Key Points
Patients with primary myelofibrosis and intermediate-2 or high IPSS risk have a median life expectancy of 4 years or less.
PMF patients with higher IPSS risks who receive ruxolitinib treatment have longer survival than those who receive conventional therapy.
Abstract
The international prognostic scoring system (IPSS) provides reliable risk assessment in patients with primary myelofibrosis (PMF). Recent clinical trials in PMF patients with intermediate-2 or high IPSS risk have shown a survival advantage of ruxolitinib over placebo (COMFORT-1) or best available therapy (COMFORT-2). Because crossover was allowed in these studies, we analyzed the cohort of ruxolitinib-naive patients used for developing the dynamic IPSS (DIPSS). By adopting ad hoc statistical analyses, we compared survival from diagnosis of 100 PMF patients receiving ruxolitinib within COMFORT-2 with that of 350 patients of the DIPSS study. Subjects were properly matched, and both left-truncation and right-censoring were accounted in order to compare higher IPSS risks exclusively. Patients receiving ruxolitinib had longer survival (5 years, 95% confidence interval [CI]: 2.9-7.8 vs 3.5 years, 95% CI: 3.0-3.9) with a hazard ratio of 0.61 (95% CI: 0.41-0.91; P = .0148). This observation suggests that ruxolitinib may modify the natural history of PMF.
Introduction
Survival of patients with primary myelofibrosis (PMF) is stratified in 4 risk categories using the international prognostic scoring system (IPSS) model1 at diagnosis or dynamic IPSS (DIPSS)2 and DIPSS-plus3 time-dependent models during follow-up. The median PMF survival for intermediate-2 or high IPSS risks is shorter than 4 years. Conversely, survival of patients with secondary myelofibrosis (sMF) post-polycythemia vera4 and essential thrombocythemia5 is unknown.6-8
Among JAK-inhibitors,9-11 ruxolitinib was the only one approved for the treatment of MF (PMF and sMF). The 2 prospective, randomized, phase III studies with ruxolitinib, named COMFORT-1 (vs placebo)12 and COMFORT-2 (vs best available therapy [BAT]),13 included patients with intermediate-2 and high IPSS risk MF with circulating blast cells <10%. Despite the fact that many patients switched, per study protocols, from the control arm to ruxolitinib, the intention-to-treat analysis showed better survival for patients randomized to ruxolitinib than for the comparators.13-16 Two additional survival comparisons of ruxolitinib-treated patients12 vs historical controls have become available, both calculating survival from different time points for the 2 groups being compared: from ruxolitinib initiation for JAK inhibitor-treated patients and from the initial referral to an academic center for the control cohort.17,18 One study did not disclose any survival advantage,18 whereas the second demonstrated survival benefit.17
In this study, we compared survival from diagnosis of PMF patients who received ruxolitinib (COMFORT-2 cohort) with that of a comparable group of conventionally treated PMF patients (DIPSS cohort).2
Study design
The COMFORT-2 study included 219 patients with MF (PMF/sMF) at IPSS intermediate-2 and high risk randomized 2:1 to receive ruxolitinib or BAT. Patients were allowed to cross from BAT to ruxolitinib if qualified as per the study protocol. The date of diagnosis was extracted from the documented medical history. Novartis Corporation provided COMFORT-2 data. In this study, we included all patients with PMF who received ruxolitinib, either in the randomized treatment arm or after crossover from BAT, with an available date of diagnosis. This subset of COMFORT-2 patients will be referred to as the COMFORT-2 cohort. The multicenter DIPSS database includes 519 PMF patients not receiving any experimental drug at data cutoff and censored at the time of hematopoietic stem cell transplantation. All patients had IPSS factors collected at diagnosis and thereafter. The study was approved by the Institutional Review Board of Varese and conducted in accordance with the principles of the Declaration of Helsinki.
To allow a fair comparison of overall survival from diagnosis, we selected a subset of patients of the DIPSS cohort comparable with the COMFORT-2 group and used statistical methods taking the specific situation of this retrospective comparison into account. In detail, patients who entered COMFORT-2 might have had any IPSS risk at time of diagnosis but became intermediate-2 or high IPSS risk during follow-up, maintaining a blast count <10% (both were inclusion criteria for COMFORT-2). We consider these parameters as the most relevant, and, by applying them to the DIPSS database, 350 (67%) of 519 patients were selected to define an appropriate control cohort, referred to as DIPSS cohort. The date of diagnosis was considered as origin of the time scale, and patients entered the analysis when starting treatment with ruxolitinib (COMFORT-2 cohort) or at the time of acquisition of an IPSS intermediate-2 or high risk (DIPSS cohort). By backdating COMFORT-2 data from enrollment to the date of diagnosis, we generated left-truncated data, excluding potentially eligible patients dying before they had the chance to enter COMFORT-2. Similarly, in selecting intermediate-2 or high risk patients for the DIPSS cohort, we have to account for the situation that patients who did not worsen to these risk categories by the time of data cutoff for our analysis were excluded. Therefore, standard survival methods may lead to biased results. To avoid this bias, Kaplan-Meier estimates and other statistical methods for left-truncated (and right-censored) survival data were applied.19 Entry time for the analysis is the start of ruxolitinib in the COMFORT-2 cohort and first documentation of intermediate-2 or high risk status in the DIPSS cohort. Statistical analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX) software.
Results and discussion
Overall, 100 PMF patients receiving ruxolitinib were studied: 76 from randomization and 24 after crossover. The median time between PMF diagnosis and study entry was 5 years (range, 0.1-38 years). Demographics of the COMFORT-2 and DIPSS cohorts are reported in Table 1. Age, the only parameter evaluable at diagnosis for comparison, was significantly different between the 2 populations: 67 years (range, 29-30) in DIPSS and 61 years (range, 27-76) in the COMFORT-2 cohort (Wilcoxon rank sum test, P < .001). The median time at risk (from time of entering analysis to last contact/death) was 2.6 years (range, 0.1-23) for DIPSS and 2.5 years (range, 0.1-3.3) for COMFORT-2, which was not statistically different.
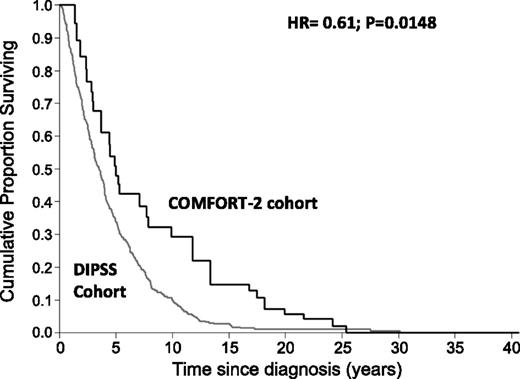
We observed 30 (30%) deaths in the COMFORT-2 cohort and 258 (86%) in the DIPSS cohort. Survival from diagnosis of patients who received ruxolitinib was better than that of patients who received only conventional therapy (Figure 1; hazard ratio [HR]: 0.61, 95% confidence interval [CI]: 0.41-0.91, P = .0148). Median survival was 3.5 years (95% CI: 3.0-3.9) for the DIPSS cohort and 5 years (95% CI: 2.9-7.8) for the COMFORT-2 cohort. The 8-year survival probability from initial diagnosis was 32.2% (95% CI: 16.5-49.1) for COMFORT-2 and 15.9% (95% CI: 11.6-20.8) for DIPSS. After adjusting for age at diagnosis and IPSS risk at the time of entering the analysis, multivariate Cox regression indicated that ruxolitinib still maintained an effect on survival (HR 0.64, 95% CI: 0.4-0.96, P = .034).
Survival estimate from diagnosis of PMF patients who become intermediate-2 and high risk IPSS with a blast cell count <10% at any time of their follow-up according to the COMFORT-2 (n = 100) and DIPSS (N = 350) cohorts.
Survival estimate from diagnosis of PMF patients who become intermediate-2 and high risk IPSS with a blast cell count <10% at any time of their follow-up according to the COMFORT-2 (n = 100) and DIPSS (N = 350) cohorts.
This result adds information on the use of ruxolitinib for patients in the unfavorable risk groups. The update of the COMFORT-1 trial (median follow-up, 2 years)14 was still consistent with the prior observation that ruxolitinib is associated with survival advantage (HR to placebo, 0.58).15 Similar results have been obtained in the 3-year update of the COMFORT-2 trial (HR to BAT, 0.52).16 The HRs reported in those 2 prospective trials are consistent with the 0.61 HR we obtained in this analysis.
These figures of HR indicate that the risk of death might be reduced by 40% to 50% by introducing ruxolitinib into the treatment of PMF patients. To find the same HRs when comparing ruxolitinib with different comparators (placebo, BAT, historical controls) suggests that non-JAK inhibitor therapies do not affect the natural disease course, similarly to placebo. In fact, little improvement of splenomegaly, symptoms, or quality of life with BAT vs placebo has been demonstrated.20 Concerning previous historical-controlled analyses, investigators compared survival in ruxolitinib-treated patients from the time of enrollment with that of a control cohort from the initial referral, either unmatched18 or matched for COMFORT-2 entry criteria.17 In the present analysis, the advantage of using the DIPSS cohort as control is that IPSS stratification is available anytime. This offers the opportunity to select comparable patients with the same characteristics acquired over time. Ruxolitinib influences survival outcome, leaving unaffected the JAK2V617F clone.12 However, giving the best doses of ruxolitinib for a very long time, a prolongation of survival has been documented with a direct relationship with greater reduction of splenomegaly.17 Again, the marked improvement of the general condition, assessed by quality of life and symptomatic scores, might make patients less vulnerable to PMF complications.
In conclusion, patients treated with ruxolitinib at some point during their disease history had a better survival when compared with those who continued standard treatment of the whole duration of follow-up, ultimately suggesting that ruxolitinib affects PMF natural history.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Research reported in this publication was supported by the Associazione Italiana Leucemie Onlus Varese. Studies performed at the Department of Hematology Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Department of Molecular Medicine, University of Pavia, Pavia, Italy and at the Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy were supported by grants from Associazione Italiana per la Ricerca sul Cancro (Special Program Molecular Clinical Oncology 5x1000, project no. 1005).
Authorship
Contribution: F.P. designed research, performed research, and wrote the paper; M.M., D.C., B.M., and C.P. performed research and analyzed data; F.C., A.M.V., E.M., T.B., E.R., H.G., L.K., C.H., and M.C. provided and analyzed single institutional series included in the whole study; and N.H. provided statistical support. All authors drafted and approved the manuscript.
Conflict-of-interest disclosure: F.P. has participated in advisory boards for Novartis, Sanofi, and Celgene; F.C. has participated in advisory boards for Novartis, Sanofi, Celgene, and AOP Orphan Pharmaceuticals and has participated in speakers bureaus for Novartis; A.M.V. has participated in advisory boards for Novartis; L.K. has participated in advisory boards for Novartis; N.H is an employee of Novartis; and C.H. received honoraria and research grant from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Francesco Passamonti, Division of Hematology, Department of Medicine, University Hospital Ospedale di Circolo e Fondazione Macchi, Viale L. Borri 57, 21100 Varese, Italy; e-mail: francesco.passamonti@ospedale.varese.it.