In this issue of Blood, Capitini et al use mouse models to demonstrate that STAT1 loss or inhibition causes dendritic cell (DC) modulation, resulting in lesser GVHD.1
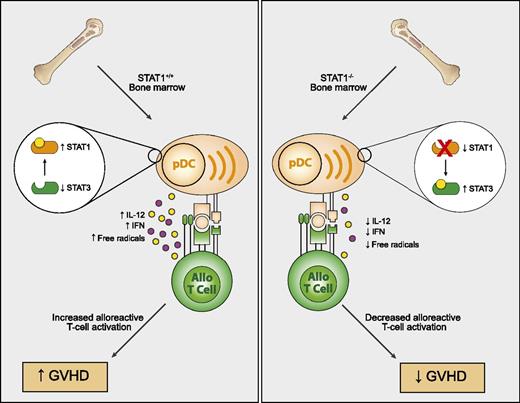
Impact of STAT1 on GVHD. STAT1−/− bone marrow cells give rise to pDCs with a tolerogenic phenotype after allogeneic HSCT in mice. This results in increased STAT3 and lesser production of interferon, IL12, and free radical formation culminating in lesser activation of alloreactive Th1 cells and GVHD pathology.
Impact of STAT1 on GVHD. STAT1−/− bone marrow cells give rise to pDCs with a tolerogenic phenotype after allogeneic HSCT in mice. This results in increased STAT3 and lesser production of interferon, IL12, and free radical formation culminating in lesser activation of alloreactive Th1 cells and GVHD pathology.
Graft-versus-host disease (GVHD) remains a significant cause of morbidity following allogeneic hematopoietic stem cell transplantation (HSCT). Much of the earlier research and approaches focused on the alloreactive donor T cell itself as the principal driver and mediator of GVHD. Approaches have ranged from simple removal of all T cells (which unfortunately also abrogates graft-versus-tumor [GVT] responses), blockade of costimulation or cytokine pathways, interfering with lymphocyte trafficking to GVHD target tissues, use of purified T-cell subsets (ie, memory cells, T regulatory [Treg] cells, or T-helper [Th]2 subsets), and modulation of T cells ex vivo to targeting intracellular signaling via Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways.2 Recently, more attention has been given to a principal and pivotal collaborator in the fueling of alloreactions after HSCT: the dendritic cell (DC). Once viewed as simple antigen-presenting vehicles, it is clear there is a tremendous complexity among DC subpopulations that profoundly affects T cells through promoting, inhibiting, and modulating T-cell responses. Initial studies in allogeneic HSCT focused on the host DC as the culprit in sensitizing the donor T cell,3 but subsequent investigations demonstrated that both donor and host DCs can contribute to GVHD and GVT responses.4 This may be the case particularly with chronic GVHD, which is becoming a more prominent complication in allogeneic HSCT. The study by Capitini et al1 nicely demonstrates the role of STAT1 in this process. STAT1 is a critical transcriptional regulator of Th1-type pathways and has been shown to directly impact CD4 T-cell responses in GVHD.5 However, when using bone marrow from STAT1 knockout mice (STAT1−/−), Capitini et al showed, across several strain combinations, a significant diminution of acute GVHD when normal donor T cells were later given as a delayed lymphocyte infusion (DLI). They further demonstrated that the absence of STAT1 markedly altered the development of donor-derived DCs as CD9−SiglecHhi plasmacytoid DCs (pDCs) predominated. These STAT1−/− pDCs also displayed important functional alterations and exhibited a tolerogenic phenotype by expressing elevated STAT3. STAT1−/− pDCs produced less interleukin (IL)12, type I interferon, and free radicals (see figure). Not only did this impair the Th1-driven GVHD processes when the normal T cells were later given as DLI, but the lesser free radical formation likely also diminished tissue damage so often associated with fueling the GVHD cascade. Tregs were also expanded. Importantly, the investigators demonstrated maintenance of GVT effects and that pharmacologic STAT1 inhibition also prevented GVHD. These studies offer a new pathway that can be targeted in GVHD modulating DC generation after HSCT. Using small molecule inhibitors or small interfering RNA, it may offer means to modulate STAT1 in a transient fashion, making it a more clinically applicable approach.
Several important questions remain to be considered. What are the long-term effects on GVT when the systemic STAT1 inhibitor is used? Is prolonged suppression of STAT1 required or is the induction of CD9−SiglecHhi pDCs enough to maintain the protective effect of STAT1 inhibition? Can it be used to modulate ongoing GVHD? The study used a DLI model where the role of the donor-derived DCs may be greater compared with host DCs. It is important to determine effects on chronic GVHD as in other murine models; the effects of STAT1 deficiency on the development of chronic GVHD have been contradictory.6,7 All of these questions are critical next steps before clinical application can be attempted. The study by Capitini et al indicates that bringing out the “softer side” to the DC may be an attractive approach in GVHD prevention through indirect control of donor T cells following DLI.
Conflict-of-interest disclosure: The author declares no competing financial interests.