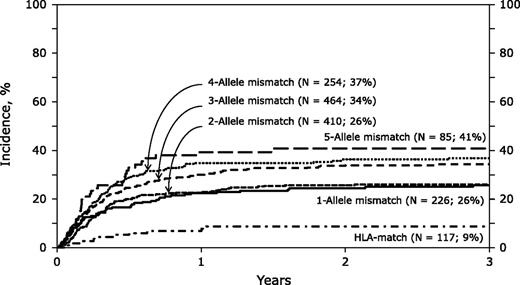
In this issue of Blood, Eapen et al report that improved allele-level matching for 4 HLA loci (-A, -B, -C, and -DRB1) produces better outcomes in single cord blood transplants (CBTs). We applaud the investigators for advancing cord blood selection criteria that will improve patient survival.1
The 3-year cumulative incidence of nonrelapse mortality was increased by more allele-level mismatches after single CBT. See Figure 1A in the article by Eapen et al that begins on page 133.
The 3-year cumulative incidence of nonrelapse mortality was increased by more allele-level mismatches after single CBT. See Figure 1A in the article by Eapen et al that begins on page 133.
The authors report the outcomes of 1568 CBTs using myeloablative conditioning for acute leukemia and myelodysplastic syndrome in patients with a median age of 9 years. Center for International Blood and Marrow Transplant Research and Eurocord-European Group for Blood and Marrow Transplantation data were used. Of note, donor-recipient HLA typing at allele level was not available in 784 cases (50%), and in those cases, donor-recipient HLA matching was estimated using the HaplogicSM III developed by the National Marrow Donor Program based on the available low/intermediate level testing that might have confounded the outcomes. The investigators showed that the frequency of neutrophil recovery was lower for recipients of mismatches at 3, 4, or 5 but not at 1 or 2 alleles compared with those of HLA-matched units. Nonrelapse mortality (NRM) was higher with units mismatched at 1 to 5 alleles compared with matched units (see figure). Overall mortality was not significantly different among the majority of cohorts except being higher for those that received units mismatched at 5 alleles. This retrospective study confirms the clinical importance of selecting better HLA allele-matched units for single CBT, an observation already well described for bone marrow and peripheral blood progenitor cell transplantation.2
The authors conclude that CB transplantation with ≥3 allele level mismatches should be avoided due to unacceptable NRM with inferior survival. Using this principle, approximately half of the patients in their study would not have received a CBT. This policy would have its impact primarily on the 4/6 matched patients using the current standard with intermediate level testing for HLA-A and -B and high-resolution testing for DRB1; 90% of the 4/6 matched patients were reported to have ≥3 allele-level mismatches in their series. This information underscores the fact that additional high-quality CB units need to be added to the global inventory for optimal CBT outcomes.
Eapen et al also report that CB unit cell dose has an impact on NRM independent of HLA matching. In fact, among patients transplanted with CB units mismatched at 3 alleles, units with total nucleated cell (TNC) dose ≥3 × 107 cells/kg had an NRM of 29% to 35% compared with an NRM of 52% observed with lower TNC doses, comparable with an NRM incidence of 26% observed with 1 or 2 allele mismatches. Thus, unit cell dose may overcome the negative impact of allele mismatches. A matrix of cell dose and allele matching will need to be considered to optimize CBT outcome.
Many patients deemed eligible for hematopoietic stem cell transplantation (HSCT) do not proceed due to unavailability of adequately matched related and unrelated donors. Patients of racial and ethnic minorities are more disadvantaged because of their relatively lower representation in donor registries. CB has been a great resource for extending the access to HSCT, especially to minorities, because mismatched CB is better tolerated than similar mismatches when bone marrow or peripheral blood progenitor cells are used. The increased use of CBT over the last decade reflects this fact.
It is obvious that with stricter criteria for allele-level matching applied, fewer patients will be eligible to proceed with CBT. Two strategies might help us to overcome this barrier until we have better units in the global inventory. First would be to identify possible permissive mismatches if units mismatched at ≤2 allele levels are not available. CBT with units matched with noninherited maternal HLA antigens3 or mismatched at graft-versus-host-only direction are reported to have lower NRM and improved survival.4 Second, graft manipulation might allow us to use smaller CB units with better HLA allele level matches. Currently, there are a number of promising graft manipulation strategies in the clinic for the ex vivo expansion5,6 and enhanced homing of CB units.7,8
In conclusion, Eapen et al suggest that allele-level matching improves transplant outcomes but at the price of reducing the number of available units for a given patient. The continued procurement of high-quality units with higher TNC doses in the global inventory will offset this restriction in the years to come. Additional analysis of allele matching in the double CBT setting is needed, particularly for older patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.