Abstract
Background: CPI-613 is a first in class agent that targets mitochondrial metabolism through inhibition of pyruvate dehydrogenase and a-ketogluterate dehydrogenase. In a single agent phase I study CPI-613 was found to be well tolerated and several patients with myeloid malignancies had a response.This trial was designed to determine the maximum tolerated dose, safety, and efficacy of CPI-613 given in combination with HiDAC and mitoxantrone in patients with relapsed or refractory AML.
Methods: CPI-613 is given daily on days 1 through 5 starting at a dose of 500 mg/m2. Beginning on day 3, HiDAC at 3,000 mg/m2 (or 1,500 mg/m2 for age ≥60) is administered every 12 hours for 5 doses and mitoxantrone at 6 mg/m2 is given daily for 3 doses. To date the CPI-613 dose has been escalated to 2250 mg/m2. If residual disease is present on day 14 re-induction with the same or a three day abbreviated course can be given. Patients who achieved a complete remission with or without complete count recovery (CR or CRi) could receive additional consolidation cycles with the goal to get responders to stem cell transplant whenever possible.
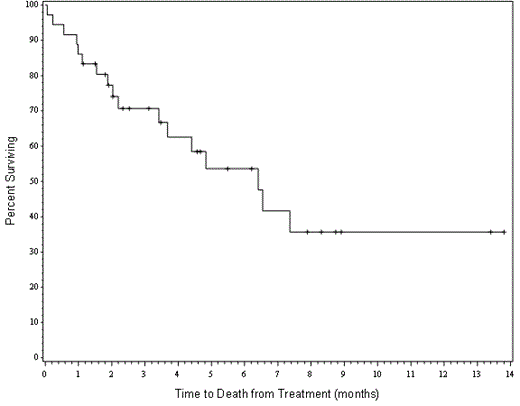
Results: A total of 36 patients have been enrolled to date. The median age is 60 (range 21-76). Eight patients had refractory disease and nine received two or more previous lines of therapy. In patients with relapsed disease where data was available, the median duration of CR1 was 8.4 months. Cytogenetics were poor risk in 17 patients, intermediate in 15, good in 3 and one patient had CML in myeloid blast crisis. The overall intention to treat response rate was 50% (16CR+2CRi). This was the same for patients ≥60 years old with a CR/CRi rate of 50% (10/20). Surprisingly, the response rate was slightly higher for patients with poor risk cytogenetics with a CR/CRi rate of 53% (9/17). In a historical cohort of patients treated with HiDAC, mitoxantrone and asparaginase at our institution, only 25% (4/16) of patients with poor risk cytogenetics achieved a CR/CRi. Median survival for the entire cohort is 6.4 months with a median of 3.1 months of follow-up (see figure 1). Five patients (14%) died on or before day 30. The regimen is well tolerated with no dose limiting toxicities observed to date. Ten patients went on to allogeneic stem cell transplantation (28%). This compares favorably with our historical data where only 17% of patients went on to transplant. Four patients with circulating blasts had blood samples taken on day one, before and after CPI-613 infusion. Three of these patients had demonstrable increases in the level of phosphorylation of pyruvate dehydrogenase complex consistent with its inhibition. Additionally, one patient who cleared her marrow demonstrated robust phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) consistent with depletion of ATP.
Conclusions: CPI-613 in combination with HDAC and mitoxantrone is a promising salvage regimen, especially in older patients and those with high risk disease. Induction of AMPK phosphorylation may serve as a predictor of response. This trial continues to accrue patients.
Figure 1. Overall survival of all patients.
Pardee:Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Off Label Use: CPI-613 is a novel mitochondrial metabolism inhibitor.. Powell:Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.