Key Points
We developed an approach of T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin.
Outcomes of suitably matched URD-HSCT and HRD-HSCT are similar, and HRD-HSCT improves outcomes of patients with high-risk leukemia.
Abstract
We developed an approach of T-cell-replete haploidentical hematopoietic stem cell transplantation (HSCT) with low-dose anti-T-lymphocyte globulin and prospectively compared outcomes of all contemporaneous T-cell-replete HSCT performed at our center using matched sibling donors (MSDs), unrelated donors (URDs), and haploidentical related donors (HRDs). From 2008 to 2013, 90 patients underwent MSD-HSCT, 116 underwent URD-HSCT, and 99 underwent HRD-HSCT. HRDs were associated with higher incidences of grades 2 to 4 (42.4%) and severe acute graft-versus-host disease (17.2%) and nonrelapse mortality (30.5%), compared with MSDs (15.6%, 5.6%, and 4.7%, respectively; P < .05), but were similar to URDs, even fully 10/10 HLA-matched URDs. For high-risk patients, a superior graft-versus-leukemia effect was observed in HRD-HSCT, with 5-year relapse rates of 15.4% in HRD-HSCT, 28.2% in URD-HSCT (P = .07), and 49.9% in MSD-HSCT (P = .002). Furthermore, 5-year disease-free survival rates were not significantly different for patients undergoing transplantation using 3 types of donors, with 63.6%, 58.4%, and 58.3% for MSD, URD, and HRD transplantation, respectively (P = .574). Our data indicate that outcomes after HSCT from suitably matched URDs and HRDs with low-dose anti-T-lymphocyte globulin are similar and that HRD improves outcomes of patients with high-risk leukemia. This trial was registered at www.chictr.org (Chinese Clinical Trial Registry) as #ChiCTR-OCH-12002490.
Introduction
An HLA-identical sibling is the preferred donor of allogeneic hematopoietic stem cell transplantation (allo-HSCT), with overall survival (OS) rates at 1 year >70%. However, patients have only a 25% likelihood of HLA matching with a sibling and a <1% chance of HLA matching with another relative. For patients without a suitably matched related donor, options include starting the search for an unrelated donor (URD), cord blood units, or a haploidentical-related donor (HRD). The application of URD transplants has been limited by the difficulty of finding a phenotypically matched URD by chance, which varies from 60% to 70% for whites to under 10% for ethnic minorities, and the time involved in identifying, typing, and harvesting cells from an URD creates a time delay of ∼4 months.1,2 Unrelated cord blood is a new and promising stem cell source; however, the limited unit size contributes to engraftment failure, and the high incidence of posttransplant infection and slow immune recovery are major obstacles to its broader application in the adult population.3,4
The present increased interest in transplantation using HRDs arises from the immediate availability of a suitable donor for virtually all patients, particularly for those who urgently need transplantation. Although HRD-HSCT may be complicated by high rates of graft-versus-host disease (GVHD), treatment-related mortality and poor immune reconstitution, recent advances in conditioning regimen, prophylaxis of GVHD, and supportive care have significantly enhanced therapeutic benefits of HRD-HSCT.5-7 Here we developed an approach to HRD-HSCT, which uses T-cell-replete peripheral blood grafts in combination with low-dose anti-T-lymphocyte globulin to prevent GVHD and graft rejection. Furthermore, we performed the first prospective treatment trial to compare the potential beneficial effect of T-cell-replete HRD-HSCTs with all contemporaneous T-cell-replete HSCTs using matched sibling donors (MSDs) and URDs.
Methods
Patients
From March 2008 to March 2013, all consecutive patients with hematologic malignancies who were suitable candidates for allo-HSCT were included. The patients fulfilled the additional criteria: (1) no cases of low-risk acute myeloid leukemia (AML) or acute promyelocytic leukemia in the first complete remission (CR1); (2) no chronic myeloid leukemia (CML) in the first chronic phase; (3) no decompensated organ diseases. All the patients gave their written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethics review committee of the First Affiliated Hospital of Zhejiang University School of Medicine and registered at Chinese Clinical Trial Registry.
Treatment protocols and donor selection
High-resolution DNA typing for HLA-A, -B, -C, -DRB1, and -DQB1were performed in all patients and donors in our center. The treatment schedule was as follows: If a fully MSD was available, patients were assigned treatment with MSD-HSCT. If an MSD was unavailable, a suitably matched URD was used as the alternative, where a suitable match involved matching more than 8 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele loci (≥8/10) and at least 5 of 6 matching HLA-A, -B, and -DRB1 antigen loci. If an MSD or a suitably matched URD was unavailable within the time frame appropriate for the patient’s malignancy and clinical circumstances (that is patients achieved CR after induction chemotherapy and have received 2-3 courses of postremission consolidation therapy), patients were allowed treatment with HRD-HSCT.
Risk classification
Patients were classified as standard and high risk on the basis of cytogenetic abnormalities, white blood cell (WBC) count at diagnosis, response to induction chemotherapy, and relapse after CR1. High risk was defined by the presence of adverse cytogenetics and/or failure to achieve CR after 2 cycles of induction chemotherapy and/or patients in CR2 or beyond.8 Cytogenetic abnormalities were graded using established criteria.9,10 For acute lymphoblastic leukemia (ALL) patients, high risk was also defined by a WBC count >30 × 109/L for B-lineage ALL or >100 × 109/L for T-lineage ALL at diagnosis, and/or central nervous system leukemia at diagnosis.11 The high-risk group also included CML in other than chronic phase, myelodysplastic syndrome (MDS) with International Prognostic Scoring System scores >2.5, and relapsed non-Hodgkin lymphoma. Standard risk included patients who were ineligible for high risk.
Conditioning regimen and stem cell harvest
All patients were given myeloablative conditioning. For patients receiving MSD-HSCT or URD-HSCT, the main myeloablative conditioning regimen used involved busulfan (Bu; 3.2 mg/kg/d IV on days –7 to –4), cyclophosphamide (Cy; 60 mg/kg/d IV on days –3 to –2). For those patients with central nervous system leukemia or a high WBC count at diagnosis or failure to achieve CR before HSCT, the conditioning regimen consisted of cytarabine (2 g/m2 per day IV on days −8 to −7), Bu (3.2 mg/kg per day IV on days –6 to –4), Cy (1.8 g/m2 per day IV on days –3 to –2), and methyl-N-(2-chloroethyl)-N-cyclohexyl-N-nitrosourea (250 mg/m2 orally on day −1). Rabbit antithymocyte globulin (ATG, Thymoglobulin; Genzyme, Cambridge, MA) was also administered to patients receiving URD-HSCTs (4.5-6 mg/kg total dose).
For patients receiving HRD-HSCTs, the conditioning regimen consisted of cytarabine (4 g/m2/d IV on days −10 to −9), Bu (3.2 mg/kg per day IV on days –8 to –6), Cy (1.8 g/m2 per day IV on days –5 to –4), methyl-N-(2-chloroethyl)-N-cyclohexyl-N-nitrosourea (250 mg/m2 orally on day –3), and anti-T-lymphocyte globulin (ATG-F; Fresenius, Bad Homburg, Germany) (2.5 mg/kg per day IV on days −5 to −2).
Donors were treated with rhG-CSF (Filgrastim; Kirin, Japan; 5 µg/kg per day) injected subcutaneously for 5 to 6 consecutive days from day –4, peripheral blood stem cells were collected with a COBE Blood Cell Separator (Spectra LRS; COBE BCT Inc., Lakewood, CO) from a total blood volume of 10 to 12 L. The target mononuclear cell count and CD34+ cell count were 4 × 108 to 6 × 108/kg and 3 × 106 to 4 × 106/kg of recipient weight, respectively. No graft was subjected to ex vivo T-cell depletion.
GVHD prophylaxis
As described previously,12-14 in our center, patients in the MSD, URD, and HRD transplantation cohorts all received the same GVHD prophylaxis, consisting of cyclosporin A (CSA), methotrexate (MTX), and low-dose mycophenolate mofetil. CSA was scheduled to be given intravenously at 2.5 mg/kg/d from day −7, with a target blood level of 200 to 300 ng/mL. According to chimeric status and evidence of GVHD, the dosage of CSA was tapered during the second month posttransplantation, ending in complete withdrawal during the sixth month after MSD- or URD-HSCT, and during the ninth month after HRD-HSCT. Mycophenolate mofetil was initiated orally at 500 mg/d on day −9 for HRD-HSCT and on day +1 for MSD- or URD-HSCT, and withdrawn on day +100. MTX was given at 15 mg/m2 on day +1 and 10 mg/m2 on days +3 and +6. For patients receiving HRD-HSCT, an additional 10 mg/m2 MTX was administered on day +9 or +11.
Study end points, definitions, and statistical analysis
The primary end points of this study were OS, and disease-free survival (DFS) after HSCT. Only patients with successful engraftment were included in the analysis of acute GVHD (aGVHD). aGVHD was diagnosed and graded using established criteria.15 Given that grade 1 typically refers to patients with clinically uncertain aGVHD and most clinicians do not treat these patients systemically, only aGVHD patients of grades 2 and higher were considered for analysis. Patients who survived ≥100 days were analyzed for chronic GVHD (cGVHD).
Cumulative incidence using the competing risk method,16 as described by Gooley,17 was used to determine the difference in probabilities of aGVHD, cGVHD, and relapse in the 3 transplantation cohorts. OS and DFS were estimated using the Kaplan-Meier method. Multivariate Cox regression models using a forward stepwise procedure were applied to analyze the effects of these characteristics and other known clinical and biological factors on HSCT outcomes. All variables in the univariate analysis with a P value ≤.2 were included in the multivariate analysis.
Results
Patient, disease, and transplantation characteristics
A total of 305 patients met the study eligibility criteria. Ninety patients underwent MSD-HSCT, 116 patients underwent URD-HSCT, and 99 patients underwent HRD-HSCT. Table 1 shows the patient, disease, and transplantation characteristics of the study patients.
In the URD cohort, 71 patients (61.2%) received stem cells from a fully 10/10 HLA allele loci matched URD, 33 patients (28.4%) had a single HLA allele locus mismatched from their donors (9/10 HLA-A, B, -C, -DRB1, and -DQB1 loci matching), and 12 patients (10.3%) had 2 mismatching allele loci from their donors (8/10 loci matching). In the HRD cohort, patients received stem cells from a direct family member, mother (n = 22), father (n = 27), brother (n = 19), sister (n = 17), son (n = 8), or daughter (n = 6), who had more than 3 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele loci (>3/10) mismatched from the patient.
The MSD-, URD-, and HRD-HSCT cohorts were well matched, and there were no significant differences in terms of underlying disease, risk classification, time from diagnosis to HSCT, disease status at HSCT, or conditioning regimen between the 3 cohorts. There were only significant differences between 3 cohorts in terms of patient’s age, donor-patient gender relationship, and the number of CD34+ cells in stem cells, and these variables were not identified as significant in univariate or multivariate outcome analysis.
The end point of the last follow-up for all of the surviving patients was September 1, 2013. The median follow-up for surviving patients was 28.5 (range 5.5-66) months, 34.5 (range 5-65.5) months, and 26.5 (range 5.5-64.5) months in the MSD-, URD-, and HRD-HSCT cohorts, respectively.
Transplantation outcomes
Engraftment.
Patients engrafted to absolute neutrophil counts exceeded 0.5 ×109/L in a median time of 12 days (range, 7-17 days) in the MSD cohort, 12 days (range, 8-19 days) in the URD cohort, and 12 days (range, 8-24 days) in the HRD cohort. Although 100% patients receiving any source of stem cells all achieved myeloid recovery, the cumulative 15-day myeloid engraftment incidences were 97.8% in the MSD cohort, 97.4% in the URD cohort, and 78.6% in the HRD cohort. Patients receiving HRD-HSCT experienced significantly delayed myeloid recovery compared with those receiving MSD-HSCT or URD-HSCT (P < .001).
After myeloid recovery, all patients achieved sustained, full donor chimerism by day 30 after HSCT except for 1 patient in the HRD cohort who died before day 30. Among the patients surviving beyond 30 days, 7 patients experienced primary platelet engraftment failure (6 receiving HRDs, 1 receiving MSD). The median time and the cumulative 30-day incidences of platelet engraftment were 12 days (range, 7-58 days) and 95.5% in MSDs, 13days (range, 6-24 days) and 100% in URDs, and 15 days (range, 6-53 days) and 95.7% in HRDs, respectively. Patients receiving HRD-HSCT also experienced delayed platelet recovery compared with those receiving MSD-HSCT (P = .004) or URD-HSCT (P < .001). There was no significant difference between the MSD and URD cohorts in myeloid and platelet engraftments.
GVHD.
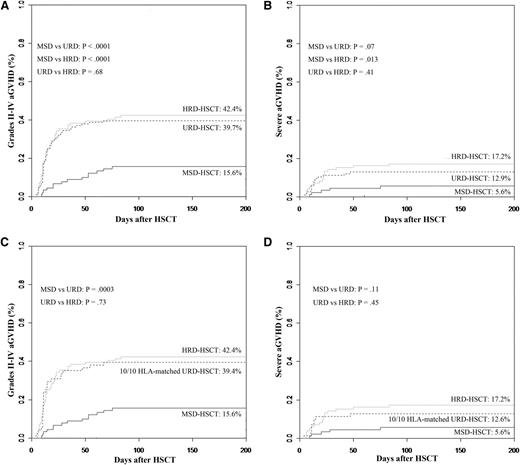
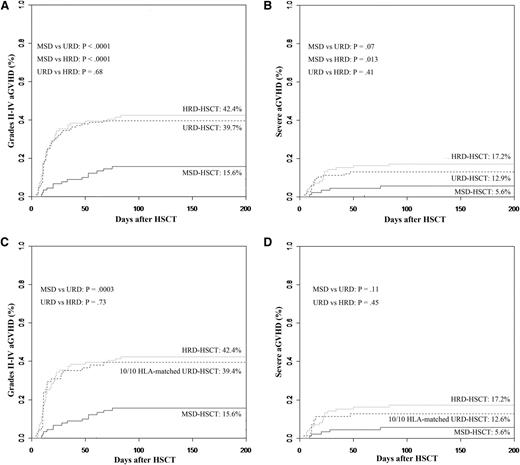
Grades 2 to 4 aGVHD were significantly more frequent in patients undergoing URD-HSCT or HRD-HSCT compared with those undergoing MSD-HSCT. Cumulative incidences of grades 2 to 4 aGVHD at 3 months were 15.6%, 39.7%, and 42.4% for patients undergoing transplantation using MSDs, URDs, and HRDs, respectively (P < .0001 for MSDs vs URDs and P < .0001 for MSDs vs HRDs, Figure 1A). Cumulative incidences for severe aGVHD at 3 months were 5.6%, 12.9%, and 17.2% in MDS-, URD-, and HRD-HSCT cohorts, respectively. Compared with MSD-HSCT, rates of severe GVHD were also significantly higher in HRD-HSCT, and less significantly higher in URD-HSCT (P = .013 for MSDs vs HRDs and P = .07 for MSDs vs URDs, Figure 1B). However, the incidences of grades 2 to 4 and severe aGVHD were comparable in patients receiving transplants from HRDs to those from URDs (P = .68 and P = .41).
Cumulative incidences of grades 2 to 4 and 3 to 4 aGVHD stratified according to the donor types. (A) Grades 2 to 4 aGVHD. (B) Grades 3 to 4 aGVHD. (C) Grades 2 to 4 aGVHD controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (D) Grades 3 to 4 aGVHD controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (Analyzed by the competing risk method.)
Cumulative incidences of grades 2 to 4 and 3 to 4 aGVHD stratified according to the donor types. (A) Grades 2 to 4 aGVHD. (B) Grades 3 to 4 aGVHD. (C) Grades 2 to 4 aGVHD controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (D) Grades 3 to 4 aGVHD controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (Analyzed by the competing risk method.)
Even if controlling for 71 patients who received stem cells from a fully 10/10 HLA-matched URD, cumulative incidence of grades 2 to 4 aGVHD was 39.4%, which was also higher than MSDs (P = .0003), but severe aGVHD was 12.6%, comparable to MSDs (P = .11). Rates of grades 2 to 4 and severe aGVHD were not significantly different among patients undergoing transplantation using a fully 10/10 HLA-matched URD and a HRD (P = .73 and P = .45) (Figure 1C-D).
According to different related donors for HRD, cumulative incidences of grades 2 to 4 aGVHD were 37% for father donors, 63.6% for mother donors, 31.6% for brother donors, 35.3% for sister donors, 37.5% for son donors, and 50% for daughter donors. Mother donors were associated with a less significantly higher incidence of aGVHD than father donors (P = .06).
The incidence of cGVHD was not significantly affected by donor types. The cumulative incidences of cGVHD at 2 years were 24.3%, 41.7%, and 41.4%, respectively, in patients receiving transplants from MSDs, URDs and HRDs.
Multivariate Cox regression analysis for aGVHD in the total cohort of 304 patients (except for 1 patient who died before day 30) including major known risk factors (Table 2) confirmed that alternative donors had adverse impact on the risk of grades 2 to 4 aGVHD (for URD: P < .001, RR = 3.525; for HRD: P < .001, RR = 3.659). Matching a female donor with a male patient was also found to be a significant risk factor (P = .031).
Relapse.
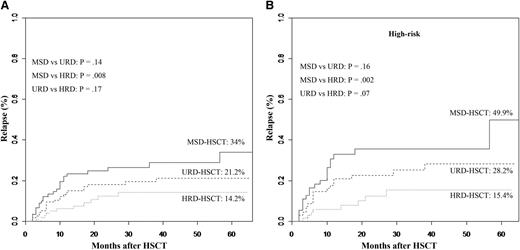
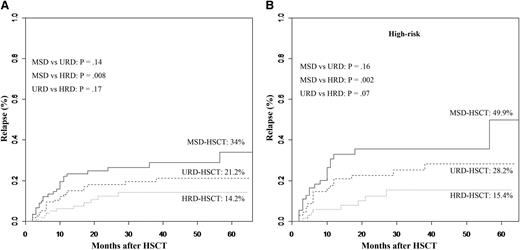
The 5-year incidence of relapse was significantly affected by donor types (34% in the MSD cohort, 21.2% in the URD cohort, 14.2% in the HRD cohort, Figure 2A). HRD-HSCT carried a superior graft-versus-leukemia (GVL) effect compared with MSD-HSCT (P = .008), but comparable to URD-HSCT (P = .17). Even after controlling for high-risk patients (n = 193), a superior GVL effect was also observed in high-risk patients undergoing HRD-HSCT. In high-risk patients receiving MSD (n = 55), 49.9% experienced relapse, as did 28.2% in the URD cohort (n = 69), but the incidence decreased to 15.4% in the HRD cohort (n = 69) (MSD vs HRD, P = .002; URD vs HRD, P = .07, Figure 2B). URD-HSCT did not carry a superior GVL effect compared with MSD-HSCT neither in all patients (P = .14) nor in high-risk patients (P = .16).
Cumulative incidence of relapse at 5 years after transplantation stratified according to the donor types. (A) All patients. (B) Controlling for high-risk patients. (Analyzed by the competing risk method.)
Cumulative incidence of relapse at 5 years after transplantation stratified according to the donor types. (A) All patients. (B) Controlling for high-risk patients. (Analyzed by the competing risk method.)
In multivariate analysis of the relapse rate (Table 2), high-risk (P = .004, RR = 2.482), patients never experiencing cGVHD (P = .001, RR = 2.685) and patients receiving stem cells from an MSD (P = .044, RR= 1.343) contributed independently to the risk of relapse for all patients. According to high-risk patients, patients receiving stem cells from an HRD (P = .036, RR = 2.276), and those experiencing cGVHD (P = .005, RR = 2.772) were protective from relapse.
Long-term follow-up and survival.
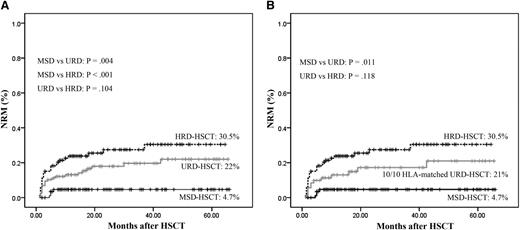
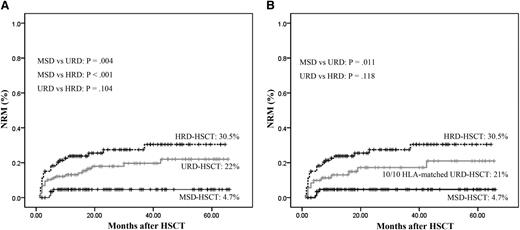
Patients undergoing MRD-HSCT had the lowest incidence of nonrelapse mortality (NRM) compared with those undergoing URD-HSCT (4.7% vs 22%, P = .004) or HRD-HSCT (4.7% vs 30.5%, P < .001), whereas patients undergoing URD-HSCT and HRD-HSCT had comparable incidences of NRM (22% vs 30.5%, P = .104, Figure 3A). According to 71 patients received a fully HLA-matched URD, the incidence of NRM was also higher than MSDs (21% vs 4.7%, P = .011), and comparable to HRDs (21% vs 30.5%, P = .118, Figure 3B).
Cumulative incidence of NRM stratified according to the donor types. (A) All patients. (B) Controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (Analyzed by Kaplan-Meier method.)
Cumulative incidence of NRM stratified according to the donor types. (A) All patients. (B) Controlling for patients received stem cells from a fully 10/10 HLA -matched URD. (Analyzed by Kaplan-Meier method.)
A total of 78 patients died and causes of death ordered by the number of patients were relapse (n = 30), infectious diseases (n = 21), aGVHD (n = 10), early complications of vascular origin (n = 7), organ failure (n = 6), bronchiolitis obliterans syndrome (n = 3), and posttransplantation lymphoproliferative disorders (n = 1) (Table 3).
The reconstitution of natural killer cells returned to normal within 4 weeks after transplantation. At 3 months after HSCT, the median count of CD4+ T cells in the peripheral blood was 179.00/μL (range, 32.64-539.68/μL). The median time of CD8+ T-cell counts >200.00 /μL was at 3.5 months after HSCT.
Five-year OS rates after transplantation were 77.2%, 63.5%, and 60.8% for MSD, URD, and HRD transplantation, respectively. Patients receiving stem cells from an MSD had a superior OS compared with those receiving stem cells from an HRD (P = .026), but equivalent to those receiving a URD (P = .379). However, 5-year OS rates were comparable between URD and HRD transplantation (P = .124) (Figure 4A). Furthermore, 5-year DFS was not significantly different for patients undergoing transplantation using 3 types of donors. Five-year DFS rates after transplantation were 63.6%, 58.4%, and 58.3% for MSD, URD, and HRD transplantation, respectively (P = .574) (Figure 4B).
OS and DFS at 5 years after transplantation stratified according to the donor types. (A) OS. (B) DFS. (Analyzed by Kaplan-Meier method.)
OS and DFS at 5 years after transplantation stratified according to the donor types. (A) OS. (B) DFS. (Analyzed by Kaplan-Meier method.)
In multivariate analysis for DFS (Table 2), lymphoblastic malignancies (P = .009, RR=1.701) and patients experiencing aGVHD had adverse effects on DFS (P = .017, RR=1.625). In contrast, cGVHD had beneficial effects on DFS (P < .001, RR=2.875). More importantly than all of that, DSF were not significantly affected by different donor types (P > .05).
Discussion
To our knowledge, our study is the first prospective clinical trial to compare allo-HSCT using HRDs with allo-HSCT using conventional MSDs and URDs in an unselected population of patients undergoing first allo-HSCT for hematologic malignancies. In our prospective clinical trial, as in most institutions and cooperative group studies, if a matched sibling donor is unavailable, the order of alternative donor selection is a suitably matched URD followed by an HRD. With technical advances in HLA typing, improved immunosuppression and supportive care, outcomes using URDs have approached those using MSDs in many studies.18-21 Our results also suggested that URD transplantations achieved comparable OS and DFS compared with MSD transplantations. However, URD-HSCT did not carry superior GVL effect compared with MSD-HSCT. There were no significant differences in relapse rate either in all patients or in high-risk patients. Although caution needs to be exercised in the interpretation of these data because of the limited number of patients, our findings are similar to a recent Center for International Blood and Marrow Transplant Research study, which assessed 4099 leukemia patients undergoing a myeloablative HSCT from a URD or MSD and demonstrated that URD-HSCT carried a higher risk of HSCT-related mortality, but did not have a more potent GVL effect than MSD-HSCT.22
The past few years have seen growing improvement and increasing interest in HRD-HSCT; however, it remains debatable whether HRDs should be used on an equal basis to URDs when an MSD is unavailable. The results of our trial suggest 3 important aspects of HRD-HSCT. First, compared with MSD-HSCT, patients receiving stem cells from an HRD were likely to suffer significantly higher incidences of grades 2 to 4 and severe aGVHD and NRM. Secondly, compared with a suitably matched URD, even a fully 10/10 HLA-matched URD, HSCT from an HRD seem to be equally efficacious, meaning that they resulted in comparable rates of grades 2 to 4 and severe aGVHD, NRM, OS, and DFS. Thirdly, for patients with high-risk leukemia, HRD-HSCT carried a significantly superior GVL effect compared with MSD- and URD-HSCTs.
There are 2 major approaches to HRD-HSCT used in bone marrow transplantation centers worldwide. Investigators in Perugia, Italy, achieved the best survival rates of ∼55% for AML and 28% for ALL in adults transplanted in CR by using effective in vitro T-cell depletion and “megadoses” of CD34+ stem cells.23 The rates of graft failure and grades 2 to 4 aGVHD were <6% and 8%, respectively.5,24,25 However, delayed immune recovery resulting in a high relapse rate and transplantation-related mortality rates in these depletion techniques need to be further improved.26-28 Another approach to HRD-HSCT is without in vitro T-cell depletion. Recently, a promising approach to HRD-HSCT was developed, which uses T-cell-replete bone marrow grafts in combination with post-HSCT high-dose cyclophosphamide to achieve the selective depletion of alloreactive T cells in vivo. Investigators at Johns Hopkins University achieved 58% of OS in relapsed or refractory Hodgkin lymphoma29 and 35% of DFS in older patients with acceptable rates of NRM (15% at 1 year) and grades of 2 to 4 aGVHD (∼30%)30,31 after nonmyeloablative T-replete HRD-HSCT by incorporating high-dose cyclophosphamide. On the other hand, myeloablative T-replete HRD-HSCT combined with high-dose cyclophosphamide also conferred 67% of DFS on standard-risk patients with 0% of NRM at 1 year,32 and 68% of DFS on patients in remission and 37% on patients with active disease at 22 month.33 Furthermore, Bashey et al recently reported that HRD-HSCT performed using T-cell-replete grafts and post-HSCT cyclophosphamide achieved 7% of NRM, 11% of severe aGVHD, 64% of OS and 60% of DFS, which were equivalent to those of contemporaneous transplantation performed using MSDs and URDs.34
We and Huang et al at Peking University (China) focused on T-replete HRD-HSCT by using ATG to achieve depletion of infused donor T lymphocytes in vivo. Huang et al recently reported that in their study of 756 leukemia patients,35 the 3-year probabilities of DFS for standard-risk and high-risk patients were 68% and 49%, respectively. The incidences of grades 2 to 4 aGVHD and 2-year cGVHD were 43% and 53%. Our data also suggested that the approach without in vitro T-cell depletion could yield improved OS and DFS (60.8% and 58.3%) with acceptable incidences of aGVHD and cGVHD after HRD-HSCT. Several factors may be related to our superior outcomes of HRD-HSCT. First, T-cell repletion might be expected to result in maintenance of a GVL effect. Secondly, we used a myeloablative conditioning regimen which fully immunosuppresses the recipient to prevent graft rejection and reduce the number of leukemia cells. Thirdly, we used low-dose anti-T-lymphocyte globulin (ATG-F, Fresenius) to balance GVHD toxicity and the risk of relapse and infections and yielded similar incidences of aGVHD and cGVHD to those using regular doses of antithymocyte globulin (ATG, Genzyme). The different ATG preparations possess differential immunomodulatory potencies and are thus used in different doses. Although very low doses may lose the immunosuppressive effects, very high doses of ATG may aggravate the delay in immune recovery and increase the risk of relapse and infections. The recommended dose for antithymocyte globulin is 6 to 8 mg/kg body weight. For ATG-F, a total dose of 30 to 60 mg/kg is recommended.36-39 It is important to note that the recommended doses of ATG are based on a few studies; thus, further investigation is required to establish optimal dosing, especially for HRD-HSCT, which lacks the recommended dose. Huang et al at Peking University (China) used a total dose of 10 mg/kg of antithymocyte globulin (ATG, Genzyme) to patients undergoing HRD-HSCT and achieved ∼40% of grades 2 to 4 aGVHD and 50% of 2-year cGVHD,35,40,41 which is comparable to incidences of grades 2 to 4 aGVHD (42.4%) and cGVHD (41.4%) after HRD-HSCT in our study using low-dose (10 mg/kg) ATG-F. Furthermore, our results indicate that HRD-HSCT performed using T-cell-replete grafts in combination with a total dose of 10 mg/kg of ATG-F achieves comparable rates of grades 2 to 4 aGVHD, cGVHD, and NRM to URD-HSCT using a total dose of 4.5 to 6 mg/kg of antithymocyte globulin, equivalent 5-year DFS to MSD-HSCT and URD-HSCT. Given that patients undergoing HRD-HSCT received an additional 10 mg/m2 MTX on day +9 or +11 and longer period of CSA treatment, and URD-HSCT used the dose of antithymocyte globulin on a little bit on the lower site, we could not rule out the possibility that if URD patients had received the same treatment they could have yielded better outcomes. Future clinical studies are also warranted to determine the possibility of further improvement of URD and HRD results with regular doses of ATG.
Patients with high-risk leukemia always urgently need transplantation, which does not allow a URD to be organized if a matched sibling donor is not available. The results of our patients who underwent HRD-HSCT were particularly intriguing because patients with high-risk leukemia benefited from HRD-HSCT which had a significantly superior GVL effect compared with MSD- or URD-HSCT. The priority use of HRD-HSCT, either with or without an in vitro T-cell depletion approach, in high-risk patients has been reported by other groups.25,42 Furthermore, Huang et al suggested that HRD-HSCT can achieve a stronger GVL effect than MSD for high-risk acute leukemia patients. The 3-year OS was higher in HRD-transplanted patients (42%) than in MSD-transplanted patients (20%).43 Although the underlying mechanisms of the superior antileukemia effect of HRD needs to be further elucidated, potentially confounding factors, including underlying disease, risk classification, time from diagnosis to HSCT, disease status at HSCT and conditioning regimen, between the MSD-, URD-, and HRD-HSCT cohorts in our trial were not significantly different. This observation was strengthened by multivariate analysis of relapse, showing that high-risk patients receiving stem cells from an HRD experienced a protective effect against relapse.
In conclusion, our study demonstrates that a matched sibling donor is the best source of stem cells. Outcomes after allo-HSCT from suitably matched URDs and HRDs with low-dose anti-T-lymphocyte globulin are similar and HRD improves the outcomes of patients with high-risk leukemia. Our data provide convincing clinical evidence to support the use of transplantation from HRDs, suggesting that they can be selected as a first-line alternative donor, especially for high-risk patients. T-replete HRD-HSCT with new immunosuppression strategies aiming at balance GVHD toxicity and the risk of relapse and infections and improving posttransplantation immune recovery need to be further improved.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded in part by the Key Project of the National Natural Science Foundation of China (81230014), the National High Technology Research and Development Program of China (2012AA020905), the National Natural Science Foundation of China (81100387, 81170501), the Major Technology Program (Key Social Development) of the Science Technology Department of Zhejiang Province (2012C13021-1), and Natural Science Foundation of Zhejiang Province (Y2110183, 2008C23047).
Authorship
Contribution: Y.L., H.X., and H.H. were responsible for the conception and design of the study; Y.L., H.X., X.L., and H. H. collected, analyzed, and interpreted the data; H.X. and H.H. drafted the manuscript; Y.L., X.L., J.S., Y.T., J.H., W.X., W.Z., Y.Z., X. Ye, X. Yu, Z.C., M.L., and H.H. provided study material or patients; and Y.L., H.X., and H.H. obtained funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: He Huang, Bone Marrow Transplantation Center, The First Affiliated Hospital, Zhejiang University School of Medicine, No 79 Qingchun Rd, Hangzhou, 310003, Zhejiang Province, People’s Republic of China; e-mail: hehuangyu@126.com.
References
Author notes
Y.L. and H.X. contributed equally to this study.