Key Points
B-cell tolerance plays a critical role in controlling production of PF4/heparin-specific antibodies.
Abstract
Immune complexes consisting of heparin, platelet factor 4 (PF4), and PF4/heparin-reactive antibodies are central to the pathogenesis of heparin-induced thrombocytopenia (HIT). It is as yet unclear what triggers the initial induction of pathogenic antibodies. We identified B cells in peripheral blood of healthy adults that produce PF4/heparin-specific antibodies following in vitro stimulation with proinflammatory molecules containing deoxycytosine-deoxyguanosine (CpG). Similarly, B cells from unmanipulated wild-type mice produced PF4/heparin-specific antibodies following in vitro or in vivo CpG stimulation. Thus, both healthy humans and mice possess preexisting inactive/tolerant PF4/heparin-specific B cells. The findings suggest that breakdown of tolerance leads to PF4/heparin-specific B-cell activation and antibody production in patients developing HIT. Consistent with this concept, mice lacking protein kinase Cδ (PKCδ) that are prone to breakdown of B-cell tolerance produced anti-PF4/heparin antibodies spontaneously. Therefore, breakdown of tolerance can lead to PF4/heparin-specific antibody production, and B-cell tolerance may play an important role in HIT pathogenesis.
Introduction
Heparin-induced thrombocytopenia (HIT) is the most common drug-induced, immune-mediated thrombocytopenia and usually occurs after 3 to 6 days of heparin treatment.1,2 Antibodies that recognize platelet factor 4 (PF4) and heparin to form immune complexes are central to the pathogenesis of HIT.2 PF4/heparin-specific antibodies in HIT patients are predominantly polyclonal immunoglobulin G1 (IgG1) isotype with some IgG2.2-4 IgG/PF4/heparin immune complexes bind FcγRIIA on the platelet surface and induce platelet activation, resulting in thrombocytopenia and a high risk of serious arterial and/or venous thrombosis and thromboembolism.5,6
Glycosaminoglycans (GAGs) other than heparin are widely expressed on endothelial cells and elsewhere in the body and react with PF4 to produce structural changes recognized by HIT antibodies.7 Other non-GAG polyanionic macromolecules such as DNA and RNA similarly modify PF4.8 Thus, humans are exposed to structurally modified, immunogenic PF4 throughout their lives, and B-cell tolerance to epitopes recognized by HIT antibodies is likely established early in life, possibly explaining why only 3% of patients given heparin experience HIT.9 B-cell tolerance to self-antigens is mediated by clonal deletion and receptor editing, leading to elimination of PF4/heparin-specific B cells, and by anergy, in which B cells are reversibly inactivated.10
Study design
Mice
Protein kinase Cδ (PKCδ)-deficient mice were derived from Anning Lin, University of Chicago, with permission from Dr Keiichi Nakayama (Kyushu University),11 and maintained on a C57BL/6 genetic background. Experimental and control mice were 8 to 10 weeks old. Mice were maintained in the Biological Resource Center at the Medical College of Wisconsin. Animal protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Activation of B cells and detection of PF4/heparin-specific antibodies
In vitro activation of human or mouse B cells, in vivo activation of mouse B cells by deoxycytosine-deoxyguanosine (CpG) challenge, and measurement of human PF4 (hPF4)/heparin- or mouse PF4 (mPF4)/heparin-specific antibodies by enzyme-linked immunosorbent assay (ELISA) were performed as previously described.12-16 G. Arepally (Duke University) kindly provided mPF4. Human studies were approved by the Institutional Review Board of BloodCenter of Wisconsin. This study was conducted in accordance with the Declaration of Helsinki.
Estimation of PF4/heparin-specific B cell frequencies in PBMCs
The frequency of B cells in cultured human peripheral blood mononuclear cells (PBMCs) that produce PF4/heparin-specific antibodies was estimated by most probable number (MPN) analysis (supplemental Table 1 on the Blood Web site).
Statistical analysis
Statistical analysis was performed with the Mann-Whitney test for unpaired data and the Wilcoxon signed-rank test for paired data. The predetermined α level of significance is 0.05.
Results and discussion
Proinflammatory signals from Toll-like receptors can break down B-cell anergy and lead to autoantibody production.17,18 To investigate whether anergy regulates HIT immune responses, we examined whether there are preexisting, inactive PF4/heparin-specific B cells in healthy adults. PBMCs from 16 healthy adult donors were fivefold serially diluted, and three fivefold dilutions were analyzed. Each dilution of PBMC samples was cultured in 24 or 36 wells of 96-well plates with proinflammatory molecules containing CpG. An experiment from a typical healthy subject demonstrated that hPF4/heparin-specific immunoglobulin Ms (IgMs) were detected in the culture supernatant in 83.3% of wells (20 of 24) containing 1 × 105, 16.7% of wells (6 of 36) containing 2 × 104, and 0% of wells (0 of 36) containing 4 × 103 PBMCs after 6 to 7 days of culture (Figure 1A). These IgM antibodies consistently reacted more strongly with hPF4/heparin than with hPF4 alone and were inhibited by excess heparin (Figure 1B) as is typical of antibodies from patients with HIT. B cells purified from PBMCs by fluorescence-activated cell sorter sorting behaved similarly following in vitro stimulation (data not shown), indicating that T cells are not essential for antibody production under the conditions of our experiments. Experiments involving a total of 16 healthy blood donors showed that hPF4/heparin-specific IgMs were consistently detected in the culture supernatant in almost all wells containing 1 × 105 cells, in a high percentage of wells containing 2 × 104 cells, and a few of wells containing 4 × 103 cells. However, PF4/heparin-specific IgG antibodies were rarely detected in the culture supernatant of any wells (data not shown). We used the most probable number (MPN) mathematical method to analyze these serial dilution data and estimated the minimum proportion of human PBMC B cells that were PF4/heparin-specific and found that PF4/heparin-specific B cells were present at a frequency ranging from 0.1 to 1.0 per 1000 B cells in PBMCs (supplemental Table 1). Therefore, PBMCs from healthy human adults in this study contain relatively large numbers of tolerant hPF4/heparin-specific B cells that can be activated to produce IgM antibodies following in vitro polyclonal stimulation.
Production of PF4/heparin-specific antibodies by PBMCs from healthy human adults or by splenic B cells from unmanipulated wild-type mice following in vitro polyclonal stimulation. PBMCs isolated from healthy donors were cultured at concentrations of 1 × 105, 2 × 104, or 4 × 103 cells per well in 24 or 36 wells of a 96-well plate in the presence of CpG. After 6 to 7 days of culture, supernatants were collected and tested for hPF4/heparin-specific IgM production by ELISA. (A) A representative experiment performed with PBMCs from 1 healthy donor. Each dot represents 1 well, and the horizontal lines indicate the mean values. Based on the values obtained from nonstimulated PBMCs, OD values above the cutoff value of 0.3 were considered positive. Data shown are representative of 16 healthy donors. (B) Antigen specificity of the IgMs. Specificity of the IgMs toward hPF4/heparin complexes, hPF4 alone, or hPF4 in the presence of excess heparin was examined. Data shown are from 6 positive wells with 2 × 104 cells in A and are representative of positive wells from 16 healthy donors. (C-D) Splenic B cells isolated from unmanipulated wild-type mice were cultured at concentrations of 5 × 104 or 1 × 104 cells per well in 12 wells of a 96-well plate in the presence of CpG or lipopolysaccharide (LPS). After 4 days of culture, supernatants were collected and tested for mPF4/heparin-specific IgM production by ELISA. (C) A representative CpG stimulation experiment performed with 1 wild-type mouse is shown. Each dot represents 1 well, and the horizontal lines indicate the mean values. Data shown are representative of 3 independent experiments. (D) A representative LPS stimulation experiment performed with 1 wild-type mouse is shown. Each dot represents 1 well, and the horizontal lines indicate the mean values. Data shown are representative of 3 independent experiments with 3 wild-type mice. (E-F) Antigen specificity of the IgMs. Specificity of the IgMs generated by (E) CpG or (F) LPS stimulation toward mPF4/heparin complexes, mPF4 alone, or mPF4 in the presence of excess heparin was examined. Data shown are from 6 positive wells with 5 × 104 B cells stimulated with CpG in C (left) or stimulated with LPS in D (right) and are representative of positive wells from 3 independent experiments with 3 wild-type mice. Statistical analysis was performed with (A,C,D) the Mann-Whitney test for unpaired data and (B,E,F) the Wilcoxon signed-rank test for paired data.
Production of PF4/heparin-specific antibodies by PBMCs from healthy human adults or by splenic B cells from unmanipulated wild-type mice following in vitro polyclonal stimulation. PBMCs isolated from healthy donors were cultured at concentrations of 1 × 105, 2 × 104, or 4 × 103 cells per well in 24 or 36 wells of a 96-well plate in the presence of CpG. After 6 to 7 days of culture, supernatants were collected and tested for hPF4/heparin-specific IgM production by ELISA. (A) A representative experiment performed with PBMCs from 1 healthy donor. Each dot represents 1 well, and the horizontal lines indicate the mean values. Based on the values obtained from nonstimulated PBMCs, OD values above the cutoff value of 0.3 were considered positive. Data shown are representative of 16 healthy donors. (B) Antigen specificity of the IgMs. Specificity of the IgMs toward hPF4/heparin complexes, hPF4 alone, or hPF4 in the presence of excess heparin was examined. Data shown are from 6 positive wells with 2 × 104 cells in A and are representative of positive wells from 16 healthy donors. (C-D) Splenic B cells isolated from unmanipulated wild-type mice were cultured at concentrations of 5 × 104 or 1 × 104 cells per well in 12 wells of a 96-well plate in the presence of CpG or lipopolysaccharide (LPS). After 4 days of culture, supernatants were collected and tested for mPF4/heparin-specific IgM production by ELISA. (C) A representative CpG stimulation experiment performed with 1 wild-type mouse is shown. Each dot represents 1 well, and the horizontal lines indicate the mean values. Data shown are representative of 3 independent experiments. (D) A representative LPS stimulation experiment performed with 1 wild-type mouse is shown. Each dot represents 1 well, and the horizontal lines indicate the mean values. Data shown are representative of 3 independent experiments with 3 wild-type mice. (E-F) Antigen specificity of the IgMs. Specificity of the IgMs generated by (E) CpG or (F) LPS stimulation toward mPF4/heparin complexes, mPF4 alone, or mPF4 in the presence of excess heparin was examined. Data shown are from 6 positive wells with 5 × 104 B cells stimulated with CpG in C (left) or stimulated with LPS in D (right) and are representative of positive wells from 3 independent experiments with 3 wild-type mice. Statistical analysis was performed with (A,C,D) the Mann-Whitney test for unpaired data and (B,E,F) the Wilcoxon signed-rank test for paired data.
We examined whether PF4/heparin-specific B cells like those found in humans can be demonstrated in healthy wild-type mice. Splenic B cells were purified from unmanipulated wild-type mice and serially diluted fivefold. Each dilution was cultured in 12 wells of 96-well plates with media containing CpG or LPS. After 4 days of culture, mPF4/heparin-specific IgMs were detected in the culture supernatant in all wells containing 5 × 104 or 1 × 104 cells (Figure 1C-D) but not in wells containing fewer cells (data not shown). These IgMs reacted more strongly with mPF4/heparin than with mPF4 alone, and binding was inhibited by excess heparin (Figure 1E-F). As in the human studies, mPF4/heparin-specific IgGs were rarely detected in the culture supernatants (data not shown). Thus, unmanipulated wild-type mice, like humans, possess preexisting, tolerant PF4/heparin-specific B cells that can be activated to produce IgM antibodies following polyclonal stimulation in vitro.
We also examined whether the preexisting, tolerant PF4/heparin-specific B cells in wild-type mice can produce antibodies following polyclonal stimulation in vivo. Wild-type mice had basal levels of PF4/heparin-specific IgM antibodies and markedly increased production of these IgMs following CpG injection (Figure 2A). Some wild-type mice produced PF4/heparin-specific IgG antibodies following CpG challenge (Figure 2A). These IgMs and IgGs reacted specifically with mPF4/heparin complexes but less well with mPF4 alone, and their reactions were inhibited by excess heparin (Figure 2A). Thus, preexisting, inactive PF4/heparin-specific B cells in wild-type mice can be activated by polyclonal stimulation to produce IgMs and even switch to IgGs in vivo.
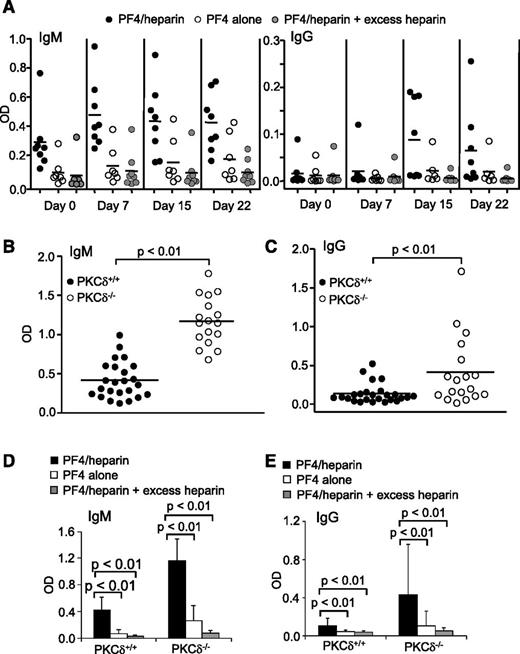
Production of PF4/heparin-specific antibodies in wild-type mice by in vivo CpG challenge and spontaneous production of PF4/heparin-specific antibodies in PKCδ-deficient mice. (A) Production of PF4/heparin-specific antibodies in wild-type mice by in vivo CpG challenge. Wild-type mice were intraperitoneally injected with CpG. At the indicated time points following CpG treatment, sera were collected and tested for mPF4/heparin-specific (left) IgM or (right) IgG production by ELISA. Specificity of the antibodies toward mPF4, mPF4/heparin, and mPF4 in the presence of excess heparin was examined. Each dot represents a mouse, and the horizontal lines indicate the mean values. Data shown are obtained from 8 wild-type mice. (B-E) Spontaneous production of PF4/heparin-specific antibodies in PKCδ-deficient mice. Sera were collected from unmanipulated wild-type (PKCδ+/+) or PKCδ-deficient (PKCδ−/−) mice and tested for mPF4/heparin-specific (B) IgM or (C) IgG production by ELISA. Specificity of the (D) IgMs or (E) IgGs toward mPF4, mPF4/heparin, and mPF4 in the presence of excess heparin was examined. Each dot represents a mouse, and the horizontal lines indicate the mean values. Data shown are obtained from 24 wild-type and 17 PKCδ-deficient mice. Statistical analysis was performed with (B-C) the Mann-Whitney test for unpaired data and (D-E) the Wilcoxon signed-rank test for paired data.
Production of PF4/heparin-specific antibodies in wild-type mice by in vivo CpG challenge and spontaneous production of PF4/heparin-specific antibodies in PKCδ-deficient mice. (A) Production of PF4/heparin-specific antibodies in wild-type mice by in vivo CpG challenge. Wild-type mice were intraperitoneally injected with CpG. At the indicated time points following CpG treatment, sera were collected and tested for mPF4/heparin-specific (left) IgM or (right) IgG production by ELISA. Specificity of the antibodies toward mPF4, mPF4/heparin, and mPF4 in the presence of excess heparin was examined. Each dot represents a mouse, and the horizontal lines indicate the mean values. Data shown are obtained from 8 wild-type mice. (B-E) Spontaneous production of PF4/heparin-specific antibodies in PKCδ-deficient mice. Sera were collected from unmanipulated wild-type (PKCδ+/+) or PKCδ-deficient (PKCδ−/−) mice and tested for mPF4/heparin-specific (B) IgM or (C) IgG production by ELISA. Specificity of the (D) IgMs or (E) IgGs toward mPF4, mPF4/heparin, and mPF4 in the presence of excess heparin was examined. Each dot represents a mouse, and the horizontal lines indicate the mean values. Data shown are obtained from 24 wild-type and 17 PKCδ-deficient mice. Statistical analysis was performed with (B-C) the Mann-Whitney test for unpaired data and (D-E) the Wilcoxon signed-rank test for paired data.
Activation of preexisting, inactive PF4/heparin-specific B cells to produce antibodies by polyclonal stimulation suggests B-cell tolerance breakdown. To obtain further evidence that HIT antibody production is normally restricted by B-cell tolerance, we examined the effect of PKCδ deficiency on PF4/heparin-specific antibody production. Signaling molecule PKCδ controls B-cell anergy and its deficiency impairs B-cell tolerance, leading to autoantibody production in humans and mice.11,19,20 Unmanipulated PKCδ-deficient mice spontaneously produced significantly higher levels of PF4/heparin-specific IgMs than wild-type controls (Figure 2B). A significantly higher number of unmanipulated PKCδ-deficient mice also spontaneously produced elevated levels of PF4/heparin-specific IgGs relative to wild-type controls (Figure 2C). These IgMs and IgGs specifically reacted with mPF4/heparin complexes but not mPF4 alone, and their binding was inhibited by excess heparin (Figure 2D-E). These findings provide additional evidence that B-cell tolerance controls PF4/heparin-specific B cells and that tolerance breakdown leads to spontaneous production of PF4/heparin-specific HIT antibodies.
PF4/heparin-specific B-cell tolerance can be broken down by an inflammatory stimulus. By analogy, the inflammatory milieu associated with tissue trauma may account for the increased incidence of HIT antibodies and clinical HIT in patients given heparin during cardiopulmonary bypass or orthopedic surgery.21,22 The tolerance breakdown concept is consistent with the previously observed atypical immune response features of HIT patients, such as rapid generation and loss of anti-PF4/heparin antibodies and the IgG character of antibodies produced by most patients exposed to heparin for the first time.1,2
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Aniko Szabo for assistance with MPN and statistical analysis.
This work is supported in part by National Institutes of Health grants PO1 (National Heart, Lung, and Blood Institute) HL44612 (to D.W. and D.K.N.) and R01 (National Institute of Allergy and Infectious Diseases) AI079087 (to D.W.).
Authorship
Contribution: Y.Z. contributed to research design, performed the research, and analyzed the results; A.W.W. designed MPN analysis of the data and also contributed to other research design and data analysis; M.Y. performed some initial experiments; A.P. contributed to the research of human samples; B.E.T. performed some initial experiments; D.K.N. critically reviewed the manuscript; G.C.W. provided intellectual input; R.H.A. provided intellectual input and critically reviewed the manuscript; R.W. provided intellectual input, supervised the study, performed the research, and critically reviewed the manuscript; and D.W. conceived and supervised the study, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renren Wen, Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI 53226; e-mail: renren.wen@bcw.edu; or Demin Wang, Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI 53226; e-mail: demin.wang@bcw.edu.