Key Points
GO before transplant improves outcome of CBF-AML patients in first relapse.
Abstract
Although core-binding factor-acute myeloid leukemia (CBF-AML) (t[8;21] or inv[16]/t[16;16]) represents a favorable cytogenetic AML subgroup, 30% to 40% of these patients relapse after standard intensive chemotherapy. The encouraging results of gemtuzumab ozogamicin (GO) in newly diagnosed AML, and particularly in CBF-AML, incited us to retrospectively investigate the impact of GO-based salvage in these patients. We retrospectively analyzed the outcome of 145 patients with CBF-AML (59 t[8;21], 86 inv[16]/t[16;16]) in first relapse. As salvage, 48 patients received GO-based chemotherapy and 97 patients received conventional chemotherapy. Median age was 43 years (range, 16-76). Median first complete remission duration was 12.1 months (range, 2.1-93.6). Overall, second complete remission (CR2) rate was 88%. With a median follow-up from relapse of 3.5 years, the estimated 5-year disease-free survival (DFS) was 50% and 5-year overall survival (OS) was 51%. Older age and shorter first complete remission duration was associated with a shorter OS. Patients treated with GO had similar CR2 rate but significantly higher 5-year DFS (68% vs 42%; P = .05) and OS (65% vs 44%; P = .02). In multivariate analysis, GO salvage was still associated with a significant benefit in DFS and OS. In the 78 patients who received allogeneic hematopoietic stem cell transplantation in CR2, GO before transplant significantly improved posttransplant DFS and OS without excess of treatment-related mortality.
Introduction
Core-binding factor (CBF) acute myeloid leukemia (AML) include 2 major subtypes, respectively associated with translocation t(8;21) (CBFA) and inversion inv(16)/t(16;16) (CBFB), that are both associated with the disruption of genes encoding subunits of the CBF, a heterodimeric transcriptional factor involved in the regulation of hematopoiesis.1,2 CBF-AML is considered to be a favorable cytogenetic subgroup, characterized by a low rate of primary drug resistance, a reduced relapse risk, and an increased overall survival (OS).3,4 Although never formally demonstrated, patients with CBF-AML have shown a markedly improved outcome when treated with consolidation regimens containing repetitive cycles of high-dose cytarabine.5-8 More recently, the adjunction of gemtuzumab ozogamicin (GO) to standard first line chemotherapy has been reported as associated with a marked benefit in CBF-AML patients treated in the large British Medical Research Council (MRC) AML-15 study.9 No advantage has been shown for autologous or allogeneic hematopoietic stem cell transplantation (autoSCT/alloSCT) in frontline treatment.10-14
About 60% to 70% of CBF-AML patients are alive at 5 years, with disease recurrence being the major treatment failure.12,15,16 Advanced age, higher white blood count, additional deletion in the long arm of chromosome 9, associated KIT and FLT3 gene mutations, and high minimal residual disease (MRD) level after induction or rising MRD level on serial monitoring have been reported as adverse prognostic factors.17,18 Two prior studies reported that patients with CBFA had a significantly shorter OS than patients with CBFB after first relapse.15,16 Postrelapse strategies have not been evaluated so far in this specific subtype. As in other AML subgroups, alloSCT is recommended in second complete remission (CR2),3 with the role of autoSCT being uncertain.13 GO was not approved in Europe, as the European Medicines Agency considered the risk-benefit balance of GO to be unfavorable. However, a patient-named compassionate program was available in France for patients with AML relapse.
The aim of our study was to retrospectively evaluate the use of GO before transplantation in CBF-AML patients in first relapse, treated in 33 centers from the French AML Intergroup.
Patients and methods
Patients
A total of 145 patients were included in this study according to the following criteria: 1) CBF-AML was diagnosed according to the French-American-British criteria with t(8;21), inv(16)/t(16;16), and/or their molecular equivalents in first relapse; 2) previous first line treatment with at least one course of anthracycline, plus cytarabine-based induction chemotherapy; and 3) no prior history of treatment with GO or auto/allo SCT. Salvage therapy was defined as the first intensive treatment of AML recurrence.
The presence of t(8;21)(q22;q22), inv(16)(p13q22), or t(16;16)(p13q22) at first diagnosis was detected either by standard cytogenetics or by reverse transcriptase-polymerase chain reaction detection of corresponding fusion transcripts. Karyotypes at relapse and gene mutations were not included in this analysis due to lack of consistent data.
First-line therapy
Patients were mostly treated according to the following protocols: Acute Leukemia French Association (ALFA)-9801, ALFA-9802, LAM-2001, and CBF-2006.17,19-21 All patients underwent induction chemotherapy with an anthracycline plus cytarabine-based regimen. This study was conducted in accordance with the Declaration of Helsinki.
Salvage therapy
The type of salvage chemotherapy was the investigator’s choice. Following approval of GO by the US Food and Drug Administration for the treatment of AML in 2000, the French health agency (French Health Products Safety Agency) opened a compassionate, patient-named program (authorization for temporary utilization program) of GO in relapsed CD33+ AML. In this authorization for temporary utilization program, potential combination of GO with standard chemotherapy was also the investigator’s choice.
The 33 centers involved in this study retrospectively included 1 to 20 patients per center. Seven centers included more than 5 patients (N = 89 of 145, 61%) and 26 included 5 patients or less (N = 56 of 145, 39%). Patients who received GO were well balanced between these subgroups: 27 of 89 (30%) were in centers that included more than 5, and 21 of 56 (38%) were in centers that included 5 or less (Fisher’s exact test, P = .47).
Salvage regimens were grouped into the following categories: 1) Forty-eight patients were treated with GO, combined in 35 patients (75%) with high-dose cytarabine alone without anthracycline; GO dose was 6 mg/sqm (single-dose) in 31 patients and fractionated doses (3 mg/sqm on days 1, 4, and 7) in 4 patients. The other 13 patients received GO in combination with cytarabine with additional anthracycline; GO dose was 9 mg/sqm in 7 patients, fractionated 3 mg/sqm on days 1, 4, and 7 in 1 patient, and 6 mg/sqm in 5 patients. 2) Ninety-seven patients were treated with chemotherapy alone, defined by standard dose cytarabine and anthracycline (n = 44) or high-dose cytarabine and anthracycline (n = 53), without GO.
Criteria for response and relapse
Morphologic response was evaluated after salvage therapy. Response was classified as CR or failure, including resistant disease and early death. Patients were considered in CR when the bone marrow examination was normal with <5% blasts and no Auer rods, recovery of ANC >1 G/L and platelets >100 G/L, and all extra-medullary disease had resolved. Relapse was defined as the reappearance of leukemic cells in the bone marrow (> 5%).
Definition of clinical end points
OS was measured on the date of relapse until date of death or date last known alive, censoring patients at last follow-up if alive. Disease-free survival (DFS) was measured only in patients who achieved a CR2, from the date of CR2 to date of relapse or death, censoring patients at last follow-up if alive.
Statistical methods
Patient characteristics and CR rate comparisons were performed using the Fisher’s exact test for binary variables and the Mann-Whitney U test for continuous variables. DFS and OS were estimated by the Kaplan-Meier method. To evaluate the impact of alloSCT and autoSCT, outcome data were estimated by the Mantel-Byar method, considering alloSCT or autoSCT as a time-dependent covariate.22,23 The method described by Simon and Makuch was applied for appropriate graphical representation of alloSCT and autoSCT impact on OS and DFS.24 Differences in OS and DFS between subgroups were assessed by univariate Cox proportional hazard regression models. Proportional-hazards assumption was checked before conducting multivariate analyses.25 For multivariate analyses, a Cox regression model according to the method of Andersen and Gill was used, including first complete remission (CR1) duration (as continuous variable), GO administration, alloSCT (as time-dependent variable), age at relapse (as continuous variable), CBF subtype (CBFA vs CBFB), and primary trial (ALFA-9801 as baseline) as covariates.26 Year of treatment was used as continuous variable to stratify all multivariate analyses. A P value ≤ .05 was considered to indicate statistical significance. Statistical analysis was performed on the STATA/SE 11.0 (StataCorp, College Station, TX) software package.
Results
Pretreatment characteristics
Between 1994 and 2011, 145 patients with first relapsing CBF-AML were analyzed with a median CR1 duration of 12.1 months (range, 2.1-93.6). Patient characteristics are summarized in Table 1. Median age at relapse was 43 years (range, 16-76). Male/female ratio was 1.13. Translocation t(8;21) was present in 59 patients (CBFA patients), including 37 with other chromosomal abnormalities (loss of sexual chromosome and del(9q) were observed in 27 [46%] and 10 [17%] CBFA patients, respectively). Inversion 16 was present in 86 CBFB patients, 26 of who had other chromosomal abnormalities (trisomy 22 and trisomy 8, in 9 [10%] and 9 [10%] CBFB patients, respectively).
Outcome after relapse
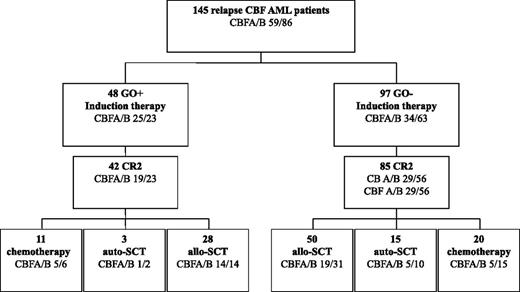
Overall, 127 of 145 patients (88%) achieved CR2 (Figure 1). In univariate analysis, patients with CBFB showed a trend toward higher CR2 compared with patients with CBFA (92% vs 81%; P = .07), whereas age at relapse (P = .62) and CR1 duration (P = .31) had no prognostic impact.
Patient flowchart. Induction and consolidation therapy according to GO administration in salvage therapy.
Patient flowchart. Induction and consolidation therapy according to GO administration in salvage therapy.
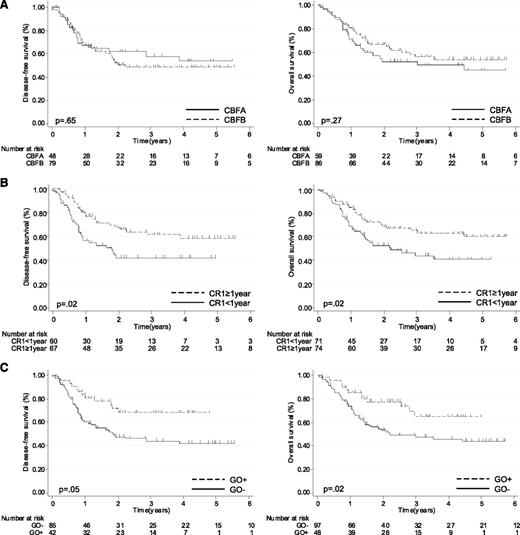
With a median follow-up from relapse of 3.5 years, the estimated 5-year DFS was 50% (95% CI, 41-60) and 5-year OS was 51% (95% CI, 41-60). In univariate analysis, CBF subgroup had no prognostic impact: 1) Five-year DFS was 54% (95% CI, 37-70) in the CBFA subgroup and 49% (95% CI, 37-61) in the CBFB subgroup (P = .65); 2) Five-year OS was 45% (95% CI, 29-60) in the CBFA subgroup and 54% (95% CI, 29-60) in the CBFB subgroup (P = .27) (Figure 2A and Tables 2 and 3). First-line trial had no prognostic impact either (Tables 2 and 3). On the other hand, shorter CR1 duration (P = .005) and older age (P = .03) were significantly associated with a shorter OS, whereas only shorter CR1 duration was associated with a shorter DFS (P = .002) (Figure 2B; Tables 2 and 3). In patients with CR1 duration < 1 year, 5-year DFS was 42% (95% CI, 29-55) and 5-year OS was 41% (95% CI, 27-54) vs 59% (95% CI, 46-72) and 60% (95% CI, 47-73) in patients with CR1 duration ≥ 1 year (Figure 2B).
Outcome of CBF-AML patients after first relapse (N = 145). DFS and OS according to (A) CBFA vs CBFB status, (B) CR1 duration (<1 vs ≥1 year), and (C) administration of GO in salvage therapy.
Outcome of CBF-AML patients after first relapse (N = 145). DFS and OS according to (A) CBFA vs CBFB status, (B) CR1 duration (<1 vs ≥1 year), and (C) administration of GO in salvage therapy.
Impact of GO therapy
Forty-eight of 145 patients (33%) were treated with a combination of GO and standard chemotherapy (GO+ subgroup). There was no significant difference between both GO+ and GO− subgroups in term of age, gender, and CBF subtype (Table 1). The CR1 duration was shorter in GO+ patients (11.1 vs 12.7 months; P = .04). As the rate of patients to whom GO was proposed as salvage increased with time, patients who received GO were more likely treated in the CBF-2006 trial than in previous ALFA-9801, ALFA-9802, or LAM-2001 trials (P < .001).
There was no impact of GO on the CR2 rate. Overall, 42 of 48 patients (88%) treated with GO and 85 of 97 patients (88%) treated with standard chemotherapy alone achieved CR2 after 1 salvage cycle (P = .99). Respectively, 6 (12%) and 11 (11%) patients failed to reach CR because of resistant disease (P = .99). One early death (due to infection) was reported during salvage in the standard chemotherapy (GO−) group. Two cases of reversible hepatic sinusoidal obstruction syndrome were documented and occurred during salvage therapy. One patient died after relapse and the other is alive and in CR. The use of GO had no significant impact on CR2 rate according to CBF subtype, even though patients with CBFB showed a trend toward higher CR2 compared with patients with CBFA (92% vs 81%; P = .07)
Despite the absence of impact of GO on CR2 rate, long-term outcome was significantly better in patients receiving GO (Figure 2C; Tables 2 and 3). The 5-year DFS in GO+ patients was 68% (95% CI, 53-84) compared with 42% in GO− patients (95% CI, 31-54; P = .05). Five-year OS was 65% in GO+ patients (95% CI, 48-82) compared with 44% in GO− patients (95% CI, 33-55; P = .02). When the analysis was restricted to patients with CBFA, GO+ was associated with a significantly higher 5-year DFS (84% [95% CI, 68-100] vs 38% [95% CI, 19-57]; P = .01) and OS (70% [95% CI, 51-89] vs 33% [95% CI, 16-51]; P = .06). In patients with CBFB, GO+ subgroup only showed a trend toward higher 5-year DFS (58% [95% CI, 36-79] vs 45%; P = .24) and OS (65% [95% CI, 41-88] vs 50% [95% CI, 37-64]; P = .08).
Feasibility and impact of SCT after relapse
We analyzed the role of alloSCT since this is typically regarded as the only potentially curative therapeutic option after relapse. Allogeneic SCT was performed in 78 of the relapsing patients. Among them, 77 patients received alloSCT as consolidation performed after achieving a CR2 following first salvage. One patient underwent alloSCT while in active disease after first salvage failure. There was no difference between patients who proceeded to alloSCT or not in term of sex ratio (P = .25), CBFA/B (P = .74), CR1 duration (12.4 vs 12 months; P = .43), or GO administration (58% vs 42%; P = .48). As expected, patients who proceeded to alloSCT were significantly younger (41 vs 52 years; P = .0002). Median time from first relapse to transplant was 2.2 months (range, 0.4-11.3). Conditioning regimens before alloSCT included standard myeloablative regimens (MAC) such as TBI or busulfan and cyclophosphamide (n = 55), or reduced-intensity conditioning (RIC) regimens with fludarabine (n = 23). Forty patients underwent alloSCT from a related donor (sibling donor [SIB]), and 35 patients underwent SCT from an unrelated donor (URD).
Eighteen patients received autoSCT in CR2. In time-dependent analysis, there was no benefit of autoSCT when compared with chemotherapy alone (N = 49) in terms of DFS (autoSCT vs chemotherapy, 5-year DFS 41% vs 39%, Hazard ratio [HR] 1.06, [95% CI, .48-2.35]; P = .88) and only a trend for OS (autoSCT vs chemotherapy, 60% vs 36%, HR .63, [95% CI, .27-1.50]; P = .30) (Figure 3A). Therefore, in order to evaluate the impact of alloSCT, and due to the low number of patients who received autoSCT in CR2, patients who received autoSCT were analyzed with those who were treated with chemotherapy alone. For patients who received alloSCT in CR2, the Mantel-Byar 5-year DFS estimate was 57% (95% CI, 43-69) compared with 42% (95% CI, 27-56) in nonallografted patients (HR .58, [95% CI, .34-.99]; P = .05; Figure 3B). Mantel-Byar 5-year OS estimates were 59% (95% CI, 44-72) in allografted patients and 45% (95% CI, 31-57) in nonallografted patients (HR .55, [95% CI, .32-.94); P = .03; Figure 3B). The benefit of alloSCT in terms of DFS was observed in CBFB patients (alloSCT vs nonalloSCT: HR .43 [95% CI, .22-.86]; P = .02), but not in CBFA patients (alloSCT vs nonalloSCT: HR .97 [95% CI, .37-2.50]; P = .94). Neither the type of donor (ID vs SIB) nor the conditioning regimen (RIC vs MAC) had an impact in terms of DFS (URD vs SIB: HR .91 [95% CI, .42-1.98]; P = .81; RIC vs MAC: HR .86 [95% CI, .36-2.04]; P = .73) or OS (URD vs SIB: HR 1.00 [95% CI, .44-2.30]; P = .99); and RIC vs MAC, HR 1.00 (95% CI .42-2.46), P = .98).
Impact of autoSCT, alloSTC, and GO administration in CBF-AML patients after relapse. Plots by Simon and Makuch for DFS and OS in nonallogeneic transplanted patients (N = 67) according to autoSCT. (A) For the whole cohort of patients according to allogeneic transplantation and (B) in allogeneic transplanted patients (N = 77) according to GO administration before transplant (C).
Impact of autoSCT, alloSTC, and GO administration in CBF-AML patients after relapse. Plots by Simon and Makuch for DFS and OS in nonallogeneic transplanted patients (N = 67) according to autoSCT. (A) For the whole cohort of patients according to allogeneic transplantation and (B) in allogeneic transplanted patients (N = 77) according to GO administration before transplant (C).
In patients receiving alloSCT in CR2 (Figure 3B), those who received GO before transplant (N = 27) had a significantly higher 5-year DFS (83% [95% CI, 60-93]) than those who received conventional chemotherapy (44% [95% CI, 28-60]; P = .01). Mantel-Byar 5-year OS was 82% (95% CI, 53-94) in patients who received GO before transplant, as compared with 48% (95% CI, 30-63; P = .01) in patients who received chemotherapy alone (Figure 3C). This benefit of GO administration before transplant was mostly observed in CBFA patients (GO vs no GO: HR .10 (95% CI .01-.77); P = .03) when compared with CBFB patients (GO vs no GO: HR .49 (95% CI .14-1.76); P = .25), although the numbers were small. There was no impact of cumulative dose of GO received before alloSCT on DFS or OS (data not shown). Less treatment-related mortality (TRM) was observed in patients who received GO before transplant (5-year TRM at 7% [95% CI, 0-19] vs 26% [95% CI, 10-41]; P = .05).
We performed a multivariate analysis including age, CBFA/B subtype, first-line trial, CR1 duration, use of GO, and of alloSCT (as time-dependent variable) as covariates. Younger age, CBFB subtype, longer CR1 duration time, and use of GO were independently associated with a longer DFS (P = .03, P = .05, P = .01, and P = .03, respectively). Longer CR1 duration, CBFB subtype, and use of GO were independently associated with a longer OS (P < .001, P = .02, and P = .002, respectively) (Tables 2 and 3).
Discussion
This study of 145 adult patients with CBF-AML in first relapse is the second largest cohort presented so far,14 with a median follow up of 3.5 years. Should there be a large consensus to consider CBF-AML at favorable risk, relapses have been reported to occur in up to 35% of patients.15-17 There is no established salvage treatment at this stage of the disease. First relapse is generally treated by chemotherapy based on a combination of anthracycline with standard- or high-dose cytarabine, followed or not by alloSCT. New therapeutic approaches are being developed, and more particularly, antibody-directed chemotherapy that offers the prospect of delivering chemotherapy to the target while sparing collateral toxicity. The British MRC AML-15 study was the first to demonstrate the superiority of GO in CBF-AML patients in CR1.9 In the French ALFA-0701 trial, GO was shown to benefit patients with favorable cytogenetic.27 Our observation suggests that GO may also benefit patients in first relapse, and that GO combined with chemotherapy followed by alloSCT, is safe and efficient.
The aim of this retrospective study was to assess the outcome of patients eligible for intensive salvage in order to evaluate the place of GO and allo-SCT in these patients. Thus, patients, none of whom had a prior history of allo-SCT, were all treated intensively and salvage treatment was left to the physician’s choice. Due to increasing arguments supporting the use of GO in CBF-AML patients, the rate of patients treated with GO at relapse increased with time, justifying that all multivariate analyses were stratified on the year of treatment. A high overall CR2 rate of 88% was observed and no patient or disease characteristics were associated with salvage failure. This rate was very similar to the 82% recently reported by the MRC study.14 No difference in CR2 rate between the two CBF subtypes were observed unlike Schlenk et al who reported a lower second CR rate of 33% in CBFA patients compared with 78% in CBFB patients. As already reported in the literature, white blood count at diagnosis was not a prognosis factor for CR2 rate.15
Due to the fact that all patients were intensively treated at relapse, relatively favorable long-term survival was noted in our cohort, with about half of the patients still alive at 5 years. In our study, prognosis after relapse seemed to be preferentially driven by treatment received as salvage therapy and the duration of the first CR. The CBFB subgroup was also associated with a better survival in multivariate analysis. A shorter survival of CBFA patients after first relapse was already noted in a previous study. Marcucci et al reported a 5-year postrelapse survival of 14% compared with 34%, respectively.16 Similar results were also shown by Schlenk et al.15 Interestingly, in the present study, GO seemed to have a marked benefit in CBFA patients. This may have contributed to minimize the difference of outcome between CBFA and CBFB patients in univariate analysis. The reasons for this difference of GO benefit between CBF subgroups remain unexplained and require further investigation. The importance of receptor tyrosine kinase mutations to predict outcome in front-line CBF patients has been highlighted by many retrospective and more recent prospective studies.28 Furthermore, more recently, MRD monitoring has been shown to be the most powerful prognostic factor in these patients.17 Due to the lack of available blast samples at diagnosis and relapse, we were unable to evaluate these parameters in our study.
It is usually considered that CBF-AML patients do not benefit from alloSCT in first CR. In a prospective study by the French Intergroup, despite a relapse incidence >30%, OS from CR remained as high as 85%, underlying the relatively good salvage rate in CBF-AML patients.17 Indeed, our observation suggests that intensive salvage in relapsed CBF-AML patients, including GO and alloSCT, may lead to favorable long-term outcomes with an estimated 5-year DFS of 83%. In relapsing patients, alloSCT is widely regarded as the only therapeutic option with curative potential.29 However, for CBF-AML patients, this remains a point of debate. A retrospective study from the ALFA group showed that donor availability was a positive factor for survival after first relapse in CBF-AML.12 This was not confirmed by a recent MRC study that did not observe any benefit of allogeneic transplant analyzed as time-dependent variable in this subgroup of patients, whatever the CBFA/B subgroup.14 In the present study, the benefit of alloSCT was restricted to the CBFB subgroup of patients. Previous studies suggested that a combination of GO and alloSCT can be applied in heavily pretreated patients with relapsed AML.30,31 In our experience, TRM after alloSCT was not increased by previous administration of GO. The 2 cases of hepatic sinusoidal syndromes occurred during induction therapy, and none after allogeneic SCT.
In conclusion, this study shows that half of CBF-AML patients can still be cured at time of first relapse. These results suggest that alloSCT should probably be reserved to postrelapse strategy or to front-line very high-risk patients. GO, combined with intensive chemotherapy, followed by allogeneic SCT in patients achieving CR2 seems to give the best chances of curability. Whether CBFA and CBFB differentially benefit from GO or alloSCT as salvage therapy should be further investigated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the clinicians of the Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang and ALFA groups who referred their patients and all clinical research assistants for their help with data management: Marie-Anne Hospital, Nicolas Boissel, Hervé Dombret, Karine Celli-Lebras (Paris), Thomas Prebet (Marseille), Sarah Bertoli and Christian Recher (Toulouse), Xavier Thomas (Lyon), Emmanuelle Tavernier (Saint Etienne), Thorsten Braun (Bobigny), Cécile Pautas and Ludovic Cabanne (Creteil), Aurore Perrot (Nancy), Bruno Lioure (Strasbourg), Philippe Rousselot and Sandra Gautier (Versailles), Thibaut Leguay (Bordeaux), Jérôme Tamburini (Hôpital Cochin, Paris), Jacques Delaunay and Marie-Aude Rambaud (Nantes), Thomas Cluzeau (Nice), Johanna Konopacki (Percy), Edouard Radriamalala (Poitiers), Sandrine Vaudaux (Rouen), Céline Berthon (Lille), Marie-Pierre Gourin, Florence Bosselut, and Céline Philippon (Limoges), Céline Haby (Mulhouse), Magda Alexis (Orleans), Chantal Himberlin (Reims), Norbert Ifrah and Thibaud Lecerf (Angers), Eric Deconinck and Monique Peria (Besançon), Jean-Christophe Ianotto (Brest), Emilie Marin (Caen), Romain Guièze (Clermont Ferrand), Sabine Camara (Colmar), Jean-Yves Cahn and Pierre Pittet (Grenoble), Julien Lazarovici (Villejuif), Eric Jourdan (Nimes), Madalina Uzunov (Pitié-Salpétrière), and Isabelle Plantier (Roubaix).
Authorship
Contribution: M.-A.H. and N.B. designed the research, collected and analyzed the data, and wrote the manuscript; T.P., S.B., X.T., E.T., T.B., C.P., A.P., B.L., P.R., J.T., T.C., J.K., E.R., C.B., M.-P.G., C.R., J.-Y.C., N.I., and H.D. assisted in data collection and manuscript preparation; and all the authors approved the final draft of the manuscript.
Conflict-of-disclosure: The authors declare no competing financial interests.
Correspondence: Nicolas Boissel, Department of Hematology, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, Paris 75010, France; e-mail: nicolas.boissel@sls.aphp.fr.