Key Points
A staging system based on proteinuria and glomerular filtration rate discriminates patients at different risk of progression to dialysis.
Changes in proteinuria and glomerular filtration rate allow early assessment of renal response to therapy.
Abstract
The kidney is involved in 70% of patients with immunoglobulin light-chain (AL) amyloidosis, but little is known on progression or reversibility of renal involvement, and criteria for renal response have never been validated. Newly diagnosed patients from the Pavia (n = 461, testing cohort) and Heidelberg (n = 271, validation cohort) centers were included. Proteinuria >5 g/24 h and estimated glomerular filtration rate (eGFR) <50 mL/min predicted progression to dialysis best. Proteinuria below and eGFR above the thresholds indicated low risk (0 and 4% at 3 years in the testing and validation cohorts, respectively). High proteinuria and low eGFR indicated high risk (60% and 85% at 3 years). At 6 months, a ≥25% eGFR decrease predicted poor renal survival in both cohorts and was adopted as criterion for renal progression. A decrease in proteinuria by ≥30% or below 0.5 g/24 h without renal progression was the criterion for renal response, being associated with longer renal survival in the testing and validation populations. Hematologic very good partial or complete remission at 6 months improved renal outcome in both populations. We identified and validated a staging system for renal involvement and criteria for early assessment of renal response and progression in AL amyloidosis that should be used in clinical practice and trial design.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is caused by a usually small plasma cell clone synthesizing light chains undergoing conformational changes that lead to their aggregation and deposition in tissues.1 This process leads to progressive multiple organ dysfunction and death if left untreated. Advanced, irreversible organ damage at presentation is the main limitation to improve the outcome of patients with AL amyloidosis. Early diagnosis and recognition of reversible organ damage is crucial to improve patients’ survival and quality of life.2 Treatment should be risk adapted, based on accurate stratification of organ dysfunction.2
Light-chain amyloidosis is the commonest disease among the recently defined monoclonal gammopathies of renal significance,3 and the kidney is involved in 70% of patients, manifesting with nephrotic syndrome and progressive renal failure.4 Renal involvement results in significant morbidity, and renal failure limits the therapeutic options.5 However, the current staging system of patients with AL amyloidosis that is used to guide the treatment strategy is based on the severity of heart involvement, which is the major determinant of survival, and does not take into account renal dysfunction.6,7 Although a recent consensus of the International Society for Amyloidosis (ISA) revised the criteria for hematologic and cardiac response to treatment based on patients’ survival,8 to date, there is no validated prognostic score for renal damage in this disease. Furthermore, the criteria for renal response to chemotherapy have not been updated since 2005 and have never been validated.9 Thus, there is a need for a simple and reliable means of stratifying the severity of renal involvement, as well as criteria for assessing the efficacy of treatment in preventing progression of renal damage, similarly to what has now become common practice for heart involvement after the introduction of cardiac biomarkers. However, because renal involvement has less relevant impact on patients’ survival compared with cardiac involvement, the criteria of renal response and progression should predict progression to dialysis and not necessarily death. Moreover, considering that the median time to a profound reduction in proteinuria is approximately 1 year,10-13 long after hematologic response is assessed,8 it is important to identify earlier markers of renal response that can allow timely changes in the therapeutic regimen.
Based on these considerations, we designed the present study in order to identify and validate criteria for assessing the risk of dialysis as well as criteria for early identification of renal response and progression in 732 consecutive, previously untreated patients with AL amyloidosis and renal involvement evaluated at 2 European referral centers: the Pavia Amyloidosis Research and Treatment Centre and the Heidelberg Amyloidosis Centre.
Methods
The databases of the Pavia Amyloidosis Research and Treatment Centre and of the Heidelberg Amyloidosis Centre were systematically searched for subjects with renal involvement diagnosed between 2004 and 2012. All the patients had biopsy-proven amyloidosis, and the deposits were characterized as AL-type by light-microscopy immunohistochemistry,14 immunoelectron microscopy,15 or proteomics16 analysis. A monoclonal light chain of the same isotype of that found in the deposits needed to be detected in serum and/or urine by immunofixation and or circulating free light-chain (FLC) quantitation.17,18 Renal involvement was defined as a urinary protein loss of >0.5 g/24 h, predominantly albumin, according to the 2005 ISA criteria.9 Patients who were on dialysis at the time of diagnosis were excluded, as well as those in whom progression to dialysis during follow-up was unknown and those who lacked data on serum creatinine and 24-hour urinary protein loss. All patients gave written informed consent as approved by the institutional Ethics Committee, in accordance with the Declaration of Helsinki.
Baseline evaluation was standardized at each center and included a complete physical examination, assessment of amyloid organ involvement,9 echocardiography, measurement of amino-terminal pro–natriuretic peptide type B19 and troponins,20,21 measurement of serum creatinine concentration and of 24-hour urinary protein excretion, serum and urine immunofixation electrophoresis, and quantitation of FLC. Hematologic response was assessed according to the 2012 ISA criteria 3 and/or 6 months after treatment initiation.8
The study end point was renal survival, defined as the time from diagnosis to dialysis initiation. Patients who died without requiring dialysis were considered censored for the purpose of the analysis of renal survival.22 We assessed the impact on renal survival of baseline variables and of their changes at the time of response assessment. The Italian cohort was used as the testing population and the German series as the validating cohort.
Cox models were fitted to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for progression to dialysis, identifying baseline variables predicting renal survival. Receiver operating characteristic (ROC) analyses based on progression to dialysis at 2 years identified the thresholds of baseline variables best predicting renal survival. A staging system for progression to dialysis based on the cutoffs derived from the ROC analyses was created. Survival curves were plotted according to Kaplan-Meier.
The evaluation of renal response and progression criteria after frontline therapy was based on a 6-month landmark analysis, as was recently done for establishing the international criteria for hematologic and cardiac response.8 Changes in baseline variables that were associated with renal survival were identified by means of ROC analyses based on progression to dialysis at 24 months.
The baseline predictors of renal survival, the renal staging system, and the response and progression criteria identified in the testing sample (Italian cohort) were validated in the validating cohort (German series) based on renal outcome. MedCalc 12.7 (MedCalc Software, Belgium) was used for computation.
Results
A total of 748 previously untreated patients were diagnosed with AL amyloidosis at the Pavia Amyloidosis Research and Treatment Center between 2004 and 2012, and 501 (67%) had renal involvement. Of them, 21 (4%) were excluded because they were on dialysis at the time of diagnosis and 19 (4%) had an incomplete data set. In the same period of time, 441 subjects were evaluated at the Heidelberg Amyloidosis Centre, and 292 (66%) had renal involvement. Of them, 16 (5%) were on dialysis at the time of diagnosis and additional 5 subjects (2%) were excluded due to incomplete data set. A total of 732 previously untreated, newly diagnosed patients were included, 461 in the Italian cohort and 271 in the German cohort. The patients’ characteristics are summarized in Table 1.
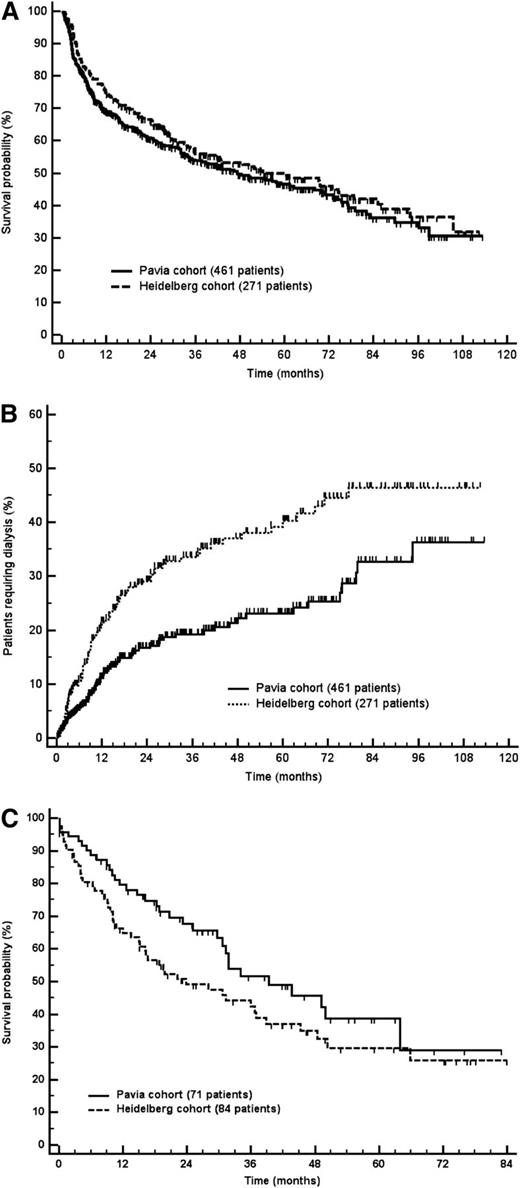
The median follow-up of living patients was 40 and 50 months in the Pavia and Heidelberg cohorts, respectively. In the Italian cohort, 226 patients (49%) died, and 136 (50%) died in the German cohort. Patients’ survival was not different between the 2 groups (Figure 1A). A total of 71 (15%) patients required dialysis in the Italian group and 84 (31%) in the German series. Renal survival was significantly longer in the Italian cohort (Figure 1B). This difference could not be explained by differences in first- and second-line treatment. In particular, when the analysis was limited to patients receiving front-line melphalan and dexamethasone, there was still a significantly higher rate of progression to dialysis in the German cohort (26% vs 12% at 2 years; P = .002).
Patients’ survival and time to dialysis. (A) Patients’ survival. Median survival was 47 months in the Pavia cohort and 54 months in the Heidelberg cohort (P = .230). (B) Renal survival (P < .001). (C) Survival from dialysis initiation. Median survival was 39 months in the Pavia cohort and 24 months in the Heidelberg cohort (P = .102).
Patients’ survival and time to dialysis. (A) Patients’ survival. Median survival was 47 months in the Pavia cohort and 54 months in the Heidelberg cohort (P = .230). (B) Renal survival (P < .001). (C) Survival from dialysis initiation. Median survival was 39 months in the Pavia cohort and 24 months in the Heidelberg cohort (P = .102).
Patients’ survival from dialysis initiation was not significantly different in the 2 cohorts (Figure 1C). Hemodialysis was chosen in most cases, and only 11 patients underwent peritoneal dialysis (9% of the 155 subjects requiring dialysis), 8 in the Italian cohort and 3 in the German cohort. Compared with patients on hemodialysis, there were no significant differences in age, cardiac biomarker concentration, proteinuria, estimated glomerular filtration rate (eGFR), bone marrow plasma cell infiltrate, and FLC concentration. However, a higher proportion of patients achieved at least a very good partial hematologic response (VGPR) in the peritoneal dialysis group (65% vs 23%; P = .014 by Fisher exact test), and this translated into a longer survival from dialysis initiation (median not reached vs 31 months; P = .015). This probably indicates that peritoneal dialysis was preferred in subjects who were less likely to require further chemotherapy.
Factors predicting renal survival
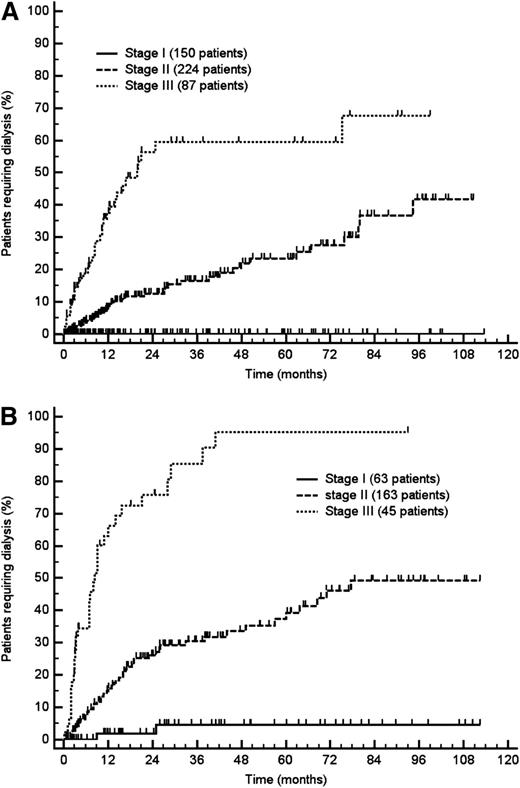
The variables predicting progression to dialysis in the Italian (testing) cohort are reported in Table 2. Renal survival was influenced by proteinuria, eGFR, and, to a lesser extent, by serum albumin. At multivariate analysis, proteinuria and eGFR independently predicted progression to dialysis (Table 2). In an ROC analysis based on progression to dialysis at 2 years, the area under the ROC curves was 0.70 (95% CI, 0.62-0.78) for proteinuria and 0.88 (95% CI, 0.82-0.93) for eGFR. The thresholds best discriminating patients who progressed were 5 g/24 h for urinary protein loss (sensitivity 77.8%; 95% CI, 64.4%-88.0%; specificity 50.0%; 95% CI, 42.3%-57.7%) and 50 mL/min per 1.73 m2 for eGFR (sensitivity 85.2%; 95% CI, 72.9%-93.4%; specificity 78.2%, 95% CI, 71.3%-84.1%). Based on these cutoffs, it was possible to design a staging system sharply discriminating 3 groups with significantly different risk of progression to dialysis (Figure 2A), with none (renal stage I), 1 (renal stage II), or both (renal stage III) risk factors, respectively (Figure 3A). The proportion of patients requiring dialysis 3 years after diagnosis was 7% in renal stage II and 60% in renal stage III, whereas no patient with baseline proteinuria ≤5 g/24 h and eGFR ≥50 mL/min per 1.73 m2 (renal stage I) became dialysis dependent. There was no significant difference in patients’ survival among the 3 renal stages (not shown).
Progression to dialysis according to renal stage. (A) Testing cohort (461 patients; P < .001). (B) Validation cohort (271 patients; P < .001). Renal stage I: both proteinuria ≤5 g/24 h and eGFR ≥50 mL/min per 1.73 m2. Renal stage II: either proteinuria >5 g/24 h or eGFR <50 mL/min per 1.73 m2. Renal stage III: both proteinuria >5 g/24 h and eGFR <50 mL/min per 1.73 m2.
Progression to dialysis according to renal stage. (A) Testing cohort (461 patients; P < .001). (B) Validation cohort (271 patients; P < .001). Renal stage I: both proteinuria ≤5 g/24 h and eGFR ≥50 mL/min per 1.73 m2. Renal stage II: either proteinuria >5 g/24 h or eGFR <50 mL/min per 1.73 m2. Renal stage III: both proteinuria >5 g/24 h and eGFR <50 mL/min per 1.73 m2.
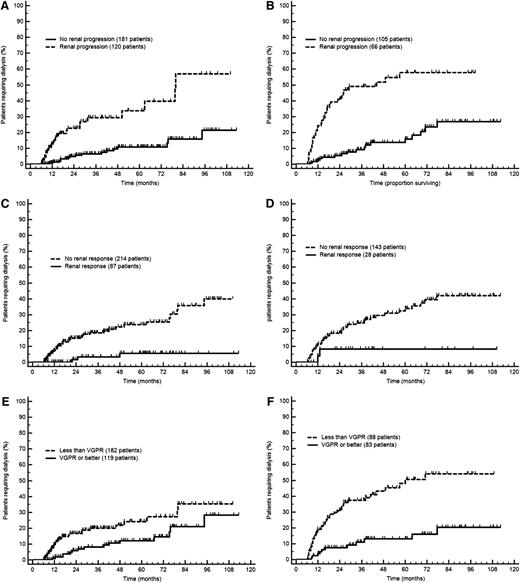
Progression to dialysis in the testing and validation cohorts according to the novel response and progression criteria (6-month landmark analysis). (A) Renal progression in the testing cohort (P < .001). (B) Renal progression in the validation cohort (P < .001). (C) Renal response in the testing cohort (P < .001). (D) Renal response in the validation cohort (P = .039). (E) Hematologic response in the testing cohort (P = .014). (F) Hematologic response in the validation cohort (P < .001). Renal progression is defined as a decrease in eGFR ≥25%. Renal response is defined as a decrease in proteinuria ≥30% or a drop of proteinuria below 0.5 g/24 h in the absence of renal progression. VGPR is defined as dFLC <40 mg/L.
Progression to dialysis in the testing and validation cohorts according to the novel response and progression criteria (6-month landmark analysis). (A) Renal progression in the testing cohort (P < .001). (B) Renal progression in the validation cohort (P < .001). (C) Renal response in the testing cohort (P < .001). (D) Renal response in the validation cohort (P = .039). (E) Hematologic response in the testing cohort (P = .014). (F) Hematologic response in the validation cohort (P < .001). Renal progression is defined as a decrease in eGFR ≥25%. Renal response is defined as a decrease in proteinuria ≥30% or a drop of proteinuria below 0.5 g/24 h in the absence of renal progression. VGPR is defined as dFLC <40 mg/L.
These results were validated in the Heidelberg cohort. An analysis of renal survival performed in the validation cohort confirmed that proteinuria >5 g/24 h (HR 7.02; 95% CI, 3.59-13.73; P < .001) and eGFR <50 mL/min per 1.73 m2 (HR 5.71; 95% CI, 3.62-9.00; P < .001) were independent determinants of progression to dialysis. The proposed renal staging system was able to detect significant differences in renal survival among renal stage I, II, and III subjects, with 4%, 30%, and 85% of patients in the German cohort becoming dialysis dependent at 3 years, respectively (Figure 2B).
Criteria for renal response and progression
For the identification of response and progression criteria, we performed a landmark analysis on 472 patients (64%), 301 in the Italian cohort and 171 in the German cohort, who were evaluated for response 6 months after treatment initiation. A total of 65 patients (9%) died, 49 (7%) started dialysis before response assessment, and 146 (22%) lacked an evaluation of response at 6 months. An ROC analysis based on progression to dialysis at 2 years from the landmark date performed in the testing cohort (Italian series) showed that changes in proteinuria, eGFR, and dFLC (difference between involved [amyloidogenic] and uninvolved free light chain) were able to discriminate subjects progressing to dialysis. The cutoffs best predicting renal outcome were a 28.8% decrease in proteinuria, a 23.1% decrease in eGFR, and a dFLC concentration after chemotherapy <44.8 mg/L. Remarkably, the best discriminating dFLC threshold was very close to the current definition of a VGPR (dFLC <40 mg/L). Based on these results and on renal survival, we devised criteria for renal response and progression as well as a target for hematologic response 6 months after treatment initiation (Table 3 and Figure 3).
In the whole cohort, 126 patients met the criterion for renal progression but did not start dialysis during follow-up. Of them, 40 (32%) died and the remaining are alive and dialysis-free after a median follow-up of 27 months. In the overall population, renal progression had similar frequency in the 3 renal stages (36% in stage I, 38% in stage II, and 45% in stage III; P = .112 compared with stage I). At multivariate analysis, renal progression (HR 4.47; 95% CI, 3.05-6.55; P < .001) and renal stage (HR 2.76; 95% CI, 2.03-3.75; P < .001) were independent determinants of renal outcome. The impact of the new criterion for renal progression in the 3 renal stages is reported in supplemental Figure 1 (available at the Blood Web site).
Achievement of a partial response (ie, ≥50% reduction of dFLC still >40 mg/L) did not confer a significant advantage over nonresponders in both groups (data not shown). Importantly, obtaining VGPR or better was able to improve renal outcome not only in renal stage II (in the whole series, median time to dialysis not reached in both groups, progression to dialysis at 3 years 4% vs 20%; P = .002) but also in renal stage III patients (median time to dialysis 30 vs 69 months in the overall population; P < .001), indicating that successful treatment can improve renal outcome also in high-risk subjects. We also tested the impact of a >90% reduction of dFLC on renal survival, which was reported to predict a better renal outcome by Pinney and coworkers.23 We observed that this pronounced dFLC decrease was associated with longer time to dialysis both in the testing (8% vs 20% at 3 years; P = .004) and in the validation (15% vs 31% at 3 years; P = .014) cohorts.
We also checked whether the novel criteria of renal response and progression were still able to discriminate between patients with different renal outcome in the subgroups of patients with low baseline values of proteinuria and eGFR. This analysis was limited by the low number of events and therefore was conducted on the overall study population. Achievement of a renal response at 6 months was associated with better renal outcome also in the subgroup of 180 patients, with baseline proteinuria <3.5 g/24 h, with 2% vs 6% of patients requiring dialysis at 2 years (P = .050). Among the 24 patients with baseline proteinuria <0.72 g/24 h whose proteinuria needed to decrease by <30% in order to drop below 0.5 g/24 h, 11 achieved a renal response, and 2, who had not achieved renal response, progressed to dialysis after 2 and 40 months, respectively. However, in this small subgroup, the difference in renal outcome between renal responders and nonresponders did not reach statistical significance (P = .116). Moreover, it was possible to demonstrate a poorer outcome for patients who met the criterion for renal progression also in the subgroup of 159 subjects with baseline eGFR >85 mL/min per 1.73 m2, with 13% vs 1% of patients requiring dialysis at 2 years (P = .038). When we looked at patients with low eGFR at baseline, we observed that in the subgroup of 36 patients with eGFR <23 mL/min per 1.73 m2, the new criterion for renal progression was still able to identify subjects with poorer outcome (40% vs 80% of patients requiring dialysis at 2 years; P = .030).
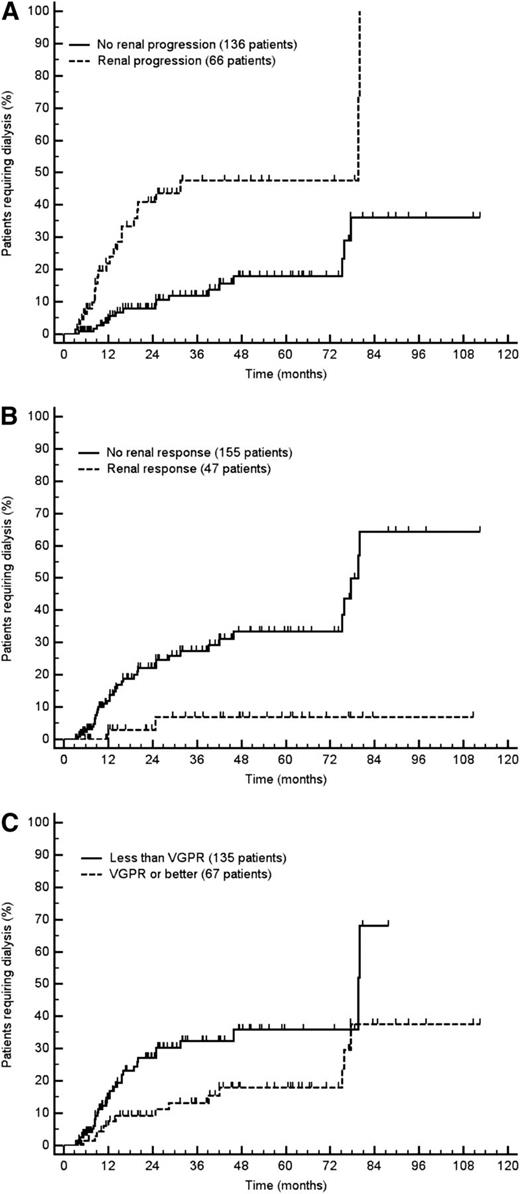
Finally, we tested the applicability of the novel renal response and progression criteria, related to renal survival, in patients who had response assessment data at an even earlier time point (3 months), 133 in the Italian cohort and 69 in the German cohort, in a 3-month landmark analysis. Given the relatively small number of cases, we performed this analysis in the overall study population of 202 evaluable patients. We found that the proposed response and progression criteria retained their prognostic significance (Figure 4).
Progression to dialysis in the overall population according to the novel response and progression criteria (3-month landmark analysis). (A) Renal progression (P = .002). (B) Renal response (P < .001). (C) Hematologic response (P = .013). Renal progression is defined as a decrease in eGFR ≥25%. Renal response is defined as a decrease in proteinuria ≥30% or a drop of proteinuria below 0.5 g/24 h in the absence of renal progression. VGPR is defined as dFLC <40 mg/L.
Progression to dialysis in the overall population according to the novel response and progression criteria (3-month landmark analysis). (A) Renal progression (P = .002). (B) Renal response (P < .001). (C) Hematologic response (P = .013). Renal progression is defined as a decrease in eGFR ≥25%. Renal response is defined as a decrease in proteinuria ≥30% or a drop of proteinuria below 0.5 g/24 h in the absence of renal progression. VGPR is defined as dFLC <40 mg/L.
Evaluation of the 2005 criteria for renal response and progression
We assessed the impact on renal outcome of the 2005 definition of renal response (>50% decrease in proteinuria in the absence of a >25% decrease in eGFR),9 and we found that it was also able to discriminate patients with better renal outcome in the testing population (2% vs 13% progressing to dialysis at 2 years; P = .004). However, the difference did not reach statistical significance in the validation cohort (7% vs 18% progressing to dialysis at 2 years; P = .073). More importantly, the patients who achieved a decrease in proteinuria between 30 and 50% and those in whom proteinuria decreased by >50% had identical outcome both in the testing (0% vs 2% progressing to dialysis at 2 years; P = .865) and in the validation (10% vs 7% progressing to dialysis at 2 years; P = .677) cohorts. This indicates that the novel response criterion correctly reclassifies as responders patients who have a good outcome even though they achieve a less pronounced decrease in proteinuria.
Increases in proteinuria at 6 months provided worse discrimination in renal outcome than decreases in proteinuria. In particular, in the overall cohort, a 50% increase in proteinuria in the absence of a ≥25% decrease in eGFR was associated with a worsening in renal survival, but this did not reach statistical significance (12% vs 15% at 2 years; P = .076).
Effect of renal response and progression on survival
Renal progression (ie, a ≥25% decrease in eGFR) at 6 months was associated with a shorter patients’ survival in the Italian cohort (median 68 vs 96 months; P = .047), but the difference was not significant in the German cohort (median 79 vs 105 months; P = .061). Renal response (ie, a decrease ≥30% or below 0.5 g/24 h of proteinuria) was not associated with different patients’ survival in both series, with the median being 66 vs 63 months (P = .988) in the Pavia group and 75 vs 60 months in the Heidelberg group (P = .476). Based on the study by Leung et al,10 we checked whether more pronounced decreases in proteinuria were associated with a survival advantage. Very few patients (4 in the Italian series and 1 in the German cohort) achieved a >95% reduction in proteinuria at 6 months. A >75% decrease in proteinuria was observed in 33 patients in the Italian series and in 18 subjects in the validation cohort and was associated with a nonsignificant survival advantage (median 61 vs 77 months in the Italian cohort [P = .061] and 60 vs 78 months in the German series [P = .174]).
Discussion
The management of patients with AL amyloidosis is based first on accurate risk evaluation at baseline to assist in the choice of therapy and second on frequent assessment of hematologic and organ response, in order to rapidly identify treatment failures and switch to effective second-line therapies.2 Simple, widely available, and validated criteria for baseline staging and for assessment of hematologic and cardiac response have markedly improved the care of patients with this disease, both in routine clinical practice and in clinical trials.8,24 However, until now, no staging system has been designed for renal involvement in AL amyloidosis. Currently used response criteria are only based on expert consensus and have not been validated, even though the kidney is involved in more than two-thirds of patients at diagnosis and is responsible for severe morbidity, reduction of quality of life, and even treatment limitations.
Two retrospective studies have addressed the issue of renal response to treatment in AL amyloidosis. The UK group showed that progression to dialysis is more likely with decreasing eGFR and less likely in patients who achieve a >90% dFLC decrease after chemotherapy.23 More recently, the Mayo Clinic group reported that a profound reduction (>95%) in proteinuria at 1 year is associated with longer patients’ survival.10 They also found that rises in serum creatinine were not associated with poorer patients’ survival. These findings challenged the current definition of renal response proposed in 2005 by the ISA panel, requiring a >50% only decrease in proteinuria in the absence of a >25% increase in serum creatinine.9 However, the Mayo Clinic study was based on patients’ survival, which is predominantly influenced by cardiac dysfunction rather than by renal involvement, and response was assessed 1 year after treatment initiation, while the assessment of hematologic response is done earlier, at 3 or 6 months.8
Consequently, we identified prognostic criteria for renal involvement based on progression to dialysis in the present study. Importantly, all results obtained in the Italian cohort were confirmed and validated in the German cohort. We were able to design a simple staging system, based on universally available measurements, proteinuria and eGFR, sharply discriminating groups of patients with different probabilities of losing renal function. Although high-risk subjects have a 60% to 80% probability of requiring dialysis within 3 years, an “early stage” of renal damage exists, defined by proteinuria ≤5 g/24 h and eGFR ≥50 mL/min per 1.73 m2, when progression to dialysis is very unlikely (0% to 4% at 3 years). This again emphasizes the need for early diagnosis, in order to identify and treat patients with AL amyloidosis before irreversible organ damage ensues, as was recently advocated.2
Moreover, we identified and validated markers of renal response and progression that can be assessed as early as 3 and 6 months after treatment initiation, the time when evaluation of hematologic and cardiac response is performed according to current guidelines.8 We observed that an early decrease by at least 30% of proteinuria or its reduction below 0.5 g/24 h was associated with increased likelihood of preserving renal function. Early increases in proteinuria did not significantly affect renal outcome. However, it is possible that later increases in proteinuria in case of relapse can have a greater effect. Moreover, as it was proposed by the 2005 ISA panel of experts, we found that an early decrease (≥25%) in eGFR portends a poor renal survival. These novel response and progression criteria allow assessing the adequacy of response to therapy very early, when the treatment strategy can still be changed if the response is unsatisfactory. Furthermore, they can be used for individual patients’ assessment and as surrogate end points in clinical trials together with the recently validated hematologic and cardiac response criteria, particularly in the studies on novel agents, which are currently being designed and will use as their primary end points both progression to organ failure and survival. Finally, the ability of predicting the rate of progression to end-stage renal disease will allow patients’ stratification and accurate calculation of sample size in these trials.
In our study, renal staging and response were not significantly associated with patients’ survival, indicating that renal involvement does not have a major prognostic impact in this disease. Also, a pronounced (>75%) decrease in proteinuria, which in the study by Leung et al10 predicted a longer survival, had only borderline significance in our study. This discrepancy could be due to our earlier evaluation of renal response (6 vs 12 months), missing patients who subsequently reach the target improvement.
As expected, hematologic response to therapy assessed according to the novel criteria8 was associated with improved renal outcome. However, partial hematologic remission is an unsatisfactory end point, being unable to reduce the risk of dialysis. Conversely, achievement of good quality response (ie, at least VGPR) is required to improve renal outcome; this is in agreement with the findings of the UK group.23
A limitation of the present study is the retrospective setting. Therefore, the renal staging system and response criteria should be validated in the large prospective phase 3 first-line trial currently being performed internationally in this disease. On the other hand, the fact that we obtained highly comparable results in 2 independent amyloidosis patient populations exposed to different regimens strengthens the findings of our study. Moreover, only a small subgroup of patients had a very low proteinuria (between 0.5 and 0.7 g/24 h), and we could not determine whether these subjects are evaluable for renal response.
In conclusion, we showed first that in AL amyloidosis, the progression of renal dysfunction is accurately predicted by baseline proteinuria and eGFR. These 2 variables identify low-risk patients who are unlikely to require dialysis and subjects who are at very high risk of renal failure. Secondly, early changes in eGFR and proteinuria should be used to assess treatment efficacy, in addition to the currently recognized hematologic and cardiac response criteria.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgments
This study was supported in part by grant from Associazione Italiana per la Ricerca sul Cancro Special Program Molecular Clinical Oncology 5 per mille n. 9965 and GERAMY Fkz 01GM1107 (BMBF, Germany). The funding institution had no role in the study design, data collection, analysis and interpretation of data, writing of the report, and the decision to submit.
Authorship
Contribution: G.P., G.M., and S.S. designed the study, evaluated patients, collected data, analyzed data, wrote the manuscript, and gave final approval; U.H. and P.M. evaluated patients, collected data, analyzed data, critically reviewed the manuscript, and gave final approval; C.K., A.F., and M.V.R. evaluated patients, collected data, critically reviewed the manuscript, and gave final approval; A.D.H. and R.M. critically reviewed the manuscript and gave final approval; and R.A. collected data, critically reviewed the manuscript, and gave final approval.
Conflict-of-interest disclosure: U.H. and S.S. received honoraria from Janssen-Cilag and Celgene, and G.M. received honoraria from Millennium-Takeda. The remaining authors declare no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, Viale Golgi, 19, 27100 Pavia, Italy; e-mail: gmerlini@unipv.it.