Key Points
In FLT3-ITD–positive AML, high allelic ratio and ITD insertion site in TKD1 predict for low complete remission rates and poor survival.
In FLT3-ITD–positive AML, allogeneic HSCT in first CR outweighs the negative impact of high allelic ratio on survival.
Abstract
The objective was to evaluate the prognostic and predictive impact of allelic ratio and insertion site (IS) of internal tandem duplications (ITDs), as well as concurrent gene mutations, with regard to postremission therapy in 323 patients with FLT3-ITD–positive acute myeloid leukemia (AML). Increasing FLT3-ITD allelic ratio (P = .004) and IS in the tyrosine kinase domain 1 (TKD1, P = .06) were associated with low complete remission (CR) rates. After postremission therapy including intensive chemotherapy (n = 121) or autologous hematopoietic stem cell transplantation (HSCT, n = 17), an allelic ratio ≥ 0.51 was associated with an unfavorable relapse-free (RFS, P = .0008) and overall survival (OS, P = .004); after allogeneic HSCT (n = 93), outcome was significantly improved in patients with a high allelic ratio (RFS, P = .02; OS, P = .03), whereas no benefit was seen in patients with a low allelic ratio (RFS, P = .38; OS, P = .64). Multivariable analyses revealed a high allelic ratio as a predictive factor for the beneficial effect of allogeneic HSCT; ITD IS in TKD1 remained an unfavorable factor, whereas no prognostic impact of concurrent gene mutations was observed. The clinical trials described herein were previously published or are registered as follows: AMLHD93 and AMLHD98A, previously published; AML SG 07-04, ClinicalTrials.gov identifier #NCT00151242.
Introduction
Internal tandem duplications (ITDs) of the FMS-like tyrosine kinase 3 gene (FLT3) occur in ∼25% of younger adult patients with acute myeloid leukemia (AML), predominantly in cytogenetically normal AML, thus being one of the most frequently affected genes in AML.1 ITD mutations result in amino acid sequence changes with intact coding frames. They result in a constitutive activation of the receptor tyrosine kinase and its downstream signaling pathways, leading to dysregulation of cellular proliferation.2-4 ITDs are located in exons 14 and 15 of the FLT3 gene and show a broad variation in the position of their insertion sites (ISs), as well as in number and sizes of the duplicated fragments. In cytogenetically normal acute myeloid leukemia (AML), FLT3-ITDs confer an unfavorable prognosis due to a high relapse rate, which translates into an inferior overall survival (OS).5-9 In addition, previous studies suggested a prognostic role for the mutant to wild-type (WT) allelic ratio (AR) and the size of the ITD.6-13 Thus far, data on the AR consistently show an association between high allelic burden,6-8,13 which implicates in general a threshold of >0.50,13,14 with, in part, loss of heterozygosity8,13 and unfavorable outcome. In the study by Thiede et al, a threshold of >0.78 was significantly associated with an unfavorable outcome for all survival end points,6 and a cut-point of 0.7 was used to stratify randomization in the large multicenter Cancer and Leukemia Group B-10603 trial (#NCT00651261), which evaluated intensive chemotherapy (CTX) with or without midostaurin in AML with activating FLT3 mutations. In contrast, the prognostic impact of low and intermediate FLT3-ITD ARs is controversial.6-8,13,14 Beyond the AR, the ITD IS within the FLT3 gene was shown to be associated with prognosis.7,15 Localization of the IS outside the juxtamembrane domain (JMD), particularly in the B1 sheet of the tyrosine kinase domain (TKD)1, is present in about one quarter of the cases.15,16 In addition, an IS in the TKD1 was associated with rewired signaling, resulting in an upregulation of the antiapoptotic protein MCL1.17 Whether a concomitant nucleophosmin-1 (NPM1) mutation adds to prognostication in AML with FLT3-ITD is currently a matter of debate. Gale et al reported a better outcome in FLT3-ITD–positive patients harboring a concurrent NPM1 mutation,13 whereas others showed that the protective effect of NPM1 in AML with higher FLT3-ITD AR (≥0.50) is diminished or gets completely lost.14,18 Recently published data suggest that FLT3-ITD retains its negative prognostic impact in intermediate-risk AML, even in the context of other genetic abnormalities, such as DNMT3A and TET2.19,20
The role of allogeneic hematopoietic stem cell transplantation (alloHSCT) to overcome the negative impact of FLT3-ITD on survival in AML patients remains controversial.21-28 In several studies, alloHSCT performed in first complete remission (CR) from matched-related donors (MRDs) has been shown to result in an improved survival compared with CTX and autologous HSCT (autoHSCT).21,23-25,27,28 However, most of these studies are comparisons to historical controls or as-treated analyses,14,23-25,27,28 whereas statistically more valid intent-to-treat approaches with donor vs no donor comparisons are rare.21,22 Thus far, only a minority of published studies has included alloHSCT from a matched unrelated donor (MUD) in first CR.24,26-28 Some authors argue that evidence supporting the approach of alloHSCT in FLT3-ITD AML is still limited and that the overall beneficial effect of alloHSCT in light of its concomitant short- and long-term risks remains elusive.22,25,26 To date, it is still unclear whether the FLT3 mutational status per se affects outcome, in particular if specific biological FLT3-ITD features, such as the AR and IS, and the molecular background of cooperating mutations are taken into account. The objectives of our study were to evaluate the relative impact of FLT3-ITD AR, ITD IS, and cooperating mutations on prognosis and to validate their value as a predictive marker in the context of alloHSCT from MRD and from MUD in a large cohort of younger adult patients.
Patients and methods
Patients and treatment
In total, 2278 younger adult patients (median age, 48 years; range: 16-62 years) with newly diagnosed AML were enrolled in 3 prospective multicenter treatment trials of the German-Austrian AML Study Group (AMLSG) between 1993 and 2009. The studies were approved by the institutional review boards of the participating centers. All patients received intensive induction and consolidation therapy (supplemental Figure 1, available on the Blood Web site) as previously described (AML HD9329 ; AML HD98A30 ; AMLSG 07-0431 ; and #NCT00151242). The diagnosis of AML was based on French-American-British Cooperative Group criteria32 for the trials AML HD93 and AML HD98A, and, after 2004, on World Health Organization 2001 criteria33 for the AMLSG 07-04 trial. Consistently throughout all AMLSG trials, patients with AML exhibiting an intermediate-risk karyotype with FLT3-ITD were intended to receive (1) a double induction therapy with idarubicin, cytarabine, and etoposide and (2) repetitive cycles of high-dose cytarabine-based consolidation therapy29-31 or an autoHSCT.30 If an HLA-matched family donor was available, an alloHSCT after a myeloablative conditioning regimen was intended. In November 2006, the algorithm for alloHSCT was modified: before the amendment, only patients with an MRD were intended to receive an alloHSCT in first CR, whereas after this date, the spectrum of donors was extended to MUDs.
Cytogenetic and molecular genetic analysis
All leukemia samples were studied centrally in the reference laboratory of the AMLSG at the University of Ulm. Chromosome banding was performed using standard techniques, and karyotypes were described according to the International System for Human Cytogenetic Nomenclature.34
To improve the accuracy of cytogenetic analysis, all patients were additionally analyzed either by fluorescence in situ hybridization35 or by polymerase chain reaction for the presence of the recurring gene fusions RUNX1-RUNX1T1, CBFB-MYH11, MLL-MLLT3, and PML-RARA.21 Leukemia samples were analyzed for mutations in FLT3 (ITD, n = 2022; TKD mutations at codons D835 and I836, n = 1890),21 NPM1 (n = 1924),21 MLL (partial tandem duplication, n = 1733),21 DNMT3A (n = 1714),20 and CEBPA (n = 1730).36 In FLT3-ITD–positive patients, the AR was quantified by GeneScan-based fragment-length analysis; in cases with >1 ITD mutation, the values of all FLT3-ITDs were added up to 1 value. The IS was determined by collection of the mutated polymerase chain reaction fragment using a denaturating high-performance liquid chromatography analyzer (WAVE System) followed by sequencing as previously described.15
Statistical analyses
CR and survival end points (OS, event-free survival [EFS], cumulative incidence of relapse [CIR] and death [CID], and relapse-free survival [RFS]) were defined as recommended.37 Cytogenetic categorization into favorable-, intermediate-, and adverse-risk groups followed the criteria published on behalf of the European LeukemiaNet.37 Pairwise comparisons between patient subgroups were performed by the Mann-Whitney or Kruskal-Wallis test for continuous variables and by Fisher’s exact test for categorical variables. Correlations between FLT3-ITD AR and continuous covariates were estimated and tested using Spearman’s rank correlation coefficient. Testing for correlations between binary covariates and the FLT3-ITD AR was done by Cochran-Armitage tests. Univariable and multivariable logistic regression models were used to test the influence of covariates on response to induction therapy. Testing and estimation of possible cutoff values for continuous variables with respect to time-to-event end points was done based on maximally selected log-rank statistics.38 The Kaplan-Meier method was used to estimate the distribution of RFS and OS.39 Confidence interval (CI) estimation for the survival curves was based on the cumulative hazard function using Greenwood’s formula for the standard error (SE) estimation.40 Stratified tests for survival analyses were applied to account for a long time period of patient accrual with the following intervals: 1993 to 1999 (n = 51), 2000 to 2003 (n = 86), 2004 to 2006 (n = 87), and 2007 to 2009 (n = 99). Stratified log-rank tests were used to compare survival curves between patient subgroups. The effect of alloHSCT on OS as a time-dependent intervening event was tested by using the stratified Mantel-Byar method for univariable and the stratified Andersen-Gill model for multivariable analyses.41,42 The method of Simon and Makuch was used to estimate survival distributions with respect to time-dependent interventions.43 CIR and CID and their SEs were computed according to the method described by Gray44 and included only patients attaining CR. Missing data were replaced by 50 imputations using multivariate imputations by chained equations applying predictive mean matching.45 Backward selection applying a stopping rule based on P values was used in multivariable regression models to exclude redundant or unnecessary variables.45 All statistical analyses were performed with the statistical software environment R, version 2.14.0, using the R packages rms, version 3.0-0, survival, version 2.35-8, cmprsk, version 2.2-1, mice, version 2.3, and lmtest, version 0.9-27.46
Results
Patient cohort
Cytogenetic analysis was available in 2076 of 2278 (91%) patients. In our analyses, only patients with normal or intermediate-risk karyotypes (n = 1396) according to the European LeukemiaNet criteria37 were included. Of those, the FLT3-ITD mutational status was available in 1276 (91%) patients, and n = 366 (30.5%) were ITD positive. The final study cohort consisted of 323 FLT3-ITD–positive patients, in whom the AR was available. Of those, the ITD IS was analyzed in 255 (79%) patients.
Pretreatment characteristics according to FLT3-ITD AR
The FLT3-ITD AR varied from 0.01 to 10.19, with a median of 0.53 and the first and third quartiles at 0.20 and 0.80. For easier interpretation, we separated FLT3-ITD ARs into 4 increasing equally sized intervals according to the quartiles of the distribution. Pretreatment patient characteristics according to the distribution of FLT3-ITD ARs are shown in Table 1. White blood counts (WBCs), serum lactate dehydrogenase (LDH) values, and percentages of peripheral blood and bone marrow blasts differed significantly with increasing ARs. Spearman rank correlation test revealed highly significant positive associations of the AR and WBC, LDH, percentages of peripheral blood, and bone marrow blasts (P < .0001, each), as well as platelet counts (P = .04). ITD ISs within the JMD and TKD1 were equally distributed within the 4 intervals (Fisher’s exact test, P = .30). Of note, there was an inverse association between the cooperating gene mutations double mutated (dm) CEBPA, IDH1, and IDH2, as well as FLT3-TKD and the AR: the higher the AR, the lower the frequency of concurrent molecular abnormalities (by Cochran-Armitage test, P = .004, P = .005, P = .03, and P = .01, respectively). Despite significantly different distributions of FLT3-ITD size among the 4 groups (Kruskal-Wallis test, P = .05), no significant correlation was present (by Spearman’s rank correlation test, P = .33). Of note, the FLT3-ITD size was significantly longer in cases with an IS in TKD1 compared with those with an IS in JMD (P < .001). Furthermore, the presence of >1 ITD (n = 33) was more frequently observed in cases with an IS in TKD1 (P = .001).
Response to induction therapy
Response could be analyzed in 321 (99.4%) of the 323 patients. Overall response was as follows: CR rate, 72% (n = 231); resistant disease (RD), 22% (n = 70); and early or hypoplastic death (ED/HD), 6% (n = 20). CR rates significantly decreased with increasing AR (logistic regression, P < .001). The highest CR rate was achieved in patients with an AR of the first interval (ARs < 0.2) with 81.5% compared with 57.5% in patients with an AR of the fourth interval (ARs > 0.8; Table 2). Lower CR rates were a result of higher RD rates associated with an increasing AR (logistic regression, P < .001), whereas ED/HD rates were comparable between the 4 intervals (P = .53).
On the basis of our previous report,15 we also analyzed response to induction therapy according to the ITD IS, categorized either as insertion in the JMD or in the TKD1. A strong trend to higher CR rates was observed in patients with FLT3-ITD IS in the JMD compared with those with insertion in the TKD1 (logistic regression, P = .06). Of note, the inverse association of AR and CR rates was significantly present in AMLs with an IS in the JMD (logistic regression, P < .001), whereas in AMLs with an insertion in the TKD1, this association was not obvious any more (logistic regression, P = .46; Table 2).
This differential effect was also reflected in the corresponding 2 multivariable logistic regression models with the end point achievement of CR after induction therapy. In the model restricted to ISs within the JMD (n = 166), significant covariables after backward selection were AR (odds ratio [OR] for an increase to the next higher interval, 0.59; P = .02) and NPM1 mutation (OR, 2.50; P = .04), whereas DNMT3A, IDH1, or FLT3-TKD mutations had no significant impact. In contrast, in the model restricted to ISs within the TKD1 (n = 88), no variable reached statistical significance.
Furthermore, FLT3-ITD size and number of ITDs per patient were significantly associated with CR rates, in that larger FLT3-ITD size (above the median) and presence of >1 ITD per patient were associated with lower CR rates (P = .02 and P = .03, respectively).
Survival analysis
The median follow-up for survival in the 323 FLT3-ITD–positive patients was 5.85 years (95% CI, 5.18-6.44 years); the estimated 4-year EFS, RFS, and OS were 21% (95% CI, 17-26%), 32% (95% CI, 27-39%), and 35% (95% CI, 30-40%).
Of the 231 patients achieving a first CR after induction therapy, 93 (40%) proceeded to alloHSCT (40 from MRD, 52 from MUD, and 1 from haplo-identical sibling donor) and 138 (60%) received other postremission therapy (n = 17, autoHSCT; n = 121, intensive CTX). The median time interval between first CR and alloHSCT was 87.5 days for MUD and 102.5 days for MRD transplantation. Pretreatment characteristics were well balanced between patients proceeding to alloHSCT and those receiving CTX/autoHSCT (age, P = .47; WBC, P = .71; AR, P = .22; FLT3-ITD IS [JMD vs TKD1], P = .25; concurrent NPM1 mutation, P = .57).
The impact of the AR on outcome of patients receiving CTX/autoHSCT or alloHSCT in first CR was analyzed separately for the end point CIR and CID (Table 3). There was a significant correlation for a higher CIR with increasing AR in patients receiving CTX/autoHSCT (P = .04), whereas this was not the case in patients proceeding to alloHSCT (P = .20). There was no statistical difference in CID in relation to the AR in patients receiving CTX/autoHSCT (P = .15) and those intended for alloHSCT (P = .45).
With respect to ITD IS, there was a trend for a higher CIR in patients with an insertion in the TKD1 compared with insertion in the JMD in patients receiving CTX/autoHSCT (P = .06) or alloHSCT (Table 4; P = .10). Again, there was no difference in CID regarding ITD IS in patients receiving CTX/autoHSCT (P = .89) or alloHSCT (P = .72). Thus, insertion in the TKD1 indicated an unfavorable prognosis not only in patients receiving CTX/autoHSCT but also in those after alloHSCT. Of note, no significant difference could be shown between alloHSCT and CTX/autoHSCT in patients in first CR with an ITD insertion in TKD1 (Mantel-Byar analysis, P = .50).
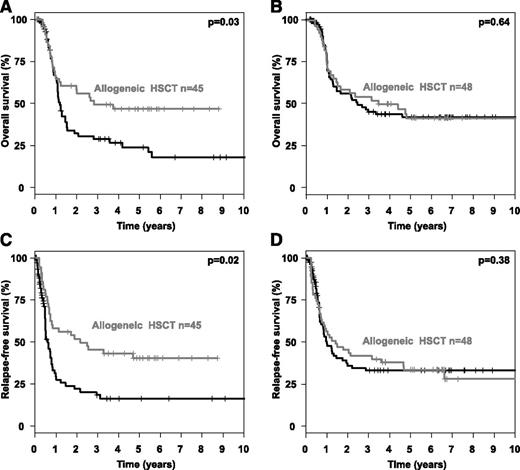
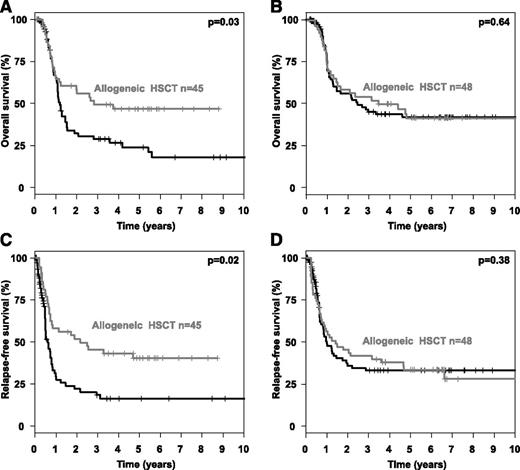
To evaluate possible optimal cut-points of the AR with respect to time-to- event end points, we used maximally selected log-rank statistics in patients who received CTX/autoHSCT for the clinical end points OS and RFS (Figure 1A). Of note, the optimal cut-point was consistent for both end points OS and RFS at an AR of 0.51 (Figure 1B-C). Subsequently, we performed 2 subgroup analyses (high AR ≥0.51 vs low AR < 0.51) evaluating the impact of alloHSCT assessed as a time-dependent covariable in comparison with CTX/autoHSCT. This is illustrated by Simon-Makuch plots for OS (Figure 2A-B) and RFS (Figure 2C-D). The Mantel-Byar tests revealed a significantly better OS and RFS for patients proceeding to an alloHSCT in first CR only in those AMLs with an AR ≥0.51 (P = .03 and P = .02, respectively), whereas no difference was evident in patients with an AR < 0.51 (P = .64 and P = .38, respectively).
Cut-point selection using maximally log-rank statistics. (A) Optimal cut-point for the AR by maximally selected log-rank statistics in intensively treated FLT3-ITD positive AMLs. Patients who proceeded to allogeneic HSCT were excluded. Maximally selected log-rank statistics performed for the continuum of the AR to test for a potential cut-point separating 2 groups with different survival distributions. The AR is shown on the x-axis and the corresponding standardized log-rank statistic on the y-axis. The estimated cutoff point was 0.51, with an M statistic of 3.15 and a corresponding corrected P value of .038. The vertical dashed line represents the optimal cut-point for AR evident on maximally selected log-rank statistics and the corresponding M statistics. (B) Impact of AR on OS in first CR according to preselected optimal cut-point in intensively treated FLT3-ITD–positive patients excluding allogeneic HSCT. (C) Impact of AR on relapse-free survival according to preselected optimal cut-point in intensively treated FLT3-ITD–positive patients excluding allogeneic HSCT.
Cut-point selection using maximally log-rank statistics. (A) Optimal cut-point for the AR by maximally selected log-rank statistics in intensively treated FLT3-ITD positive AMLs. Patients who proceeded to allogeneic HSCT were excluded. Maximally selected log-rank statistics performed for the continuum of the AR to test for a potential cut-point separating 2 groups with different survival distributions. The AR is shown on the x-axis and the corresponding standardized log-rank statistic on the y-axis. The estimated cutoff point was 0.51, with an M statistic of 3.15 and a corresponding corrected P value of .038. The vertical dashed line represents the optimal cut-point for AR evident on maximally selected log-rank statistics and the corresponding M statistics. (B) Impact of AR on OS in first CR according to preselected optimal cut-point in intensively treated FLT3-ITD–positive patients excluding allogeneic HSCT. (C) Impact of AR on relapse-free survival according to preselected optimal cut-point in intensively treated FLT3-ITD–positive patients excluding allogeneic HSCT.
Overall and relapse-free survival according to FLT3-ITD allelic ratio and type of postremission therapy. Simon Makuch plots illustrating the influence of postremission treatment modality on OS in (A-B) first CR and (C-D) RFS according to FLT3-ITD AR in FLT3-ITD–positive AMLs with (A,C) high (≥0.51) or (B,D) low (<0.51) AR.
Overall and relapse-free survival according to FLT3-ITD allelic ratio and type of postremission therapy. Simon Makuch plots illustrating the influence of postremission treatment modality on OS in (A-B) first CR and (C-D) RFS according to FLT3-ITD AR in FLT3-ITD–positive AMLs with (A,C) high (≥0.51) or (B,D) low (<0.51) AR.
In contrast, no prognostic impact of FLT3-ITD size and number of ITD per patient could be shown for the end points RFS and OS of CR patients in the CTX/autoHSCT subgroup and also in the whole study cohort.
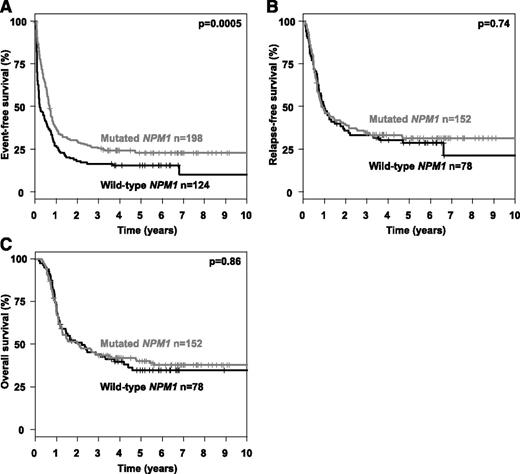
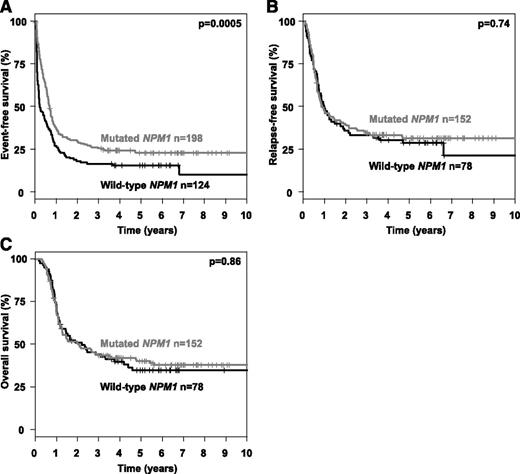
Multivariable analysis in patients in first CR after induction therapy on the clinical end point RFS and OS were performed using an Andersen-Gill model allowing for the integration of alloHSCT as a time-dependent covariable. To increase external validity and to be consistent with previous reports,13,14 we used a cut-point for the AR of 0.50. In both models, an interaction term of the variables “allogeneic HSCT in first CR” and “AR > 0.50” was included. The Andersen-Gill models revealed a significant improved RFS, as well as OS, in those patients with a high AR who received an alloHSCT in first CR (RFS: hazard ratio [HR], 0.39 [95% CI, 0.25-0.71]; OS: HR, 0.47 [95% CI, 0.28-0.81]), whereas in patients with a low AR, no statistical significant impact of alloHSCT was evident (RFS: HR, 0.86 [95% CI, 0.53-1.39]; OS: HR, 0.96 [95% CI, 0.58-1.59]). Further, significant and in-trend important variables in both models (RFS and OS) were high AR, IS in TKD1, and age. WBC was not included in the models due to collinearity. Of note, cooperating mutations in NPM1 and FLT3-TKD had no significant impact in the multivariable Andersen-Gill models (Table 5). For mutational status of IDH1/2, DNMT3A, ASXL1, and RUNX1, no significant prognostic impact was identified in univariable analyses (data not shown); due to a high rate of missing values, these variables were not included into the multivariable survival models. Based on previous reports13,14 showing a favorable impact of mutated NPM1 on the background of FLT3-ITD, we performed additional explorative subgroup analyses. The favorable impact of mutated NPM1 on CR rate (mutated NPM1, 77%; WT NPM1, 63%; P = .01) mentioned above directly translated into a significant difference in EFS (P = .0005), whereas no prognostic impact was evident for the end points CIR (P = .59), CID (P = .76), RFS (P = .74), and OS (P = .86) (Figure 3).
Event-free, relapse-free and overall survival according to NPM1 mutational status. Kaplan-Meier plots illustrating the influence of mutated NPM1 on (A) event-free, (B) relapse-free, and (C) overall survival.
Event-free, relapse-free and overall survival according to NPM1 mutational status. Kaplan-Meier plots illustrating the influence of mutated NPM1 on (A) event-free, (B) relapse-free, and (C) overall survival.
Discussion
Our study focuses on the impact of specific FLT3-ITD characteristics such as the AR and localization of ITD IS on clinical outcome in younger adult AML patients including the end points CR rates, RFS, and OS. In particular, we evaluated the impact of alloHSCT in first CR compared with CTX/autoHSCT as postremission strategies on survival end points. The analyses included 323 AML patients with intermediate-risk karyotype all harboring a FLT3-ITD.
We were able to show that the FLT3-ITD AR had an significant impact on induction success independent from other variables, which adds to recently published data.13,14,47 We found a significant proportional association of an increasing AR with increasing rates of refractory disease translating into decreasing CR rates. These findings underline the biological importance of specific FLT3 molecular characteristics in FLT3-ITD–positive AML. Hence, approaches to improve treatment should not only focus on the reduction of the relapse-risk but also on attempts to increase CR rates, in particular, in patients with a high AR. First data indicate that FLT3 TKIs such as midostaurin or sorafenib in conjunction with intensive induction therapy may increase the CR rate.48,49 Complete and sustained inhibition of FLT3-mutated AML will probably require a combination of agents, consisting of both targeted and conventional CTX, particularly in those AMLs mostly dependent on FLT3-signaling.50 From a biological point of view, an HR ratio may be associated with at least in part loss of heterozygosity8,13 and presumably provides a survival advantage for the leukemic blast cells.50 Further evidence comes from a FLT3-ITD knock-in mouse model indicating that loss of the WT allele contributes to myeloid expansion and disease aggressiveness.51 Our clinical data suggest that dependence to FLT3 signaling conferred by a high AR is predominantly seen in patients with the typical IS in the JMD (Table 2). The effect of intrinsic resistance associated with TKD1 IS may be attributable to the activation of further downstream signaling pathways.17,52,53 With respect to other gene mutations, only concurrent NPM1 mutations had a prognostic favorable impact on achievement of CR if an FLT3-ITD IS within the JMD was present (OR of 2.75), whereas there was no impact if the IS was located within the TKD1 (OR of 0.92). None of the other cooperating gene mutations, such as IDH1, IDH2, or DNMT3A, had an impact on induction success neither in the whole group nor in the subgroups as defined by IS.
By evaluating the impact of the AR on survival end points, we were able to show that an increasing AR was associated with a proportional increase in CIR, which is in line with previous reports.13,14 In our analysis, this was the case for patients receiving CTX/autoHSCT in first CR as postremission therapy. In contrast, CIR was not affected by increasing AR in patients who received an alloHSCT (Table 3). Of note, IS in TKD1 was associated with a negative prognostic impact irrespective of the postremission strategy (Table 4). Therefore, the AR in FLT3-ITD–positive AMLs may be useful as a predictive marker indicating whether an alloHSCT in first CR is beneficial in terms of overall outcome. In contrast, the IS in the TKD1 remains an unfavorable prognostic marker irrespective of the type of postremission therapy. However, an alloHSCT in first CR in these cases may be a treatment option with a significant reduction of CIR compared with CTX/autoHSCT (Table 4), although no clear beneficial effect on OS could be demonstrated (Mantel-Byar analysis, P = .50). To determine an optimal cut-point within the continuum of the AR in patients receiving CTX/autoHSCT, we used maximally selected log-rank statistics as a solid statistical method accounting for multiple testing. For both survival end points, RFS and OS, the optimal cut-point for the AR was 0.51, indicating a significantly adverse prognosis above this cut-point. Our findings are in line with others, where an AR of 0.50 was reported as the cutoff value with clinical prognostic impact.13,14 Hence, the previously reported cutoff value was confirmed in our study by statistics corrected for multiple testing and thus strongly argues for future use in clinical trials.
A major benefit of alloHSCT performed in first CR was present in patients with an AR of ≥0.51 with respect to RFS and OS, which is in line with the data recently published by Pratcorona et al.14 However, alloHSCT did not improve RFS and OS in patients with a low AR in our study, suggesting that in these patients, the risk associated with alloHSCT was not outweighed by its benefit. Thus, although alloHSCT may still be a valuable option for patients with a low AR,47 it may not be the primary recommended consolidation procedure.
In a study reported by the Medical Research Council cooperative group, in which patients were subdivided into donor and no-donor groups, no clear benefit of alloHSCT performed in first CR with a MRD in FLT3-ITD–positive AML was evident, although the relapse rate was markedly reduced compared with other treatments.22 Beside evaluating FLT3-ITD–positive AML as a homogenous group not taking into account further biological ITD characteristics, another limitation of this study was that only 35 of 68 FLT3-ITD–positive patients with a donor in fact underwent allogeneic HSCT in first remission, resulting in a treatment adherence of only 51%. In contrast, recently reported data indicate a clinical benefit in FLT3-ITD–positive AML after alloHSCT, with both RFS and OS rates being significantly improved.21,23-25,27,28 Consistently shown in all these trials was a reduction in relapse risk to values of ∼30%23,24,27 after alloHSCT, which is in line with our results. In particular, patients with an AR above the median (third and fourth interval) had a markedly reduced CIR by alloHSCT compared with CTX/autoHSCT, suggesting a strong antileukemic effect after myeloablative conditioning in combination with a graft-versus-leukemia reaction. However, the marker FLT3-ITD still retains an unfavorable prognostic value even after alloHSCT in first CR, which has been reported in a single center experience17 and also in a registry study of the European Group for Blood and Marrow Transplantation.27 To reduce the incidence of relapses after alloHSCT, approaches evaluating FLT3 inhibitors as maintenance therapy are under way (#NCT01477606, #NCT01398501, and #NCT01468467).
In several studies it has been shown that the prognostic impact of FLT3-ITD should be interpreted in the context of a cooperating NPM1 mutation,14,21,47 which is present in ∼50% of the patient populations with a normal karyotype.13,21 Retrospective studies suggest that the presence of a NPM1 mutation may somewhat mitigate the negative prognostic effect of FLT3-ITD. In contrast, in our study, the additional impact of a concurrent NPM1 mutation could not be demonstrated, irrespective of whether specific FLT3-ITD characteristics were taken into account or not (Figure 3; Table 5).
Of note, we were able to confirm our previous published findings on the negative prognostic impact of FLT3-ITD IS within the TKD1.15 In addition, we could show that the clinical course of these patients is different from those with an IS in the JMD. In patients with an IS in TKD1, there was only a limited benefit from alloHSCT performed in first CR, with a relatively high CIR of 49% and a low OS of 36% after 3 years (Table 4). The unfavorable impact of a more C-terminal localized IS, ie, in the TKD1, was also reported by Schnittger et al.7
In summary, our data provide novel clinical information that may be useful for refining prognostication and prediction with respect to induction and choice of the optimal postremission therapy in FLT3-ITD–positive AML. From a clinical perspective, the AR allows to predict achievement of CR after standard induction therapy, particularly in patients with an IS in the JMD. In addition, patients with a high AR significantly benefitted from alloHSCT performed in first CR, as both RFS and OS were improved. In contrast, FLT3-ITD IS in TKD1 is an unfavorable prognostic factor irrespective of postremission strategy with a dismal outcome even after an alloHSCT in first CR. Whether the introduction of FLT3 TKIs will significantly improve outcome in these high-risk subgroups must be shown in recently completed and currently ongoing clinical trials.
Presented in part at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2012.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all members of the German-Austrian AML Study Group (AMLSG) for providing leukemia specimens and clinical data. A list of AMLSG institutions and investigators participating in this study appears in the supplemental Appendix.
This work was supported by grants from the Else Kröner-Fresenius-Stiftung (Forschungskolleg Ulm, 2010_Kolleg.24), grants 01GI9981 (Network of Competence Acute and Chronic Leukemias) and 01KG0605 (IPD-Meta-Analysis: A model-based hierarchical prognostic system for adult patients with AML) from the German Bundesministerium für Bildung und Forschung, the German Research Foundation (DFG FI405/5-1, BU 1339/3-1, and BU 1339/5-1, SFB 1074 B3), and the Deutsche José Carreras Leukämie-Stiftung (DJCLS H 05/02).
Authorship
Contribution: R.F.S., S.K., H.D., and K.D. conceived and designed the study; R.F.S., S.K., G.K., J.C., M.R., G.H., P.B., M.L., H.R.S., T.K., H.A.H., G.W., D.N., K.G., P.P., V.I.G., V.T., D.S., J.K., A.G., H.D., and K.D. provided study materials or patients; R.F.S., S.K., and D.S. collected and assembled the data; R.F.S., S.K., A.L., A.B., H.D., and K.D. provided data analysis and interpretation; R.F.S., S.K., and H.D. wrote the manuscript; and R.F.S., S.K., L.B., G.K., J.C., M.R., G.H., P.B., M.L., H.R.S., T.K., H.A.H., G.W., D.N., K.G., A.L., P.P., V.I.G., V.T., D.S., A.B., J.K., A.G., H.D., and K.D. gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.K. is Department of Internal Medicine V, University Hospital of Heidelberg, Heidelberg, Germany.
Correspondence: Richard F. Schlenk, Department of Internal Medicine III, University Hospital of Ulm Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: richard.schlenk@uniklinik-ulm.de.
References
Author notes
R.F.S. and S.K. contributed equally to this work.