less than in patients with JAK2 mutations in the absence of concurrent mutations in epigenetic modifiers.
The goal of the current study was to analyze the longer follow-up of patients with PV or ET treated on a phase 2 clinical trial of PEG-IFN-α-2a. This allowed us to delineate the hematologic and molecular response rate with longer follow-up and to perform detailed molecular studies at diagnosis and during therapy. Most importantly, we investigated whether somatic mutations in epigenetic modifiers impacted the clinical and molecular response to PEG-IFN-α-2a.
Methods
Inclusion and exclusion criteria
Patients with a diagnosis of ET or PV, according to the PV Study Group 2005 criteria, either newly diagnosed or previously treated, were eligible for this study. Of note, the diagnosis of PV or ET did not vary when using the World Health Organization criteria. Any patient over the age of 18 was allowed to enter, and there was no upper age limit. Patients were required to have an Eastern Cooperative Oncology Group performance status <2, serum creatinine <2.0 mg/dL, serum bilirubin <2 times the upper limit of the normal range, and normal cardiac function, and they were required to be off MPN-directed therapy for at least 1 week before entering the study. However, hydroxyurea or anagrelide treatment was allowed for up to 1 month after study entry if judged necessary. Exclusion criteria included standard contraindications to the use of PEG-IFN-α-2a including a history of psychiatric disorder, particularly depression, autoimmune disorders, hypersensitivity to IFN-α, ischemic retinopathy, systemic infections (such as hepatitis B or C or HIV), pregnant or lactating women, history of severe heart disease, renal disease on hemodialysis, or seizure disorder requiring anticonvulsant therapy. All patients signed an informed consent approved by The University of Texas MD Anderson Cancer Center Institutional Review Board and in accordance with the Declaration of Helsinki.
Treatment schedule
The first 3 patients in the study received PEG-IFN-α-2a subcutaneously at 450 µg weekly (based on dosing from the phase 1 study of PEG-IFN-α-2a in chronic myelogenous leukemia patients).18 As a result of poor tolerance, the starting dose was decreased in a stepwise manner by 90-µg decrements based on tolerance.11 A dose schedule of 90 µg weekly was eventually established as the starting dose. PEG-IFN-α-2a was administered for as long as the patient obtained clinical benefit. The dose was modified based on toxicity or lack of efficacy. In the event of persistent significant grade 2 toxicity or grade 3 or 4 toxicity, therapy was discontinued until resolution of the toxicity to grade 0 or 1 and reinstated at the immediate lower dose level. Alternative dose schedules were allowed to minimize toxicity while sustaining optimal benefit (eg, 45 µg every 2-4 weeks).
Clinical response criteria
Assessment of hematologic response was performed every 3 months. The definitions of CHR and partial hematologic response (PHR) in patients with ET or PV have been reported previously.11 Briefly, a CHR for either ET or PV required normalization of peripheral blood counts (hematocrit <45% in males and <42% in females for patients with PV), complete disappearance of splenomegaly in the absence of cytoreductive therapy (ie, hydroxyurea or anagrelide), and the absence of thromboembolic events.
Molecular response criteria
Assessment of molecular response was performed at a priori specified time intervals of every 6 months as part of this clinical trial. JAK2V617F was detected in DNA samples extracted from bone marrow (BM) specimens by polymerase chain reaction (PCR) and quantified by a pyrosequencing assay (sensitivity 5%) developed at The University of Texas MD Anderson Cancer Center (Houston, TX).11 In the absence of the JAK2V617F mutation, patients were screened for exon 12 mutations by Sanger sequencing as previously described.19 In addition to pyrosequencing for quantitative assessment of the JAK2V617F mutant allele burden, we performed quantitative assessment of the JAK2V617F mutant allele burden via JAK2V617F quantitative allele-specific PCR (ASP) assay as described previously.20 Quantitative ASP was performed on both genomic DNA from BM aspirates and the peripheral genomic DNA, which was used for more extensive mutational profiling as described subsequently.
Assignment of patients to molecular response categories was done according to results from pyrosequencing, which was confirmed by results from JAK2V617F ASP. CMR required an undetectable JAK2V617F mutation; partial molecular response (PMR) required >50% reduction of baseline JAK2V617F mutation level; and minor molecular response (mMR) required 20% to 49% reduction of baseline JAK2V617F mutation level, as reported previously.21 Similar molecular response criteria have recently been proposed by the European LeukemiaNet.22
Patient evaluation
In addition to standard testing, baseline studies included BM aspiration and biopsy with cytogenetics and JAK2V617F allele burden quantitation. JAK2V617F allele burden quantitation was repeated during follow-up every 6 months, and any patients with cytogenetic abnormalities were followed for cytogenetic response or clonal evolution by assessing for cytogenetic abnormalities every 6 to 12 months.11 The cytogenetic result was considered pathological when at least 2 abnormal metaphases were identified with structural abnormality or chromosome gain, or when at least 3 metaphases of the same chromosome loss were identified. Toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Sequence analysis
DNA was isolated from peripheral blood polymorphonuclear cells and/or BM nucleated cells from 83 patients with PV (n = 43) or ET (n = 40). Approval was obtained from the institutional review boards at the MD Anderson Cancer Center and at Memorial Sloan-Kettering Cancer Center for these studies, and informed consent was provided according to the Declaration of Helsinki.
DNA resequencing of all coding exons of known mutations in ASXL1, IDH1, IDH2, JAK2, DNMT3a, EZH2, MPL, and TET2 was performed on all samples. All DNA samples were whole genome amplified using Ø29 polymerase to ensure that sufficient material was available for sequence analysis.23 Primer sequences, target regions, and PCR conditions have been described previously.24 PCR amplicons were smaller than 500 bp, and overlapping PCR amplicons were designed for all exons larger than 400 bp to ensure complete coverage. For each PCR reaction, 20 ng of genomic DNA was used for PCR amplification followed by magnetic bead purification (SPRI; Agencourt Bioscience, Beverly, MA) and bidirectional sequencing using ABI 3730 capillary sequencers (Agencourt Bioscience). Bidirectional sequence traces were analyzed for missense and nonsense mutations using Mutation Surveyor (Softgenetics Inc., State College, PA), and all traces were reviewed manually and with Mutation Surveyor for the presence of frameshift mutations. Nonsynonymous alterations not present in dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/) were annotated as somatic mutations or single nucleotide polymorphisms based on sequence analysis of matched buccal DNA extracted from T cells isolated from peripheral blood mononuclear cells, which were used as paired normal DNA as described previously.25 Nonsynonymous alterations not in dbSNP nor determined to be somatic in paired samples or in published studies were censored with respect to mutational status for that specific gene. All somatic mutations were validated by resequencing nonamplified DNA.
Methylcellulose colony assays
BM mononuclear cells (MNCs) were isolated after Ficoll-Hypaque gradient density separation (Histopaque-1077; Sigma-Aldrich, St. Louis, MO). Three million BM MNCs were plated in standard methylcellulose assay (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 20% fetal calf serum, 0.1% bovine serum albumin, stem cell factor, interleukin 3, in the presence or in the absence of 1 IU/mL erythropoietin (EPO). Erythroid colonies were enumerated at days 12 to 14.
Statistical considerations
This is a prospective, open-label, single-center, phase 2 trial of PEG-IFN-α-2a for patients with ET or PV. A response rate >35% would be accepted as evidence of acceptable efficacy for each MPN. The trial was to be stopped if responses were seen in <1 of 11 patients or <2 of 15 patients in each MPN. Otherwise, it would accrue up to 80 patients (40 with ET and 40 with PV) to better define response and toxicity profiles. An overall severe toxicity rate of >20% was deemed unacceptable, and the study was to be stopped. The statistical analysis for response was conducted by the intention-to-treat method. A P value < .05 was considered significant. The MedCalc statistical package was used for all computations.
Results
Patient characteristics
The patient characteristics of the study cohort are listed in Table 1: 83 patients have been accrued (43 with PV and 40 with ET), of which 63% had received some form of medical therapy before enrollment (in addition to aspirin), including standard IFN-α in 9% of patients with PV and 6% of patients with ET. Fifty-one percent of patients with PV were newly diagnosed or had been previously managed solely with phlebotomies, and 23% of patients with ET had not previously received medical therapy. All patients with PV and 48% of ET patients carried the JAK2V617F mutation. The median JAK2V617F allele burden was 65% in patients with PV and 23% in patients with ET, respectively. None of the ET patients were deemed to have an early form of myelofibrosis rather than ET, based on expert hematopathological review as a part of their care at MD Anderson Cancer Center.
Hematologic response to extended treatment with PEG-IFN-α-2a
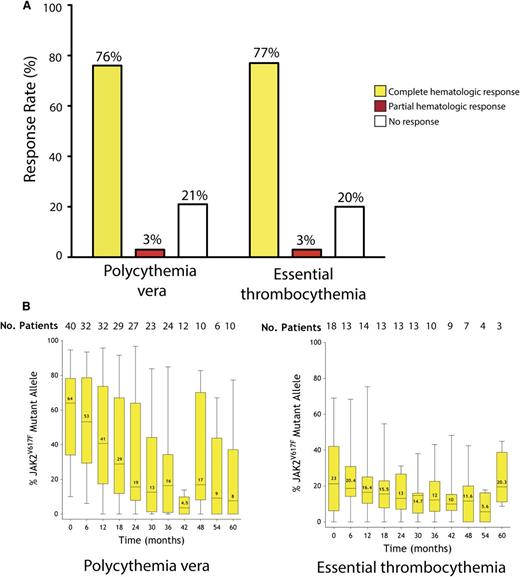
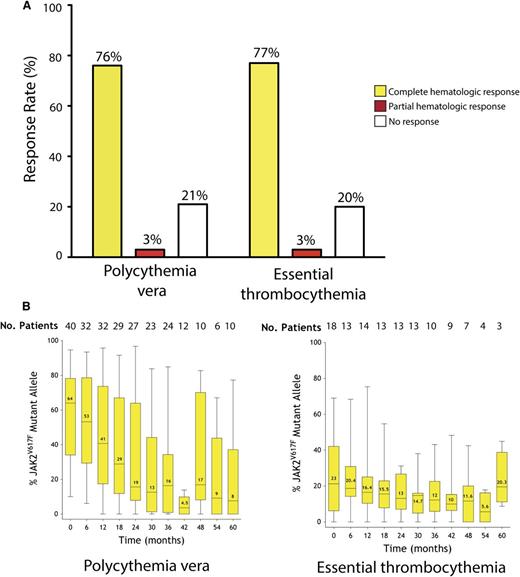
The current median follow-up time for the entire cohort is 42 months (range, 6-72 months). All patients were evaluable for response. Similar rates of hematologic response were observed in patients with PV and ET (Figure 1A). The overall hematologic response was 79% for patients with PV and 80% for patients with ET, including a CHR rate of 76% and 77% for PV and ET, respectively. The median time to complete response was 40 days with a range of 3 to 1478 days.
Long-term follow-up of response to PEG-IFN-α-2a. (A) Hematologic response in patients with PV or ET. (B) Dynamics of JAK2V617F mutant allele burden in patients with PV or ET over time. Black horizontal lines indicate median values; black bars represent minimum and maximum values; yellow rectangular boxes represent values included between the 25% and the 75% percentiles.
Long-term follow-up of response to PEG-IFN-α-2a. (A) Hematologic response in patients with PV or ET. (B) Dynamics of JAK2V617F mutant allele burden in patients with PV or ET over time. Black horizontal lines indicate median values; black bars represent minimum and maximum values; yellow rectangular boxes represent values included between the 25% and the 75% percentiles.
Toxicity during extended PEG-IFN-α-2a therapy and current dosing
PEG-IFN-α-2a administered at 90 µg weekly resulted in no grade 4 toxicities. Grade 3 side effects were also infrequent (see supplemental Table 1, available on the Blood Web site). To date, 27 (32%) patients have been taken off the study for the following reasons: depression (n = 4), stroke (vasospastic in nature and secondary to an angiogram performed to assess a venous malformation of the brain; unrelated to PEG-IFN-α-2a, n = 1), infection (n = 1), fatigue (n = 5), shortness of breath/dyspnea (n = 2), financial reasons/noncompliance (n = 2), retinal toxicity (n = 1), motor vehicle accident (n = 1), death (unrelated to PEG-IFN-α-2a, n = 2), neuropathy (n = 1), neutropenia (n = 2), lupus nephritis (related to PEG-IFN-α-2a; resolved on steroids, n = 1), and no response (n = 2). The 2 deaths that occurred during the conduction of the study occurred in (1) a patient hospitalized with pneumonia who subsequently developed central pontine myelinolysis due to rapid correction of hyponatremia and (2) a patient who died as a consequence of complications derived from severe aortic stenosis and pulmonary hypertension. No patient experienced progression to acute myeloid leukemia in this trial cohort thus far (none of the patients receiving the study drug at the time of last follow-up had blasts in peripheral blood); however, 2 patients experienced progression to myelofibrosis while on PEG-IFN-α-2a and were taken off the study as a result.
Treatment discontinuation was directly related to PEG-IFN-α-2a in 17 (20%) patients when all dose levels were considered, and in 6 (18%) of the 32 patients who started therapy at 90 µg weekly. Twenty-one (38%) of the 56 patients still being treated on the study are receiving weekly PEG-IFN-α-2a therapy (1 patient at 450 µg, 1 at 360 µg, 5 at 180 µg, 2 at 135 µg, 6 at 90 µg, and 6 at 45 µg). Eight patients are receiving PEG-IFN-α-2a at 90 µg every 2 to 4 weeks, and 27 at 45 µg every 2 to 8 weeks with adequate response. Therapy is currently on hold in 7 patients; this includes 2 patients with PV who remain in CHR and CMR 4 and 18 months after treatment discontinuation, respectively, due to grade 2 anemia and grade 3 neutropenia secondary to PEG-IFN-α-2a, respectively. None of the patients in whom the study drug was put on hold have any evidence of disease progression on regular BM biopsy examinations.
Molecular response according to JAK2V617F allele burden
JAK2V617F was detected in 62 (75%) of the 83 patients treated (43 with PV and 19 with ET). The JAK2V617F mutant allele burden was quantified on at least 2 different occasions in 58 (94%) of these 62 patients; these patients were considered assessable for molecular response (Table 2). Categorization of molecular response was performed using pyrosequencing and quantitative ASP of genomic DNA from serial BM aspirates as well as quantitative ASP of peripheral blood granulocyte DNA (supplemental Table 2). The overall molecular response rate was 60% for patients with PV and 67% for patients with ET. The JAK2V617F mutation became undetectable in 7 (18%) patients with PV and in 3 (17%) patients with ET consistent with achievement of a CMR in these patients. In addition, 35% of patients with PV and 33% of those with ET achieved a PMR (ie, a reduction of the mutant allele burden of at least 50% from baseline) (Table 2). Of note, the use of other therapies prior to PEG-IFN-α-2a in patients did not influence the likelihood of achieving CMR. Importantly, of the 10 patients achieving CMR, 4 had no evidence of MPN in concurrent BM biopsy samples.
Analysis of quantitative JAK2V617F mutant allele burden over time revealed a sustained, marked decrease of the JAK2V617F allele burden in PV patients over time. Patients with PV entered the study with a median allele burden of 64%, and treatment with PEG-IFN-α-2a therapy resulted in a decrease to 19% after 24 months (Figure 1B). Importantly, no patient who achieved CMR was observed to experience hematologic or molecular relapse after a median follow up of 57 months (range 53-72 months). Moreover, this decrement in JAK2V617F mutant allele burden was sustained; patients reaching 60 months of treatment had a median allele burden of 8% (P < .0001). In contrast, the majority of ET patients enrolled in the trial did not experience a significant decrease in the JAK2V617F allele burden (Figure 1B).
Analysis of mutations outside of JAK2 and their impact on response to PEG-IFN-α-2a
In order to further delineate the baseline mutational status of patients with PV or ET beyond the JAK2 mutations, we sequenced the entire coding regions of genes reported to be mutated at a frequency of >5% in chronic phase PV and ET (TET2, ASXL1, DNMT3A, and EZH2),26,27 as well as the regions of previously described mutations for MPL, IDH1, and IDH2 in pretreatment granulocyte DNA samples from 71 patients, including 46 patients who carried JAK2 mutations. This extended mutational profiling was performed in peripheral blood granulocyte DNA from serial samples for patients who remained on therapy. The frequency of somatic mutations was as follows: TET2 (16.9%), DNMT3A (12.6%), ASXL1 (4.2%), EZH2 (1.4%), and IDH2 (1.4%). These mutational frequencies are in accordance with prior mutational studies of each of these genes in patients with PV or ET (Table 3).25,28-34 Importantly, the frequency of mutations in genes outside of JAK2 was higher in patients failing to achieve a CMR (56%) vs those achieving CMR (30%); however, this difference did not reach statistical significance. There was no statistically significant difference in age between those patients found to have mutations in genes other than JAK2, at any point during their disease course, compared with those who were JAK2 wild type.
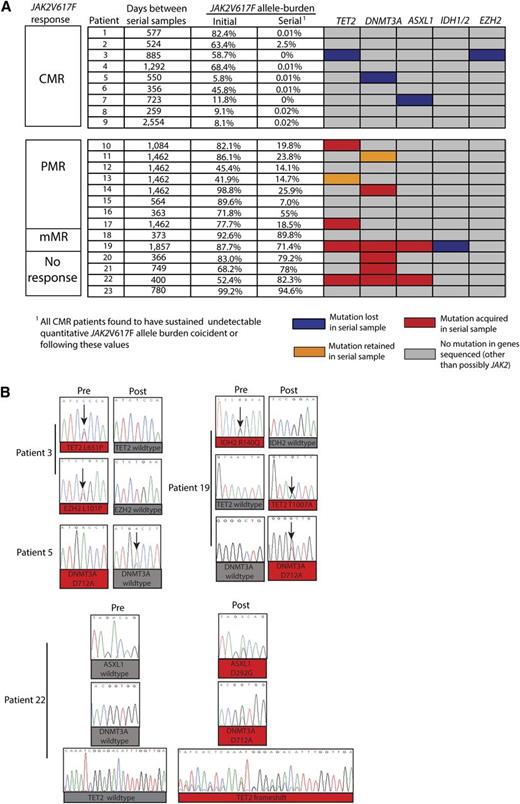
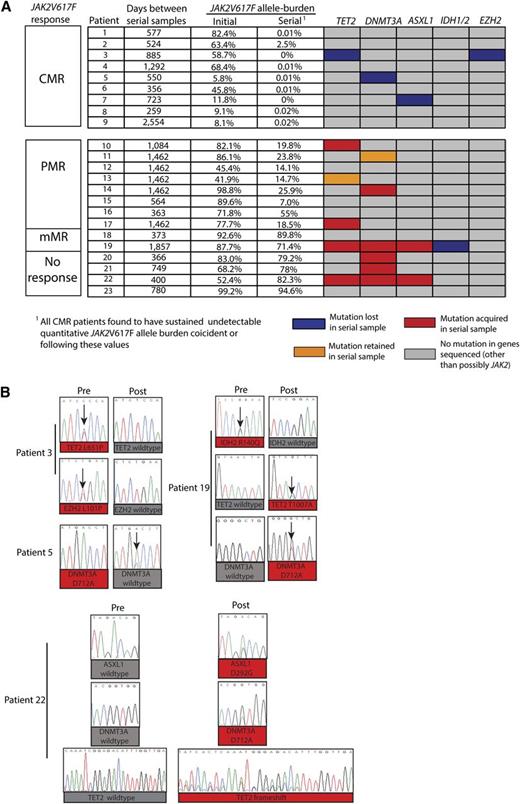
In order to examine the dynamics of acquisition of mutations different from JAK2V617F during PEG-IFN-α-2a therapy, we analyzed granulocyte DNA from paired samples obtained prior to and during PEG-IFN-α-2a treatment of the presence of mutations in the gene panel mentioned previously. Pretreatment and serial samples during therapy were available for 23 of the 46 patients with JAK2 mutations on long-term therapy (9 from patients who had achieved CMR, 8 with PMR, 2 with mMR, and 4 with no response) (Figure 2A; supplemental Tables 2 and 3). The median time between pretreatment and serial on-treatment samples used for mutational studies was 885 days, with a range of 259 to 2554 days. This analysis revealed a marked enrichment of acquisition of new mutations in serial samples in patients failing to achieve a CMR (Figure 2A-B). The mean interval between samples used for extended mutational analysis for those patients achieving CMR (mean interval between samples was 857 days) vs those not achieving CMR (mean interval between samples was 989 days) was not statistically significant. Of the 9 patients who achieved CMR, 6 patients had no mutations outside of JAK2; whereas 1 patient carried both mutant TET2 and EZH2 alleles, a second had a mutation in DNMT3a at the initiation of therapy, and a third had a mutation in ASXL1 at the initiation of therapy. Of note, all of these disease alleles outside the JAK-STAT pathway became undetectable during PEG-IFN-α-2a therapy based on Sanger capillary-based sequencing (Figure 2A-B). Of the 14 patients who failed to achieve a CMR during PEG-IFN-α-2a therapy, 9 patients were found to have additional MPN disease alleles in addition to JAK2. Seven of those 9 patients acquired the mutation during the course of PEG-IFN-α-2a therapy, whereas in 2 others, the mutations present at diagnosis remained detectable during therapy. Thus, 9 of 14 patients (64%) who failed to achieve CMR during the course of PEG-IFN-α-2a therapy had evidence of clonal evolution of disease based on acquisition of new somatic genetic abnormalities during treatment, whereas none of the analyzed patients who achieved CMR (n = 9) were found to acquire new somatic genetic abnormalities.
Serial mutational analysis of genes outside of JAK2 during therapy with PEG-IFN-α-2a in patients with PV and ET. Graphical representation of serial mutational analysis in patients based on JAK2V617F allele burden response. (A) Somatic mutations in granulocyte DNA that were acquired (red boxes), retained (orange boxes), or lost (purple boxes) in follow-up samples compared with pretreatment samples. Also displayed are the JAK2V617F mutant allele burden values (determined by JAK2V617F ASP) in peripheral blood granulocyte DNA at the time in which extended mutational profiling was performed. (B) Sanger sequencing electropherograms displaying acquisition and/or loss of mutations from individual patients during the course of PEG-IFN-α-2a therapy.
Serial mutational analysis of genes outside of JAK2 during therapy with PEG-IFN-α-2a in patients with PV and ET. Graphical representation of serial mutational analysis in patients based on JAK2V617F allele burden response. (A) Somatic mutations in granulocyte DNA that were acquired (red boxes), retained (orange boxes), or lost (purple boxes) in follow-up samples compared with pretreatment samples. Also displayed are the JAK2V617F mutant allele burden values (determined by JAK2V617F ASP) in peripheral blood granulocyte DNA at the time in which extended mutational profiling was performed. (B) Sanger sequencing electropherograms displaying acquisition and/or loss of mutations from individual patients during the course of PEG-IFN-α-2a therapy.
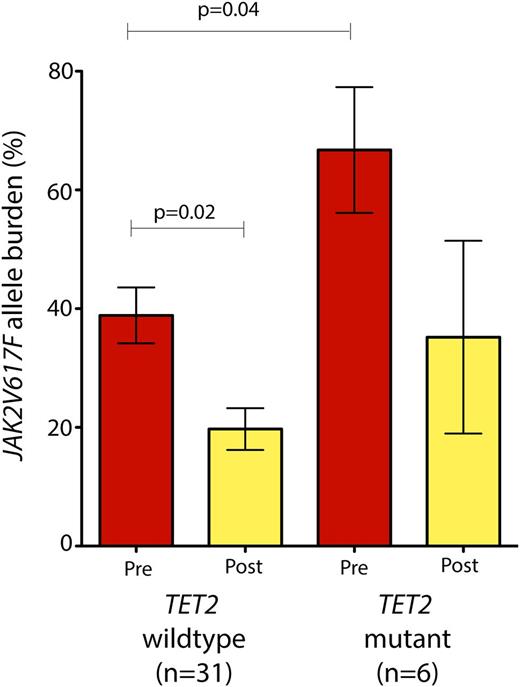
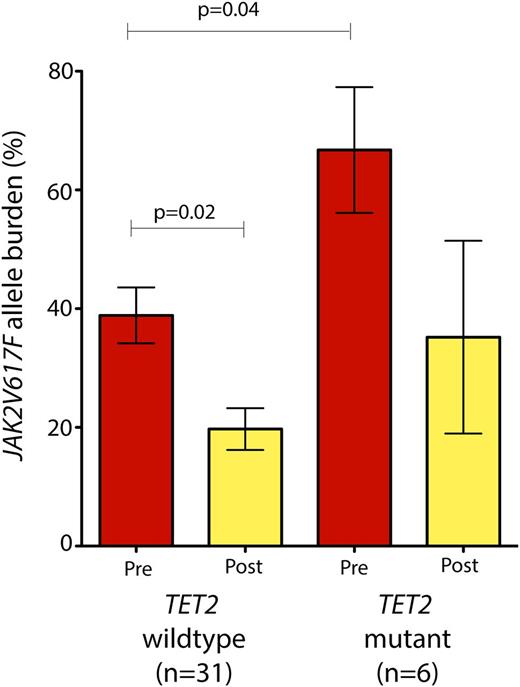
Although the number of patients with mutations in any one of these genes was too low to identify whether specific mutations were significantly enriched in patients with any particular response to PEG-IFN-α-2a, patients with mutations in TET2 (n = 6) at the onset of therapy had a higher JAK2V617F allele burden compared with their TET2 wild-type counterparts (n = 31) (Figure 3). Patients with TET2 mutations had an average JAK2V617F allele burden of 66.7% (range 18.5% to 91.8%) compared with 38.7% in TET2 wild-type patients (range 2.9% to 93.6%; P = .04, Wilcoxon rank-sum test; Figure 3). Moreover, patients with TET2 mutation had a less significant reduction in their JAK2V617F allele burden compared with TET2 wild-type patients (Figure 3).
JAK2V617F allele burden prior to and during PEG-IFN-α-2a therapy based on TET2 mutational status. Patients with JAK2V617F mutations in addition to TET2 mutations (n = 6) had a significantly higher JAK2V617F allele burden at the onset of therapy compared with their TET2 wild-type/JAK2 mutant counterparts (P = .04; Mann-Whitney U test). Moreover, patients with TET2/JAK2 comutations did not experience a significant decrement in JAK2V617F allele burden in contrast with JAK2 mutant/TET2 wild-type patients.
JAK2V617F allele burden prior to and during PEG-IFN-α-2a therapy based on TET2 mutational status. Patients with JAK2V617F mutations in addition to TET2 mutations (n = 6) had a significantly higher JAK2V617F allele burden at the onset of therapy compared with their TET2 wild-type/JAK2 mutant counterparts (P = .04; Mann-Whitney U test). Moreover, patients with TET2/JAK2 comutations did not experience a significant decrement in JAK2V617F allele burden in contrast with JAK2 mutant/TET2 wild-type patients.
In addition to molecular genetic analysis, we performed conventional cytogenetics on all patients at diagnosis. Any patients with cytogenetic abnormalities were followed for cytogenetic response or clonal evolution every 6 months. Five patients were found to have abnormalities on metaphase cytogenetics pretreatment with PEG-IFN-α-2a (supplemental Table 4). Of these 5 patients, 1 had resolution of cytogenetic abnormalities on the study. We did not observe acquisition of new cytogenetic abnormalities in these 5 patients during therapy. The patient who lost his cytogenetic abnormality on therapy was JAK2 mutation negative at baseline; other patients with cytogenetic abnormality did not achieve CMR.
Clonogenic assays for endogenous erythroid colony formation during PEG-IFN-α-2a treatment
In addition to analyzing serial samples for the acquisition and/or loss of mutations during the course of therapy with PEG-IFN-α-2a, we also performed methylcellulose colony assays for the detection of endogenous erythroid colony (EEC) formation in patients posttherapy with PEG-IFN-α-2a (Table 4). BM MNCs from a number of patients experiencing CMR as well as CHR at the time of the assay revealed clear production of EECs in the absence of EPO exposure. The generation of EECs was independent of the presence of the JAK2V617F mutations or other mutations outside the JAK2 locus, as none of the patients achieving CMR had detectable mutations outside of JAK2 in the bulk granulocytes at the time of the EEC assay. The lack of identifiable JAK2V617F mutation was confirmed in genomic DNA from all individual colonies, cultured from BM cells plated in the presence and absence of EPO, from all 3 patients with CMR who had evidence of EEC at the time of CMR. This was verified using JAK2V617F ASP, an assay validated to detect up to 0.1% JAK2V617F mutant allele burden.20
Discussion
We present here detailed clinical updated data as well as molecular data (both at initiation of treatment and in serial follow-up samples obtained during the course of therapy) from a large cohort of patients with PV or ET treated with PEG-IFN-α-2a. The results of this data indicate that PEG-IFN-α-2a is associated with significant clinical activity in PV or ET with high rates of durable hematologic and molecular response. CHR was achieved in 76% of patients with PV and in 77% of those with ET. These clinical responses were frequently accompanied by molecular responses, which were documented in 60% of patients with PV and in 67% of patients with ET: 18% of patients with PV and 17% of those with ET achieved a CMR (CMR being defined according to consensus criteria22 as eradication of the mutant JAK2V617F to below detectable levels). No patient who achieved CMR was observed to relapse during the course of PEG-IFN-α-2a. Recently updated data from the phase 2 study from the PV-Nord group, a separate phase 2 trial of PEG-IFN-α2-a in 40 patients with PV, also reported the efficacy of PEG-IFN-α-2a in producing durable complete hematologic and molecular remissions.35 Moreover, 29% of patients stopped PEG-IFN-α-2a and sustained a hematologic response without further cytoreductive therapy after a median observation time of more than 28 months (with 1 patient up to 64 months). Twenty-eight percent of the patients in this study achieved a CMR, as measured by JAK2V617F allele burden in peripheral blood granulocytes as well as BM nucleated cells. The identification that PEG-IFN-α-2a results in durable CMR in a reproducible manner across studies is remarkable as it indicates that PEG-IFN-α-2a, thus far, represents one of the few therapies known to revert all phenotypic aspects of PV or ET and to consistently induce molecular remissions, which in some cases can be sustained after treatment discontinuation. A much longer follow-up of cohorts of patients with PV or ET treated with PEG-IFN-α-2a is warranted to confirm the durability of these findings. In addition, future studies will hopefully address the effect of PEG-IFN-α-2a on MPN-related symptoms, including pruritus and overall quality of life.
At the same time that PEG-IFN-α-2a was found to induce significant CMR rate in this trial, molecular analysis indicated that 56% of patients who failed to achieve CMR during the course of PEG-IFN-α-2a therapy had evidence of clonal evolution of disease as evidenced by the acquisition of new somatic genetic abnormalities during treatment. This phenomenon was lower in patients who achieved CMR (30%; not statistically different likely due to the small number of evaluable patients). Phenotypic analysis of patients indicated that even patients achieving CMR demonstrated EEC activity at the time when JAK2V617F mutation was undetectable, suggesting that there are additional mutations that can drive clonal hematopoiesis in the setting of chronic PEG-IFN-α-2a treatment. Although we were limited to sequencing DNA from time points where adequate sample material was available, we performed mutational analysis on the entire panel of genes reported to be mutated at a frequency of 5% or more in patients with nontransformed PV or ET, including TET2, DNMT3A, ASXL1, IDH1, IDH2, and EZH2. Three of the patients found to harbor EECs at the time of CMR (ie, undetectable JAK2V617F mutation) did not have mutations in this panel of genes, suggesting that additional genetic abnormalities are responsible for cytokine-independent clonal hematopoiesis outside of the currently reported recurrent mutations in MPNs. These data are consistent with recent data reported by Kiladjian et al10 on a smaller cohort of patients who received PEG-IFN-α-2a as the first-line therapy.
More patients failing to achieve CMR were found to have acquired mutations outside of JAK2 during the course of PEG-IFN-α-2a therapy than those who have achieved CMR. We speculate that (1) these mutations may confer resistance to PEG-IFN-α-2a or (2) these mutations merely reflect clonal evolution in the persistent malignant clone that was not eradicated by PEG-IFN-α-2a therapy. Although no specific mutation could be significantly correlated with PEG-IFN-α-2a resistance, we did observe that patients with TET2 mutations had higher JAK2V617F allele burdens and experienced a more modest reduction in JAK2V617F allele burden and less significant reductions in mutant allele burden over time compared with JAK2V617F mutant/TET2 wild-type counterparts. Kiladjian et al10 recently observed that patients carrying both TET2 and JAK2 mutations experienced reduction in the JAK2 mutant clones without significant eradication of the TET2 mutant clone. Although we did observe that TET2 mutant alleles can also be eradicated by PEG-IFN-α-2a in some patients, taken together these data suggest that TET2 mutant clones most commonly persist during PEG-IFN-α-2a therapy despite eradication of JAK2V617F mutant clones. The recent discovery that mutations in TET2,13,15,16,36 DNMT3A,14 and IDH1/237 result in increased self-renewal suggests that such mutations might affect the ability of therapies such as PEG-IFN-α-2a to eliminate mutant MPN disease-initiating cells bearing these mutations or confer acquired resistance to stem-cell targeted therapies. However, whether mutations in TET2 and/or other genes that regulate the HSC compartment (such as DNMT3a and IDH1/2) result in persistence of malignant clones during PEG-IFN-α-2a therapy has yet to be directly demonstrated. Further laboratory-based studies on the influence of specific genetic alterations with and without JAK2V617F mutations as found in MPN patients with sensitivity to IFN may shed further light on the role of these mutations in response to PEG-IFN-α-2a. Moreover, larger clinical studies in MPN patients treated with PEG-IFN-α-2a and other agents, including hydroxyurea and/or JAK inhibitors, will provide further insight into the relationship between specific mutations, clonal heterogeneity, and response to MPN-directed therapies.
In conclusion, therapy with PEG-IFN-α-2a induces high CHR rates, and, in a subset of patients, it can induce CMR, which can be sustained after therapy discontinuation. The lack of complete eradication of JAK2V617F in some patients appears to be associated with the acquisition of mutations outside the JAK2 locus. However, even patients with CMR and carrying no detectable known mutations still harbor EECs, suggesting that the persistence of clonal hematopoiesis may be driven by heretofore unidentified MPN disease alleles. Additional clinical and molecular studies are needed to determine markers of sensitivity and resistance to IFN in patients with PV and ET.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients who participated in this trial as well as the dedicated nurses and nurse practitioners who cared for them.
This work was supported in part by grants from the National Institutes of Health, National Cancer Institute (U54CA143798-01/CA/NCI NIH, Physical Sciences Oncology Center; and 1R01CA138234-01/CA/NCI NIH) (R.L.L.); grants from the Peter Solomon Fund at Memorial Sloan-Kettering Cancer Center, the MPN Research Foundation, and the Starr Cancer Consortium (R.L.L.); a basic research fellowship from the American Society of Hematology (O.A.-W.); a grant from the National Institutes of Health, National Cancer Institute (K08CA160647-01/CA/NCI NIH) (O.A.-W.); the National Natural Science Foundation of China (81170490) (S.-J.Z.); and the Leukemia and Lymphoma Society (R.L.L.).
Authorship
Contribution: A.Q.-C., O.A.-W., T.M., R.L.L., and S.V. performed analysis of clinical and molecular data; A.Q.-C., O.A.-W., R.L.L., and S.V. wrote the manuscript; A.Q.-C., J.C., H.K., and S.V. enrolled patients for clinical trial and treated and cared for patients enrolled in the study; T.M., Z.E., and D.H. collected and processed the samples and performed methylcellulose colony assays; and O.A.-W., O.K., A.-L.R., S.-J.Z., and R.L.L. performed molecular analysis of patient samples.
Conflict-of-interest disclosure: S.V. received research support for the conduct of the clinical study from Roche. The remaining authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, MD Anderson Cancer Center, Unit 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: sverstov@mdanderson.org; and Ross L. Levine, Zuckerman 519, Human Oncology and Pathogenesis Program and Leukemia Service, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; e-mail: leviner@mskcc.org.
References
Author notes
A.Q.-C., O.A.-W., T.M., R.L.L., and S.V. contributed equally to this study.