The P2Y12 inhibitors, clopidogrel, prasugrel, and ticagrelor, are administered in fixed doses without laboratory monitoring. Randomized trials in acute coronary syndrome have shown that prasugrel and ticagrelor are more effective than standard-dose clopidogrel. Nonetheless, standard-dose clopidogrel remains widely used because it causes less bleeding and is less expensive. Patients treated with standard-dose clopidogrel have substantial variability in platelet inhibition, which is partly explained by genetic polymorphisms encoding CYP2C19, the hepatic enzyme involved in biotransformation of clopidogrel to its active metabolite. Some advocate tailoring P2Y12 inhibitor therapy according to the results of routine laboratory testing. Although there is good evidence for analytic, biological, and clinical validity of several phenotypic and genotypic biomarkers, the benefit of a management strategy that incorporates routine biomarker testing over standard of care without such testing remains unproven. Appropriately designed, adequately powered trials are needed but face the challenges of feasibility, cost, and the progressive switch from clopidogrel to prasugrel or ticagrelor.
Introduction
Dual antiplatelet therapy with aspirin and a P2Y12 ADP receptor antagonist is a mainstay of treatment of acute coronary syndrome (ACS). Clopidogrel has been the P2Y12 inhibitor of choice and is given in fixed doses without laboratory monitoring. Although effective, standard doses of clopidogrel fail to completely inhibit ADP-induced aggregation in up to 30% of patients, a phenomenon labeled poor response.1,2 Prasugrel and ticagrelor, the newer P2Y12 inhibitors, are more effective than clopidogrel,3,4 prompting some guidelines to recommend these agents over clopidogrel in ACS.5,,-8 Nevertheless, clopidogrel remains widely used because it causes less bleeding and costs less.9
Some experts advocate individualizing P2Y12 inhibitor therapy based on laboratory test results,10,11 justifying their approach on 2 assumptions: (1) platelet function tests1,2,12 and genetic polymorphisms13,,-16 can identify poor responders to clopidogrel and (2) intensifying treatment in poor responders improves outcome. Treatment intensification strategies include doubling the clopidogrel dose or switching to prasugrel or ticagrelor. Although intensifying treatment increases efficacy, it also increases bleeding risk. Others reject routine phenotypic and genetic testing because its clinical utility is unknown.17,-19
This review focuses on current understanding of the value of phenotypic and genetic testing to identify poor responders to clopidogrel. We limited discussion to clopidogrel because it is the most widely used P2Y12 inhibitor and shows the greatest between-patient variability in pharmacological effect.20,-22
Pharmacokinetic and pharmacodynamic variability of clopidogrel
Clopidogrel, a prodrug, requires bioactivation in the liver.23 About 50% of oral clopidogrel is absorbed in the intestine,24 of which 15% is activated via 2 sequential oxidative steps involving the hepatic CYP450 system.25,26 In a competing pathway, ∼85% of absorbed clopidogrel is converted by esterases to a carboxylic acid metabolite lacking P2Y12 antagonism. Blood levels of the active metabolite vary widely among patients,15,27,28 and the inhibitory effect of clopidogrel on ADP-induced platelet aggregation is also variable.1,2,29 Increasing the clopidogrel dose does not eliminate variability in inhibition of ADP-induced platelet aggregation.30,-32 Differences in drug absorption,33 enzyme activity,15 drug-to-drug interactions (eg, statins, proton pump inhibitors, and calcium channel blockers),1,34 age,1 body mass index,1 diabetes,35 high epinephrine states, hyperfibrinogenemia, and genetic factors contribute to the variable response to clopidogrel.14 However, substantial variability in response to clopidogrel remains unexplained.36
Prasugrel is also a prodrug, but compared with clopidogrel, bioactivation of prasugrel involves one less step, and is less susceptible to genetic variation and drug interactions.25 Like clopidogrel, the active metabolite of prasugrel binds irreversibly to P2Y12, but prasugrel exhibits less between-subject variability in peak concentration and exposure in healthy subjects. The coefficients of variation (CVs) for maximum plasma concentrations (Cmax) of prasugrel and clopidogrel are 40% and 55%, respectively, whereas those for area under curve (AUC) are 30% and 50%, respectively.27,28 Data on variability of pharmacokinetic parameters in ACS and percutaneous coronary intervention (PCI) populations are lacking. Ticagrelor is a direct-acting P2Y12 inhibitor that does not require metabolic activation and shows similar between-subject variabilities as prasugrel. The CVs for Cmax and AUC are both ∼40% in healthy subjects.37 Compared with clopidogrel, prasugrel and ticagrelor produce greater and more consistent platelet inhibition.20,-22,27,28,37
Predictive biomarkers to identify poor responders to clopidogrel
Predictive biomarkers, which can be phenotypic or genotypic, identify subgroup(s) of patients who may have a better clinical response with an intensified antiplatelet regimen.38,-40 Phenotypic biomarkers measure the inhibitory effects of clopidogrel on ADP-mediated platelet activation. Genotypic biomarkers identify characteristics that influence clopidogrel metabolism.13,-15
Conceptual framework for evaluating predictive biomarkers
We propose 4 criteria (Table 1) to evaluate phenotypic and genotypic biomarkers for identifying poor responders to clopidogrel41 :
Analytical validity focuses on test precision and accuracy for measuring the biomarker.
Biological validity informs on test ability to measure the inhibitory effect of clopidogrel on ADP-induced platelet activation (phenotypic) or the concentration of the active metabolite (genetic).
Clinical validity informs on test ability to predict clinical outcome. Although clinical validity is important, it does not prove clinical utility.
Clinical utility informs on whether modifying treatment based on the biomarker test result improves clinical outcome. In this review, we focus on the modulation of P2Y12 inhibition based on biomarker results rather than treatment modification involving alternative revascularization strategies such as avoidance of PCI or consideration of coronary artery bypass.
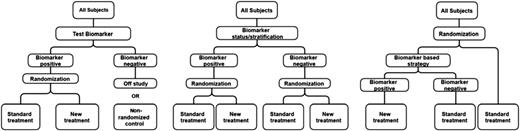
Three study designs (Table 2) have been used to evaluate the clinical utility of phenotypic and genetic biomarker testing.42,43
Design A. The biomarker enrichment design examines whether intensified treatment (high-dose clopidogrel, prasugrel, or ticagrelor) is better than standard-dose clopidogrel in poor responders identified by biomarker testing. It is limited because any observed benefit of experimental treatment cannot be attributed to biomarker testing nor does it inform on the efficacy or safety of intensified treatment relative to control treatment in normal responders.
Design B. The biomarker by treatment interaction design randomizes patients into experimental or control arms. Biomarker testing is then performed to identify poor and normal responders to clopidogrel. Because subjects are not randomized into a biomarker testing or nontesting strategy, such studies are not as rigorous as design C. Alternatively, biomarker testing could be performed prerandomization to stratify patients into poor and normal responders (biomarker-stratified design).
Design C. The biomarker strategy is the best design because it randomizes patients to use or nonuse of a biomarker strategy. If the biomarker strategy is used, poor responders receive intensified treatment and normal responders receive standard-dose clopidogrel. In contrast, patients randomized to nonuse of the biomarker strategy receive standard-dose clopidogrel. This design requires the largest sample size because only ∼30% of patients in the biomarker strategy arm will be poor responders.
As predictive biomarkers, several phenotypic tests (Table 3) and a genetic test13,-15 satisfy the first and second criteria, some satisfy the third, but to date, none has satisfied the fourth. Consensus guideline committees (and clinicians) should determine whether satisfying the first 3 criteria, without exploring the fourth, is sufficient to recommend routine screening of clopidogrel-treated patients.
Review of phenotypic biomarkers
Table 3 lists the features of 6 commonly used phenotypic assays44,45 : (1) light transmission aggregometry (LTA); (2) VerifyNow P2Y12; (3) multiplate impedance aggregometry (MEA); (4) PFA-100 (INNOVANCE P2Y cartridge); (5) thromboelastography (TEG); and (6) vasodilator-stimulated phosphoprotein (VASP) assay.
The first 5 assays measure the inhibitory effect of clopidogrel on ADP-induced platelet aggregation using different methods of detection, including light absorbance for LTA and VerifyNow, electrical impedance for MEA, closure time for PFA-100, and clot tensile strength for TEG. We consider the use of the PFA100 system in conjunction with the newer INNOVANCE P2Y cartridge rather than the conventional Dade PFA collagen/ADP test cartridge, which is insensitive to P2Y12 inhibitors.46 Using flow cytometry, the VASP assay measures downstream effects of clopidogrel on ADP-induced P2Y12 receptor activation. Of the 6 assays, only VerifyNow P2Y12 is a true point-of-care assay, being easy to perform and having a rapid turnaround time.47
Analytical validity
A systematic review by the Agency for Health and Quality Research identified >100 studies assessing the analytical performance of phenotypic assays.45 All 6 tests (Table 3) were evaluated by assessing (1) reproducibility in replicate samples (intra-assay CV), (2) correlation between LTA and other assays, and (3) test agreement between LTA and other assays, summarized by κ statistics. The intra-assay CV is reported as <11% (∼30 studies); an acceptable result in view of the wide between-subject variability in the pharmacodynamic response to clopidogrel (CV ∼ 70%).1 Although most studies reported moderate to good correlation between LTA and the other assays, test agreement was poor, in part because cutoffs were not rigorously evaluated (Table 3).
Biological validity
All 6 assays are biologically valid because each measures ≥1 consequence of P2Y12 receptor stimulation by ADP: platelet activation, platelet aggregation, or clot formation. The VASP assay quantifies phosphorylated VASP levels downstream to the P2Y12 receptor, which is a measure of platelet activation.48 The TEG measures clot tensile strength. The other assays capture clopidogrel’s inhibition of P2Y12 by measuring platelet aggregation and are susceptible to variables that influence the optical (LTA and VerifyNow) and impedance (MEA) end points. Test selection depends on feasibility in clinical trials. The most convenient test is the VerifyNow P2Y12 assay. Clinical outcome studies are required to determine a test’s cutoff values.12 An optimal cutoff value is identified by performing an exploratory study to identify the cutoff, which is then prospectively tested in a confirmatory clinical outcome study.
Clinical validity
Adverse cardiovascular outcomes.
Most studies were performed in the setting of PCI and used major adverse cardiovascular events (MACE) and stent thrombosis as efficacy outcomes.12,45 Five meta-analyses of prospective observational studies and subanalyses of randomized controlled trials (RCTs) involving >10 000 PCI patients have been published (Table 4).49,,,-53 All reported strong associations between poor response to clopidogrel and adverse cardiovascular outcomes with the 4 commonly evaluated assays (LTA, VerifyNow P2Y12, VASP, and MEA). The odds ratios (ORs) were significant for MACE (range, 2.1-8.0) and stent thrombosis (range, 3.1-7.0).
Limited information is available in medically managed patients with coronary artery disease (CAD). The largest study in medically managed ACS patients, a nested substudy (n = 2,564) of the targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes (TRILOGY ACS) trial, failed to show an independent association between poor response and MACE (adjusted hazard ratio [HR], 1.03; 95% confidence interval [CI], 0.96-1.11).54
Bleeding.
Results of studies examining the relationship between enhanced response to clopidogrel and bleeding have been inconsistent. Two observational studies support a relationship between clopidogrel response and bleeding. In the first, enhanced responsiveness to clopidogrel by MEA showed a 3.5-fold increase in major bleeding in a PCI population (n = 2533).55 The second, the assessment of dual antiplatelet therapy with drug eluting stents (ADEPT-DES) prospective registry (n = 8665), reported that poor responders had less clinically relevant bleeding (adjusted HR, 0.65; 95% CI, 0.43-0.99).56 In contrast, 2 large RCTs57,58 failed to show an association between clopidogrel response and bleeding but were probably underpowered.
Parallel comparisons of phenotypic assays in the PCI population.
The meta-analyses do not provide information about relative capacities of the various assays to predict clinical outcomes. The “Do platelet function assays predict clinical outcomes in clopidogrel pretreated patients undergoing elective PCI” (POPULAR) study performed parallel comparison of 8 phenotypic assays to predict 1-year MACE outcome and bleeding in 1069 consecutive patients.59 The assays differed in their associations with clinical outcomes. Only LTA, VerifyNow P2Y12, and Plateletworks (an uncommonly used assay because it needs to be performed within 10 minutes) showed significant associations with MACE, but the ability to differentiate between responders and poor responders was modest (AUC range, 0.61-0.63). None of the assays predicted bleeding.
Clinical utility
The clinical utility of phenotypic testing was evaluated in several older RCTs in >1500 patients using enrichment designs (design A) (Table 5).60,,,,,-66 Although poor responders to clopidogrel who were treated with an alternative P2Y12 inhibitor had improvement in clinical outcome,67 these studies do not inform on whether routine biomarker testing and treatment intensification in poor responders were responsible for the improved outcome.
Three more recent randomized studies (double randomization of a monitoring adjusted antiplatelet treatment vs a common antiplatelet treatment for DES implantation, and Interruption vs continuation of double antiplatelet therapy [ARCTIC], gauging responsiveness with a VerifyNow assay-impact on thrombosis and safety [GRAVITAS], and testing platelet reactivity in patients undergoing elective stent placement on clopidogrel to guide alternative therapy with prasugrel [TRIGGER-PCI]) used VerifyNow to identify poor responders.57,58,68 Of these, only ARCTIC used a biomarker strategy design (design C) to compare a tailored approach with standard-dose clopidogrel in all-comers. The other 2 used an enrichment design (design A).
ARCTIC study: is a phenotypic biomarker based strategy better than conventional use of antiplatelet in a PCI population?
The ARCTIC study (n = 2440), an open-labeled RCT, enrolled patients with stable angina (73%) or ACS (27%) who underwent PCI.57 Patients were randomized to either standard antiplatelet therapy (aspirin and clopidogrel) or the experimental arm of VerifyNow-directed antiplatelet therapy. Poor responders to clopidogrel in the experimental arm were identified using a cutoff of >235 platelet reactivity units (PRUs) or platelet inhibition of <15% from baseline. Prior to PCI, 34.5% of patients were identified as poor responders at initial testing and were treated with a glycoprotein IIb/IIIa inhibitor and/or an increased loading dose of clopidogrel (600 mg) or prasugrel (60 mg), in addition to either maintenance clopidogrel (150 mg daily) or prasugrel (10 mg daily). On days 14 to 30 after stent implantation, a second VerifyNow test was performed in patients allocated to the experimental arm; 15.6% were found to be poor responders. The clopidogrel dose was increased further in these patients, or they were switched to prasugrel. At 1 year, the MACE rates in the experimental and control arms were similar (34.6% and 31.1%, respectively; HR, 1.13; 95% CI, 0.98-1.29) as were the rates of stent thrombosis (1.0% vs 0.7%, respectively; HR, 1.34; 95% CI, 0.56-3.18). In addition, there was no significant difference in overall rates of bleeding between the groups (4.5% vs 3.1%, respectively; HR, 0.90; 95% CI, 0.46-1.05).
GRAVITAS study: is high-dose clopidogrel better than standard-dose clopidogrel in PCI patients identified as poor responders by VerifyNow P2Y12?
The GRAVITAS study, a blinded RCT, enrolled 2214 patients with stable angina (60.2%) or ACS (39.8%) who had undergone PCI. Poor responders identified with the VerifyNow assay (using the consensus cutoff of PRUs ≥230 at 12-24 hours after PCI) were randomized to either increased-dose clopidogrel (150 mg daily) or standard clopidogrel (75 mg daily). At 6 months, the rates of MACE, the primary outcome, in the experimental and control arms were similar (2.3% and 2.3%, respectively; HR, 1.01; 95% CI, 0.58-1.76), as were the rates of stent thrombosis (0.5% and 0.7%, respectively; HR, 0.63; 95% CI, 0.21-1.93) and bleeding (1.4% and 2.3%, respectively; HR, 0.59; 95% CI, 0.31-1.11).
GRAVITAS is limited because the MACE rate of 2.3% in the control group was lower than the projected rate of 5.0%. Furthermore, the cutoff PRU value ≥230 used to classify poor responders to clopidogrel may have been too high because a post hoc analysis identified a PRU value >208 as being a more appropriate cutoff value.69 In addition increasing the clopidogrel dose to 150 mg was not sufficient to overcome a poor response to clopidogrel because >35% of patients in the experimental arm remained poor responders when VerifyNow testing was repeated at 1 and 6 months.58
TRIGGER PCI: is prasugrel better than standard clopidogrel in PCI patients identified to be poor responders by VerifyNow P2Y12?
The TRIGGER PCI study, a blinded RCT, enrolled patients with stable angina who had received drug-eluting stents.68 Poor responders to clopidogrel, identified with the VerifyNow assay using a cutoff PRU value of >208 (the cutoff tested post hoc in GRAVITAS) were randomized to either standard clopidogrel (75 mg) or prasugrel (10 mg) starting in the morning after PCI. The trial was stopped for futility after enrollment of only 413 patients because of 6-month MACE rates of 0.5% in the control arm and 0% in the experimental arm. Therefore, TRIGGER PCI contributes little useful information.
In summary, the 3 largest studies conducted to date have failed to show clinical utility of phenotypic assays in ACS patients to identify poor responders so that they can be targeted for intensified therapy. Two ongoing RCTs are exploring the clinical utility of VerifyNow in the PCI population (dual antiplatelet therapy tailored on the extent of platelet inhibition [DANTE] and tailored antiplatelet therapy vs recommended dose of prasugrel [ANTARCTIC]),70,71 with the latter focusing on elderly patients.
Genotypic biomarkers
Most genetic biomarker testing has focused on the CYP2C19 gene because it is the only one independently associated with variability in the platelet inhibitory response to clopidogrel in genome-wide or whole-exome association studies.14,72 The CYP2C19 gene encodes an enzyme involved in both steps of conversion of clopidogrel to its active metabolite.26 This gene is highly polymorphic, with ≥34 identified polymorphisms, some of which result in loss of function (LOF) and others in gain of function (GOF).73 CYP2C19*2 and CYP2C19*3 are the most common LOF alleles (with an estimated carrier prevalence of 30% in whites, 40% in blacks, and 55% in East Asians).74 The other LOF alleles (CYP2C19*4, *5, *6, *7, and *8) are much less common (<1% allelic frequency each)75 and have not been adequately evaluated in clinical studies. Individuals who are heterozygous for LOF alleles are intermediate metabolizers, whereas those who are homozygous are poor metabolizers of clopidogrel.
Although LOF CYP2C19 genotypes are associated with reduced ADP-induced platelet aggregation in response to clopidogrel, it is estimated that the common CYP2C19*2 allele explains only 12% of the variation in platelet response.14,72 With other factors collectively explaining >70% of the variation,14 treatment modification based on CYP2C19 testing alone is unlikely to have a major impact on outcome.
CYP2C19*17, a GOF allele, occurs in 2% to 5% of Asians and 20% to 25% of whites and blacks.76 Although initially reported to be associated with an exaggerated response to clopidogrel, subjects with this GOF haplotype lack the CYP2C19*2 LOF allele, raising the possibility that the gain of effect attributed to CYP2C19*17 allele is caused, at least in part, by the absence of CYP2C19*2 allele.77
Analytical validity
A systematic review of 11 studies reported good reproducibility of CYP2C19 genotyping methods and high levels of interassay agreement.45 Two point-of-care CYP2C19 tests, the Spartan Rx (Food and Drug Administration approved) and Verigene (Food and Drug Administration cleared), identify the 2 most common LOF alleles (CYP2C19*2 and *3) and the GOF allele (CYP2C19*17). Both are appropriate for bedside use and provide results within 1 and 3 hours, respectively.78,79
Biological validity
Clinical validity
Adverse cardiovascular events.
The association between carriers of CYP2C19 LOF alleles and an increased risk of cardiovascular events in clopidogrel-treated patients has been investigated in patients with ACS, PCI, stable ischemic heart disease, and atrial fibrillation. MACE and stent thrombosis have been used as clinical outcomes in >30 observational studies and 6 genetic substudies nested in RCTs, which included >42 000 patients (Table 6).29,53,82,,,,,,,-90 There was also an association between LOF alleles and MACE, which on indirect comparison of 2 separate meta-analyses suggests a greater risk in patients undergoing PCI. Thus, in the meta-analysis by Mega et al,83 in which the majority of subjects had undergone PCI, a significant increase in the risk of both stent thrombosis (HR, 2.81; 95% CI, 1.81-4.37) and MACE (HR, 1.57; 95% CI, 1.13-2.16) in carriers of LOF alleles was observed. The meta-analysis by Holmes et al29 also showed a significant increase in risk of either stent thrombosis (relative risk [RR], 1.75; 95% CI, 1.50-2.03) or MACE (RR, 1.18; 95% CI, 1.09-1.28), but a lesser proportion (∼40%) of subjects underwent PCI, and the magnitude of the effect was comparatively lower.
Most studies included in these meta-analyses were observational and therefore subject to bias and confounding. The only meta-analysis of RCTs,29 which separately analyzed 4 placebo-controlled trials of clopidogrel (n = 11 012), failed to show a significantly higher rate of MACE in carriers of LOF alleles, but most patients included in these studies had not undergone PCI (Table 7).91,-93
Bleeding
Studies evaluating the association between LOF alleles and major bleeding were not powered to look for differences in major bleeding. The evidence for an association is limited to a meta-analysis of 3 subanalyses of placebo-controlled trials of clopidogrel in which a modest reduction in overall bleeding was reported in carriers of CYP2C19 LOF alleles compared with noncarriers (RR, 0.84; 95% CI, 0.75-0.94), but there was no reduction in severe bleeding (RR, 1.07; 95% CI, 0.92-1.25).29
Clinical utility
To date, 2 genetic substudies of larger RCTs (Table 8) have evaluated the clinical utility of CYP2C19 LOF testing.15,94,95 The majority of patients enrolled in the study of platelet inhibition and patient outcomes [PLATO] and trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel–thrombolysis in myocardial infarction [TRITON-TIMI] 38 trials underwent PCI,3,4 and the efficacy and safety of clopidogrel relative to prasugrel or ticagrelor in carriers and noncarriers of LOF alleles were reported in genetic substudies (design B).
PLATO genetic substudy.
PLATO, which compared clopidogrel with ticagrelor in 18 624 ACS patients of whom 64% underwent PCI, showed a reduction in MACE with ticagrelor (HR, 0.84; 95% CI, 0.77-0.92).4 In the genetic substudy (n = 10 285), ticagrelor produced similar estimates for efficacy as clopidogrel in the LOF (HR, 0.77; 95% CI, 0.60-0.99) and non-LOF subgroups (HR, 0.86; 95% CI, 0.74-1.01; P interaction = .46).95 Estimates for major bleeding were also similar in the LOF (RR, 1.04; 95% CI, 0.82-1.30) and non-LOF subgroups (RR, 0.96; 95% CI, 0.83-1.12; P interaction = .60).
TRITON TIMI 38 genetic substudies.
TRITON TIMI 38, which compared clopidogrel with prasugrel in 13 608 ACS patients scheduled for PCI, showed a greater reduction in MACE with prasugrel (HR, 0.81; 95% CI, 0.73-0.90).3 The effect of LOF alleles on outcome (Table 8) was published separately for the clopidogrel (n = 1477) and prasugrel arms (n = 1466). In the clopidogrel report, LOF carriers treated with clopidogrel had a worse MACE outcome than non-LOF carriers (HR, 1.53; 95% CI, 1.03-2.19),15 whereas in the report of prasugrel-treated patients, MACE outcomes in LOF and non-LOF carriers were similar (HR, 0.89; 95%, 0.66-1.31).94 The authors concluded that LOF status is a predictor of outcome in patients treated with clopidogrel, but not in those treated with prasugrel. In a subsequent report, Sorich et al estimated that compared with clopidogrel, prasugrel reduced the MACE risk in LOF carriers (RR, 0.57; 95% CI, 0.39-0.83) but not in noncarriers (RR, 0.98; 95% CI, 0.80-1.20; P interaction = .046).96 Total bleeding was increased with prasugrel in LOF carriers (RR, 1.60; 95% CI, 0.8-3.1) and noncarriers (RR, 1.38; 95% CI, 1.00-1.93).
Based on the integrated analysis of Sorich et al, the TRITON genetic substudy results could be interpreted to indicate that prasugrel is the preferred treatment of carriers of LOF alleles and clopidogrel is adequate for noncarriers. However, there is pharmacodynamic evidence that prasugrel produces greater inhibition of ADP-induced platelet aggregation than clopidogrel in both LOF carriers and noncarriers,27,28,30 and the much larger PLATO genetic substudy showed a clear benefit of ticagrelor over clopidogrel in noncarriers.95
In an ongoing RCT in ∼6000 patients undergoing PCI (TAILOR-PCI), rates of MACE and overall bleeding with a CYP2C19 genotype-based strategy (poor responders are switched to ticagrelor 90 mg twice daily) will be compared with those with a standard clopidogrel regimen (design C).97
Summary of the evidence
Phenotypic assays
Despite analytical limitations of phenotypic tests, clinical trials in patients undergoing PCI have shown that, compared with normal responders, poor responders to clopidogrel have increased risks of MACE and stent thrombosis. The evidence for this association in medically treated ACS patients is weak, and the evidence for an inverse association between platelet reactivity and bleeding is inconsistent. All 3 RCTs testing the clinical utility of using the VerifyNow assay to tailor therapy have not shown benefit, but all had limitations that could mask a true effect.57,58,68
Genetic assays
There is good evidence for analytical validity of the genetic test for LOF polymorphisms.45 There is also good evidence that LOF polymorphisms are associated with reduced levels of the active clopidogrel metabolite and with reduced on-treatment inhibition of ADP-induced platelet activation.15,80,81 In PCI populations, there is consistent evidence for an association between LOF polymorphisms and adverse clinical outcomes (stent thrombosis and MACE),83 but evidence of an association for other treatment indications is either absent or weak. Evidence for clinical utility of CYP2C19 genotyping as a predictive biomarker is limited to subgroup analyses with inconclusive findings.15,94,95
Recommendations for future research
Earlier, we outlined 3 study designs to evaluate the clinical utility of predictive biomarkers. The first, the biomarker enrichment design (design A), provides the weakest level of support for routine biomarker testing. The second design, the biomarker by treatment interaction design (design B), informs on the net benefits of alternative P2Y12 treatment strategies compared with standard-dose clopidogrel in both poor and normal responders and can be used to support routine biomarker testing if the results are definitive. The third design, the biomarker strategy design (design C), is best because patients are randomized to undergo or not undergo biomarker testing.
The sample size and complexity of a definitive trial are influenced by the trial design and type of biomarker selected, phenotypic or genetic. Phenotypic testing is expected to identify most poor responders but requires initial clopidogrel exposure and a properly validated cutoff value. Furthermore, because a reduced response to clopidogrel can be transient, phenotypic testing may be misleading if performed in the acute setting. The CYP2C19 polymorphism does not have these shortcomings, but its ability to identify poor responders is inferior; <50% of LOF carriers had evidence of poor response on LTA.98
For reasons of feasibility and clinical relevance, evaluation of biomarker testing strategies is best directed at patient groups with high event rates. Patients with ACS undergoing PCI are an acceptable group because, although the rate of stent thrombosis is low, the average 1-year MACE rate is expected to be ∼10%. Routine biomarker testing is less likely to produce a worthwhile benefit in lower risk population such as medically treated patients with ACS or stable angina. The preferred design to establish the clinical utility of biomarker testing is design C. Our calculated sample size for a design C study in ACS patients undergoing PCI using phenotypic biomarker testing and comparing prasugrel or ticagrelor with clopidogrel is ∼15 000. This sample size is based on an assumed clopidogrel event rate of 10%, an overall 15% RR reduction with prasugrel or ticagrelor, and a poor response rate of 30%. Because of study feasibility and costs and the increasing use of the newer P2Y12 inhibitors, particularly in the PCI population, such a study is unlikely to be performed.
Conclusions
Prasugrel and ticagrelor are more effective than clopidogrel in ACS patients. Nevertheless, clopidogrel is still widely used because it is less expensive and causes less bleeding.3,4 Despite the variable effects of clopidogrel on ADP-mediated platelet activation, the benefit of a management strategy that incorporates routine biomarker testing remains unproven. We recognize that we are using stringent criteria to assess the potential role of routine biomarker testing of clopidogrel and that some experts will be critical of our recommendation not to endorse such testing. We also recognize that “absence of proof is not proof of absence.” It is not our intention to imply that testing should be disallowed if recommended to individual patients by informed physicians. However, from a societal perspective, we suggest that routine phenotypic or genetic testing should not be recommended until an appropriately designed clinical trial shows that such testing provides clinical benefit to patients.
Acknowledgments
N.C.C. is supported by an educational grant from the Haematology Society of Australia and New Zealand.
Authorship
Contribution: N.C.C. and J.H. wrote the first draft of the manuscript; and all authors contributed to critical review and revisions of the manuscript.
Conflict-of-interest disclosure: J.W.E. was a member of the Steering Committee of the CURRENT-OASIS 7 trial and has received honoraria and research support from companies that market clopidogrel (Sanofi), prasugrel (Daiichi-Sankyo), and ticagrelor (Astra-Zeneca). J.I.W. has received honoraria from Daiichi-Sankyo and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: John W. Eikelboom, Population Health Research Institute, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail: eikelbj@mcmaster.ca.