Key Points
ETP-ALL, a high-risk subtype of T-ALL, is characterized by aberrant activation of the JAK/STAT signaling pathway.
The JAK1/2 inhibitor ruxolitinib demonstrates robust activity in patient-derived xenograft models of ETP-ALL.
Abstract
Early T-cell precursor (ETP) acute lymphoblastic leukemia (ALL) is a recently described subtype of T-ALL characterized by a unique immunophenotype and genomic profile, as well as a high rate of induction failure. Frequent mutations in cytokine receptor and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathways led us to hypothesize that ETP-ALL is dependent on JAK/STAT signaling. Here we demonstrate aberrant activation of the JAK/STAT pathway in ETP-ALL blasts relative to non-ETP T-ALL. Moreover, ETP-ALL showed hyperactivation of STAT5 in response to interleukin-7, an effect that was abrogated by the JAK1/2 inhibitor ruxolitinib. In vivo, ruxolitinib displayed activity in 6 of 6 patient-derived murine xenograft models of ETP-ALL, with profound single-agent efficacy in 5 models. Ruxolitinib treatment decreased peripheral blast counts relative to pretreatment levels and compared with control (P < .01) in 5 of 6 ETP-ALL xenografts, with marked reduction in mean splenic blast counts (P < .01) in 6 of 6 samples. Surprisingly, both JAK/STAT pathway activation and ruxolitinib efficacy were independent of the presence of JAK/STAT pathway mutations, raising the possibility that the therapeutic potential of ruxolitinib in ETP-ALL extends beyond those cases with JAK mutations. These findings establish the preclinical in vivo efficacy of ruxolitinib in ETP-ALL, a biologically distinct subtype for which novel therapies are needed.
Introduction
Early T-cell precursor (ETP) acute lymphoblastic leukemia (ALL) was first described in 2009 as a subtype of T-ALL with a unique immunophenotype that includes expression of myeloid and early progenitor or stem cell markers in addition to T-cell lineage markers.1 Although overall survival for the majority of T-ALL cases has improved dramatically over the last 50 years,2 largely due to intensification of chemotherapy regimens, many published studies suggest a large percentage of ETP-ALL cases have dismal outcomes.1,3-7 More recent studies suggest that children with ETP-ALL treated on contemporary protocols that intensify therapy based on minimal residual disease response may not fare as poorly as originally thought.8,9 ETP-ALL, which represents ∼10% to 15% of new T-ALL diagnoses in children1,4,5 and ∼10% to 30% in adults,3,6 accounts for a disproportionate fraction of T-ALL induction failures (ie, failure to achieve a morphologic remission at the end of the first month of chemotherapy). Novel therapies with alternative mechanistic approaches are urgently needed for chemotherapy-refractory subgroups of ETP-ALL.
In addition to an immunophenotype with myeloid/stem cell markers, ETP-ALL cases demonstrate a similar transcriptional and mutational landscape to myeloid leukemias and hematopoietic stem cells.1,6,10-12 More than 60% of ETP-ALL cases harbor activating mutations in genes involved in cytokine receptor (IL7R), RAS (NRAS, KRAS, FLT3), and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) (JAK1, JAK3, SH2B3) signaling, and gene expression profiling suggests the JAK/STAT pathway may be dysregulated in some ETP-ALL.10-12 The JAK family of tyrosine kinases activates the STAT family of transcription factors. The JAK/STAT pathway represents one of the main signaling cascades mediating cytokine receptor-derived signals and plays a role in hematopoietic cell growth, proliferation, differentiation, and survival. A variety of hematologic malignancies activate JAK pathway signaling inappropriately through several mechanisms, including activating mutations, fusions, and downregulation of negative regulators.13-17 We previously showed in preclinical models that targeting the JAK pathway is a viable treatment strategy in Philadelphia chromosome (Ph)-like B-ALL, another high-risk leukemia subset with frequent lesions in the JAK/STAT pathway.18
Given the frequent mutations in cytokine and JAK/STAT signaling pathway members and enrichment of genes involved in JAK/STAT signaling by gene expression profiling, we hypothesized that the JAK/STAT pathway is hyperactivated in ETP-ALL and that targeting JAK/STAT has therapeutic relevance. To test this hypothesis, we evaluated the JAK/STAT signaling pathway in ETP-ALL blasts collected from pediatric patients and demonstrate relative hyperactivation of the JAK/STAT pathway in ETP-ALL compared with non-ETP T-ALL. Moreover, we tested the activity of the JAK1/2 inhibitor ruxolitinib in xenograft models derived from pediatric ETP-ALL blasts, finding robust activity regardless of the presence or absence of a mutation in the JAK/STAT signaling pathway. Together, these findings suggest that hyperactivation of JAK/STAT signaling may be a common feature of ETP-ALL and thus a relevant target in this subtype of T-ALL.
Methods
Patient samples
Previously banked diagnostic specimens (peripheral blood or bone marrow) from patients treated at Sydney Children’s Hospital, University of California San Francisco (UCSF), St. Jude Children’s Research Hospital (SJCRH), or on Children’s Oncology Group (COG) protocols were used to create xenografts for biochemical and drug efficacy studies. Informed consent for use of diagnostic specimens for future research was obtained from patients or their guardians according to the Declaration of Helsinki, and protocols were approved by the National Cancer Institute and institutional review boards of participating institutions. ETP-ALL and T-ALL samples had previously been characterized by gene expression profiling, whole exome or genome sequencing, or targeted sequencing of a gene panel.12
Establishment of xenograft models
Xenograft models were established as previously described19-22 with modifications described herein. All experiments were conducted on protocols approved by the Institutional Animal Care and Use Committee and Institutional Review Board of the Children's Hospital of Philadelphia and the Human Research Ethics Committee and the Animal Care and Ethics Committee of the University of New South Wales. Primary leukemia cells from peripheral blood or bone marrow were intravenously injected into nonobese diabetic/severe combined immunodeficient (NOD.Cg-Prkdcscid, also termed NOD/SCID) or NOD/SCID/Il2rgtm1wjl/SzJ (NSG) mice. Samples were injected at a cell dose of 2.5 to 10 × 106 cells per mouse. Splenocytes harvested from successfully engrafted xenografts were reinjected (106 cells per mouse) to create secondary and tertiary xenografts for biochemical and treatment studies. Engraftment was determined by flow cytometric analysis of peripheral blood using antibodies against human CD45 and cytoplasmic CD3 (cCD3-PE; Beckman Coulter). Cells were collected on an Accuri CSampler flow cytometer (BD Accuri Cytometers), and analysis was performed with FlowJo software (Tree Star).
For biochemical and pharmacodynamic studies, splenocytes were harvested once spleens were nearly replaced with human leukemia. For pharmacodynamic studies, 3 mice per arm were treated for 72 hours (in 2 cases, 1 mouse per arm died before treatment completed). Mice were euthanized, and spleens were harvested for immunoblotting.
For efficacy studies, mice were randomized to treatment or vehicle (4-7 mice per arm) once xenografts had engrafted with sufficient disease burden to detect >1% peripheral human cCD3+ blasts. Ruxolitinib (INCB018424) was administered for the entire 3- to 4-week treatment period in chow formulation provided by Incyte. Serum levels were confirmed to be within the range achieved in humans (data not shown). Studies were terminated, and mice were euthanized after 3 to 4 weeks of treatment, when vehicle-treated mice became ill according to standard parameters set on the Institutional Animal Care and Use Committee protocol. Spleen and bone marrow were harvested at the time of death. Disease burden was assessed every 7 to 14 days by flow cytometric measurement of human cCD3+ blast count in peripheral blood, using CountBright absolute counting beads (Invitrogen) as described in the manufacturer's instructions, and at death, by measuring absolute splenic blasts (total splenic cell count × % human cCD3+/CD45+ cells).
Phosphoflow analysis
Xenografted T-ALL or ETP-ALL samples were processed as previously described.12 Briefly, cells were thawed, washed, and adjusted to 2 × 106 cells/mL. After resting for 1 hour at 37°C in serum-free media containing 0.5% bovine serum albumin, 0.5 × 106 cells were plated and stimulated with 100 ng/mL interleukin-7 (IL7) or 200 μM pervanadate for 20 minutes. Cells were subsequently fixed, permeabilized, stained with antisera to CD3, human CD45, CD127, Caspase-3, and phospho-STAT5-allophycocyanin (BD Biosciences) and subjected to flow cytometry on a FacsVerse (BD Biosciences). Data were analyzed using FlowJo 8.8.7 (Tree Star), gating on the Caspase 3-negative population of cells. For drug assays, cells were cultured in T-ALL media23 (α-minimum essential medium [Gibco, Grand Island, NY] supplemented with 10% fetal calf serum, 10% human heat inactivated AB+ serum, 1% penicillin/streptomycin, 1% Glutamax [all from Invitrogen], human IL7 [R&D Systems, Minneapolis, MN], human stem cell factor, human FMS-like tyrosine kinase 3 (FLT3) ligand [both from Peprotech, London, United Kingdom], and insulin [Sigma-Aldrich, St. Louis, MO]) with vehicle or drug at the times and concentrations indicated in the figure legends and processed as above.
Immunoblotting
Methods for preparation of whole cell extracts, determination of protein concentrations, and analysis of cellular proteins by immunoblotting have been described in detail elsewhere.24 Additional details are provided in supplemental Materials, available on the Blood Web site.
Statistical analysis
The Mann-Whitney nonparametric analysis was performed using Prism software (GraphPad) to compare phospho-protein levels by immunoblot and phosphoflow cytometry. Two-way analysis of variance was performed using Prism software (GraphPad) to determine statistical significance of peripheral blast counts. Spleen blast counts were analyzed by a 2-sided t test. The Welch-Satterthwaite t test was performed on the fold change with treatment in phospho-protein levels by immunoblot.
Results
Patient-derived xenograft models
To perform preclinical studies in ETP-ALL, we developed multiple murine xenograft models derived from pediatric ALL blasts that met the distinct immunophenotypic diagnostic criteria for ETP-ALL (lack of CD1a and CD8 expression, weak or absent CD5 expression, and aberrant expression of ≥1 myeloid or stem cell markers), independently reviewed and confirmed by T-ALL immunophenotyping experts for the COG and SJCRH.1 Diagnostic specimens from patients treated on COG and SJCRH protocols were intravenously injected into immunodeficient mice. Engraftment was determined by peripheral blood flow cytometry for human cytoplasmic CD3-positive (cCD3+) and human CD45+ blasts. Seven of 14 ETP-ALL samples successfully engrafted. The poor rate of engraftment was likely caused by a combination of decreased viability on thaw and the inherent biology of ETP-ALL blasts. Historically, we have found excellent viability (>80% of samples have >80% viability) on thaw, using samples from the same cell banks. In contrast, 6 of the 7 samples that did not engraft had poor viability (average, 55%) on thaw, whereas successfully engrafted samples had good viability (>80%). Samples with >80% viability on thaw had similar rates of engraftment to prior work published by our group and others.18,21,25 We injected between 2 and 10 million cells per mouse and did not find a difference in time to engraftment or success of engraftment based on cell dose. Once engrafted with high disease burden, splenocytes were harvested and reinjected to create secondary, tertiary, and quaternary xenografts, used for biochemical and treatment studies. Establishment of secondary xenografts was possible in 6 ETP-ALL samples. One of the 7 samples that engrafted had low levels of engraftment detected in bone marrow, and insufficient cell numbers prevented secondary engraftment. Baseline clinical characteristics and relevant genetic lesions of these 6 samples used to create patient-derived xenograft models for this study are listed in Table 1.
To ensure the populations that engrafted were the leukemic populations of interest, we performed extensive immunophenotypic and single nucleotide polymorphism microarray analyses of blasts collected from mouse spleens after multiple passages in the mice. The xenografted samples preserved the ETP genetic profile with all clonal copy number alterations retained in the corresponding xenografts (supplemental Figure 1). Immunophenotype was largely preserved except for subtle upregulation of CD5 and CD8 in some cases and downregulation of CD117 in 1 case (supplemental Table 2). Data on immunophenotype of ETP-ALL during or after treatment in patients are lacking; therefore, it is not known if similar immunophenotypic drift is unique to xenografting or is also seen in patients.
JAK/STAT pathway activation in ETP-ALL
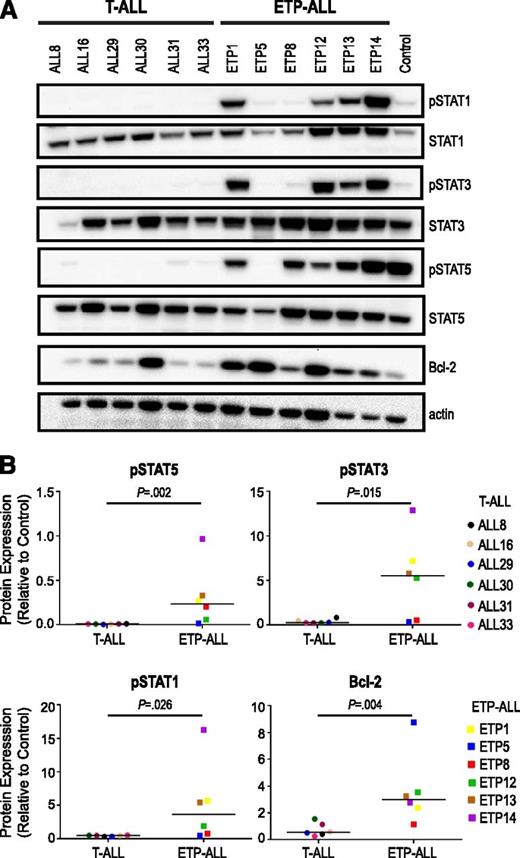
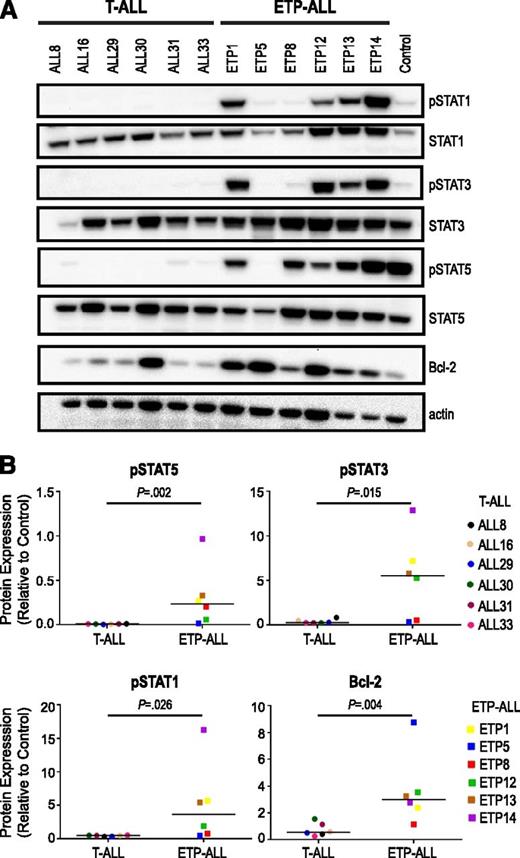
We hypothesized that JAK/STAT signaling is aberrantly activated in ETP-ALL. To study this hypothesis, we evaluated 6 ETP-ALL samples with or without JAK mutations by immunoblot and compared them with 6 non-ETP T-ALL samples. We assessed basal levels of phosphorylated downstream targets and found elevated levels of phosphorylated (p)STAT1 (P = .026), pSTAT3 (P = .015), and pSTAT5 (P = .002) in ETP-ALL samples compared with non-ETP T-ALL (Figure 1). Levels of pSTAT1 and pSTAT3 were elevated in 4 of 6 ETP-ALL samples and 0 of 6 non-ETP T-ALL samples (Figure 1). Levels of pSTAT5 were elevated in 5 of 6 ETP-ALL samples and 0 of 6 non-ETP T-ALL samples. Interestingly, JAK/STAT pathway activation was observed in the majority of samples regardless of JAK mutation status. The only ETP-ALL sample that did not have JAK/STAT pathway activation was ETP5. ETP5 has a mutation in PTPN11, which activates signaling through mitogen-activated protein kinase (MAPK).
Increased JAK/STAT activation in ETP-ALL relative to non-ETP T-ALL. (A) Levels of phosphophorylated (p) and total signaling pathway proteins in the spleens of non-ETP T-ALL xenograft models (ALL8, ALL16, ALL29, ALL30, ALL31, ALL33) and ETP-ALL xenograft models (ETP1, ETP5, ETP8, ETP12, ETP13, ETP14) by immunoblot. A CRLF2-overexpressing B-ALL xenograft with high levels of JAK/STAT pathway proteins was used as a positive control (Control). (B) Comparison of levels of pSTATs and Bcl-2 in non-ETP T-ALL and ETP-ALL xenografts. Protein levels by immunoblot of 3 replicates were quantitated relative to positive control (Control), graphed as means, and analyzed using the nonparametric Mann-Whitney test.
Increased JAK/STAT activation in ETP-ALL relative to non-ETP T-ALL. (A) Levels of phosphophorylated (p) and total signaling pathway proteins in the spleens of non-ETP T-ALL xenograft models (ALL8, ALL16, ALL29, ALL30, ALL31, ALL33) and ETP-ALL xenograft models (ETP1, ETP5, ETP8, ETP12, ETP13, ETP14) by immunoblot. A CRLF2-overexpressing B-ALL xenograft with high levels of JAK/STAT pathway proteins was used as a positive control (Control). (B) Comparison of levels of pSTATs and Bcl-2 in non-ETP T-ALL and ETP-ALL xenografts. Protein levels by immunoblot of 3 replicates were quantitated relative to positive control (Control), graphed as means, and analyzed using the nonparametric Mann-Whitney test.
IL7 signaling in ETP-ALL
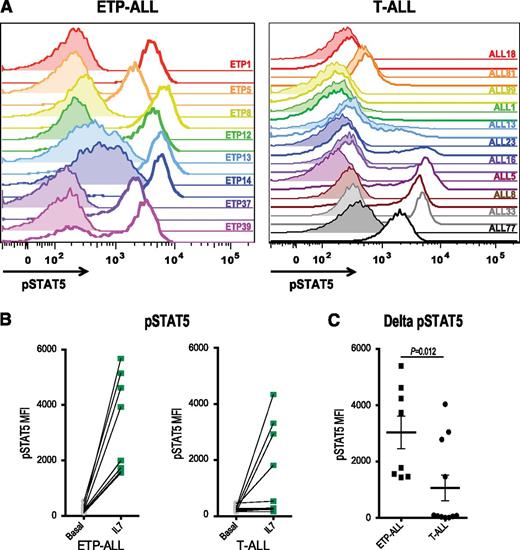
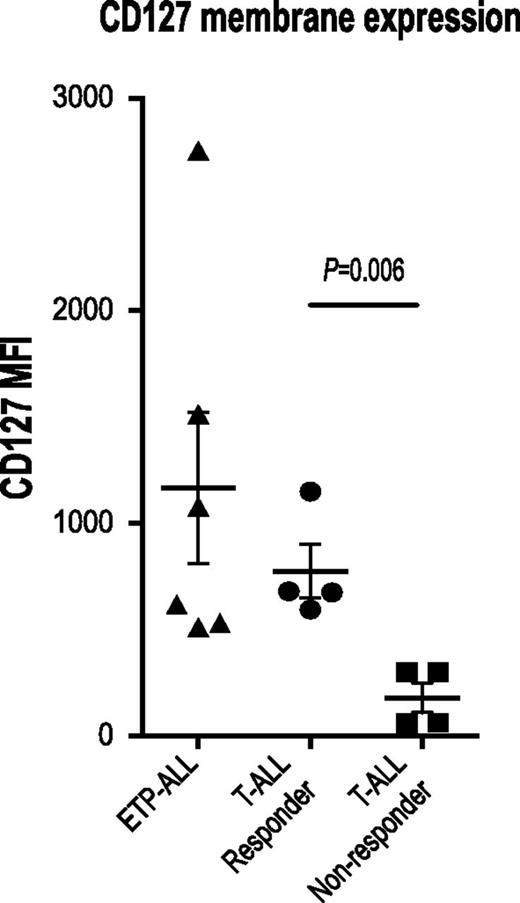
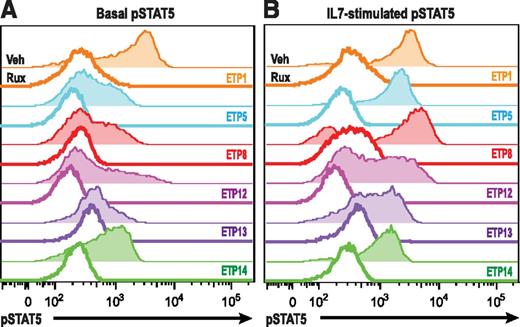
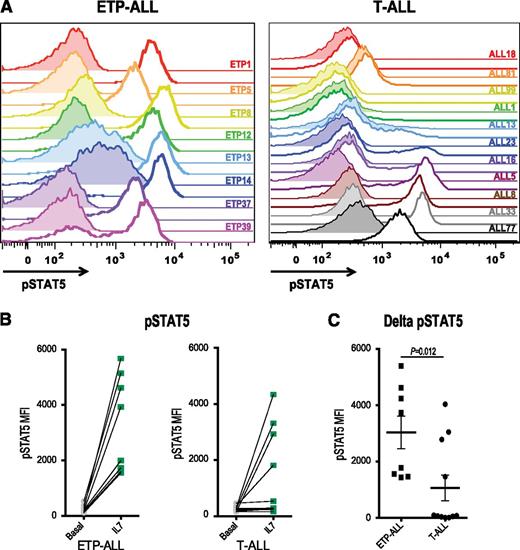
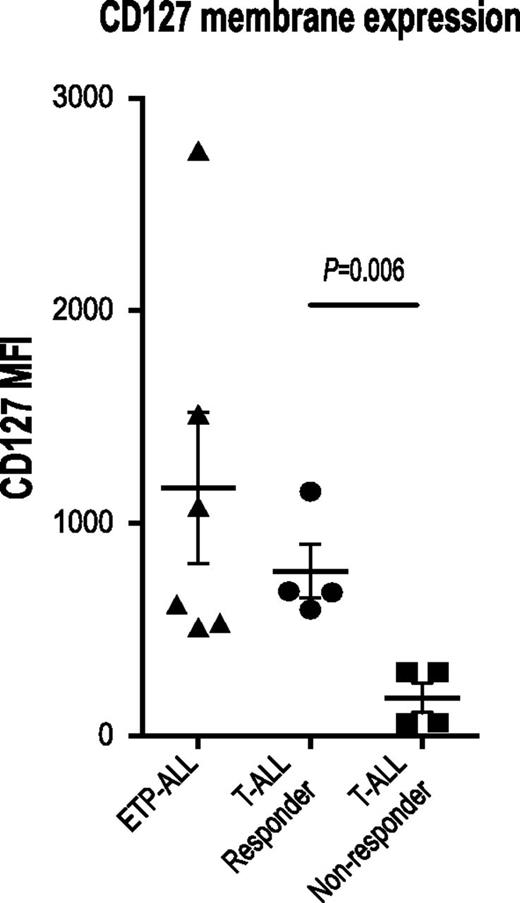
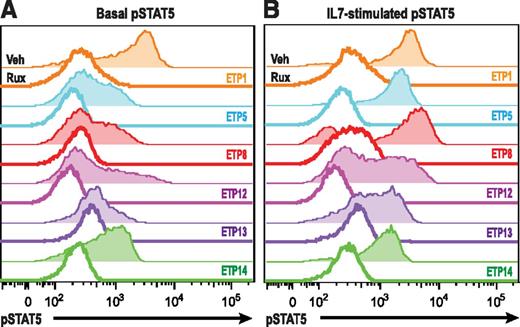
IL7R, which encodes the IL7RA subunit of the heterodimeric receptors for the cytokines IL7 and thymic stromal lymphopoietin, is mutated in a fraction of ETP-ALL.12 Expression of activating mutants of IL7R in hematopoietic cell lines results in activation of the JAK/STAT pathway.12,26 Therefore, we investigated JAK/STAT activation after IL7 stimulation in a larger panel of both ETP and non-ETP T-ALL samples ex vivo. Despite the fact that none of the ETP-ALL samples in our cohort had IL7R mutations, we demonstrate that 8 of 8 ETP-ALL samples responded to IL7 stimulation with increased pSTAT5, in contrast to only 4 of 11 non-ETP T-ALLs (Figure 2; P value for ETP vs non-ETP = .0118). Both the ETP samples and the non-ETP samples that responded to IL7 stimulation had higher levels of surface CD127 (IL7 receptor) compared with the IL7 nonresponsive non-ETP T-ALL samples (Figure 3). To determine whether the IL7-induced phosphorylation of STAT5 was mediated by JAK signaling, we treated our panel of 6 ETP-ALL samples with a JAK inhibitor. In vitro exposure to the JAK1/2 inhibitor ruxolitinib decreased basal phosphorylation of STAT5 (Figure 4A) and abrogated the IL7-induced increase in pSTAT5 (Figure 4B).
ETP-ALL hypersensitivity to IL7 stimulation. (A) Phosphoflow cytometry analysis of levels of pSTAT5 in xenografted non-ETP T-ALL and ETP-ALL leukemic blasts in the basal state (shaded) and in response to stimulation with 100 ng/mL IL7 for 15 minutes (unshaded). (B) Quantitation of mean fluorescence intensity (MFI) in the basal state and in response to IL7 exposure. (C) Change in pSTAT5 MFI with IL7 exposure compared with vehicle treatment of each xenograft. The nonparametric Mann Whitney test was used to evaluate statistical significance.
ETP-ALL hypersensitivity to IL7 stimulation. (A) Phosphoflow cytometry analysis of levels of pSTAT5 in xenografted non-ETP T-ALL and ETP-ALL leukemic blasts in the basal state (shaded) and in response to stimulation with 100 ng/mL IL7 for 15 minutes (unshaded). (B) Quantitation of mean fluorescence intensity (MFI) in the basal state and in response to IL7 exposure. (C) Change in pSTAT5 MFI with IL7 exposure compared with vehicle treatment of each xenograft. The nonparametric Mann Whitney test was used to evaluate statistical significance.
Expression of the IL7 receptor, CD127, correlates with IL7-induced STAT5 phosphorylation. Flow cytometry analysis of surface CD127 expression. Quantitation of MFI in non-ETP T-ALL samples that did or did not respond to IL7 and in ETP-ALL samples.
Expression of the IL7 receptor, CD127, correlates with IL7-induced STAT5 phosphorylation. Flow cytometry analysis of surface CD127 expression. Quantitation of MFI in non-ETP T-ALL samples that did or did not respond to IL7 and in ETP-ALL samples.
Ruxolitinib abrogates IL7-induced STAT5 phosphorylation. (A) ETP-ALL xenograft samples were treated for 30 minutes with vehicle (shaded) or 500 nM ruxolitinib (unshaded), and pSTAT5 levels were assessed by flow cytometry. (B) ETP-ALL xenograft samples treated as in A were subsequently exposed to vehicle or 100 ng/mL IL7 for 20 minutes, and pSTAT levels were measured.
Ruxolitinib abrogates IL7-induced STAT5 phosphorylation. (A) ETP-ALL xenograft samples were treated for 30 minutes with vehicle (shaded) or 500 nM ruxolitinib (unshaded), and pSTAT5 levels were assessed by flow cytometry. (B) ETP-ALL xenograft samples treated as in A were subsequently exposed to vehicle or 100 ng/mL IL7 for 20 minutes, and pSTAT levels were measured.
Bcl-2 family in ETP-ALL
A subset of patients with ETP-ALL are resistant to cytotoxic chemotherapy, including corticosteroids.7 We also found ETP-ALL blasts to be corticosteroid resistant in vitro and in vivo (S.L.M., T.V., C.D.-M., M.L.H., D.T.T., unpublished data, 2013). Dysregulation of Bcl-2 family proteins is one of the most common causes of corticosteroid resistance in ALL.27,28 Accordingly, we hypothesized that Bcl-2 family proteins may be upregulated in ETP-ALL. Increased expression of the prosurvival proteins, Bcl-2 and Bcl-xL, was recently demonstrated in a syngeneic murine model of T-ALL induced by TEL-JAK2 transgene expression (EμTEL-JAK2).29 This effect was dependent on JAK2 activity and was not seen in a Notch1-driven T-ALL that did not harbor a JAK translocation. Moreover, STAT5 has been shown to be critical for Bcl-2 expression in hematopoietic cells.30,31 In our patient-derived xenograft models, we also observed increased expression of Bcl-2 in ETP-ALL compared with non-ETP T-ALL (Figure 1). Taken together, our data demonstrate that ETP-ALL showed several markers of JAK pathway activation, including increased levels of downstream targets (pSTATs and Bcl-2 family proteins) and hyperstimulation by IL7.
Ruxolitinib efficacy in xenograft models of ETP-ALL
The JAK/STAT pathway hyperactivation observed in ETP-ALL samples led us to hypothesize that pharmacologic JAK inhibition may be effective in vivo. To determine the efficacy of ruxolitinib in ETP-ALL, we performed therapeutic studies in each of the xenograft models listed in Table 1. Once xenografted mice had sufficient disease burden to detect >1% blasts in the peripheral blood, they were randomized to receive ruxolitinib or vehicle control. At this point, bone marrow and spleen are typically heavily involved with disease.21 Disease burden was assessed by peripheral blast count weekly and by splenic blast count at death.
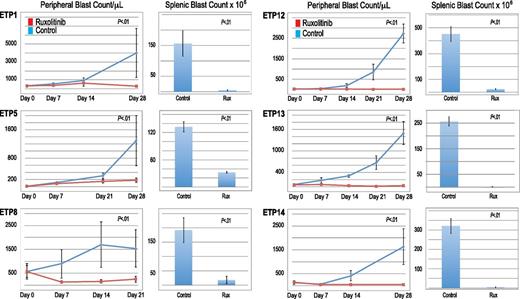
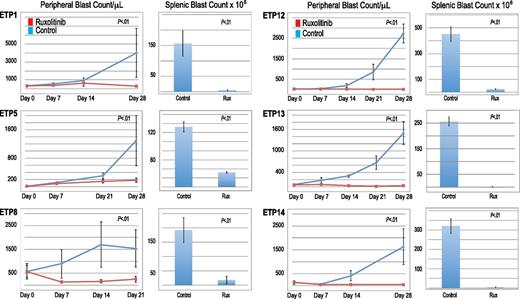
All 6 ETP-ALL xenograft models exhibited significantly decreased leukemia burden with ruxolitinib treatment compared with control mice, evidenced by lower blast counts in the blood and splenic compartments (Figure 5). Furthermore, in 5 of the 6 ETP-ALL samples, ruxolitinib decreased peripheral blast counts below pretreatment levels. Initial study of ETP5 xenografts treated with ruxolitinib demonstrated significantly decreased splenic blast counts compared with control without a significant decrease in peripheral blast counts. This experiment had a few outliers, including a control mouse whose disease spontaneously regressed. In addition, unlike the other samples, rapid progression of this leukemia caused these mice to decrease their chow consumption (and therefore, decrease drug exposure) early in the treatment course. We repeated this study, starting therapy at a lower disease burden and found a statistically significant reduction in blast count compared with control in both the blood and splenic compartments (Figure 5). However, ruxolitinib did not decrease peripheral blast count over time as it did in the other 5 ETP samples, suggesting a less robust (cytostatic) effect similar to responses often seen with other signal transduction inhibitors in xenograft models.18,21
Efficacy of ruxolitinib in xenograft models of ETP-ALL. Peripheral blood blast count over time and splenic blast count at death in ETP-ALL xenografts. Graphed are means and standard deviations with absolute blast count on the vertical axis and days of treatment on the horizontal axis.
Efficacy of ruxolitinib in xenograft models of ETP-ALL. Peripheral blood blast count over time and splenic blast count at death in ETP-ALL xenografts. Graphed are means and standard deviations with absolute blast count on the vertical axis and days of treatment on the horizontal axis.
Ruxolitinib efficacy was independent of JAK mutation status. Cases with and without identified JAK activating lesions responded to ruxolitinib treatment. In contrast, the mammalian target of rapamycin (mTOR) inhibitor rapamycin and the FLT3/Raf inhibitor sorafenib, inhibitors of other important cell growth signaling pathways, showed minimal efficacy in ETP-ALL (data not shown).
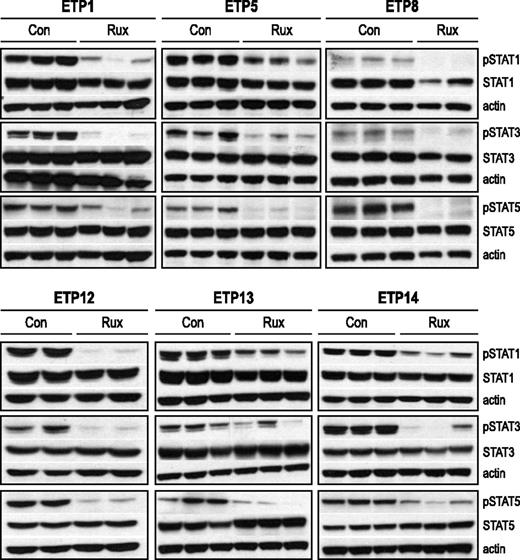
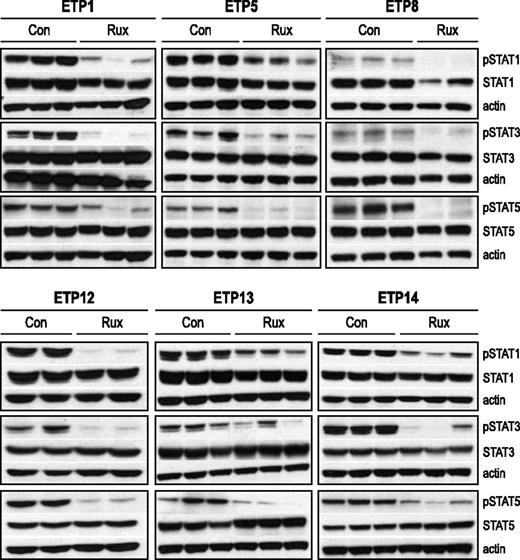
We confirmed that ruxolitinib inhibits JAK activity in vivo by assessing the phosphorylation status of STAT family members. Levels of pSTAT1, pSTAT3, and pSTAT5 by immunoblot were decreased in the spleens of ruxolitinib-treated mice relative to control mice (Figure 6). To test whether or not ruxolitinib could affect Bcl-2 expression, we also assessed Bcl-2 expression in ruxolitinib-treated mice relative to control mice and found no difference (data not shown).
Down-regulation of JAK targets with ruxolitinib treatment. Levels of phosphophorylated (p) and total STAT proteins in the spleens of ETP-ALL xenografts. Xenografts (2-3 mice per arm, replicates shown) were treated with ruxolitinib (rux) or vehicle (con) for 72 hours, spleens were harvested, and protein lysates were subjected to immunoblot. Protein levels by immunoblot of replicates were quantitated relative to actin and analyzed by the Welch-Satterthwaite t test. Levels of pSTAT1 were reduced by >50% in treated mice compared with control for all 6 samples (P < .05). There was a >50% reduction in pSTAT3, which was statistically significant (P < .05) for ETP1, 5, 8, and 14 but not significant for ETP12 and 13. There was a 70% or greater reduction in pSTAT5 for all 6 samples (P < .05).
Down-regulation of JAK targets with ruxolitinib treatment. Levels of phosphophorylated (p) and total STAT proteins in the spleens of ETP-ALL xenografts. Xenografts (2-3 mice per arm, replicates shown) were treated with ruxolitinib (rux) or vehicle (con) for 72 hours, spleens were harvested, and protein lysates were subjected to immunoblot. Protein levels by immunoblot of replicates were quantitated relative to actin and analyzed by the Welch-Satterthwaite t test. Levels of pSTAT1 were reduced by >50% in treated mice compared with control for all 6 samples (P < .05). There was a >50% reduction in pSTAT3, which was statistically significant (P < .05) for ETP1, 5, 8, and 14 but not significant for ETP12 and 13. There was a 70% or greater reduction in pSTAT5 for all 6 samples (P < .05).
Discussion
ETP-ALL accounts for 10% to 30% of T-ALL cases, is identified by a unique immunophenotype, and has been shown to portend an inferior prognosis in many studies.1,3-7 Despite their T-cell origin, very recent work suggests the genetic landscape of ETP-ALL blasts more closely resembles myeloid/early progenitor leukemia than lymphoid leukemia, with a gene expression profile similar to myeloid leukemia and stem cells and a mutational spectrum similar to myeloid leukemia, including mutations in histone-modifying proteins and in genes that regulate Ras and cytokine receptor signaling.1,6,10-12
Although survival rates for childhood T-ALL exceed 80%,2,7 data from SJCRH and older Associazione Italiana Ematologia Oncologia Pediatrica trials suggested ETP-ALL patients respond poorly to current chemotherapeutic approaches, with a 10-year overall survival of just 19%.1 More recent data from Associazione Italiana Ematologia Oncologia Pediatrica-Berlin-Frankfurt-Münster ALL 2000, UKALL 2003, and COG AALL0434 suggest ETP-ALL patients may not have worse outcome with intensified therapy.7-9 On UKALL 2003, event-free and overall survival was not significantly inferior in the ETP-ALL subgroup compared with non-ETP T-ALL. Unpublished data from the recently closed COG AALL0434 trial also demonstrate a similar overall outcome.9 Both UKALL 2003 and AALL0434 intensified therapy for patients who were slow responders by bone marrow morphology or positive for minimal residual disease (MRD) at the end of induction. Unlike the majority of patients with non-ETP T-ALL, the majority of patients with ETP-ALL were MRD positive at end induction and thus were allocated to receive more intensive therapy. Moreover, across all of these studies, there was a markedly higher rate of induction failure in ETP-ALL patients.
Most ETP-ALL treatment failures are identified very early in therapy as prednisone poor responders, induction failures, or MRD positive at the end of 2 blocks of therapy (end consolidation MRD).1,4,7,8 Consistently across studies, this group of ETP-ALL patients has a 4-year event-free survival of <50%. These chemorefractory ETP-ALL patients, as well as relapsed ETP-ALL patients, likely need alternative therapies for cure. As the biology of ETP-ALL is distinct from non-ETP T-ALL, it is not surprising that some patients with ETP-ALL likely need different therapies than other ALL subtypes. Alternative treatment strategies, including rational targeted approaches based on the underlying genomic alterations and perturbations of signaling pathways that control proliferation, differentiation, and survival, are necessary to decrease treatment failures. Accordingly, this study focused on developing agents that would improve remission induction rates.
Using patient-derived xenografts, we demonstrated that the majority of ETP-ALL cases have JAK/STAT pathway activation. In addition to the unique immunophenotype, JAK/STAT hyperactivation distinguished ETP-ALL from non-ETP T-ALL. Expression of Bcl-2 prosurvival family proteins was increased in ETP-ALL relative to T-ALL, a finding also recently observed in pediatric T-ALL and a transgenic mouse model of non-ETP T-ALL expressing the TEL-JAK2 fusion.29,32 Taken together, these findings suggest JAK/STAT pathway hyperactivation is characteristic of ETP-ALL.
Furthermore, ETP-ALL samples were hyper-responsive to IL7 stimulation compared with T-ALL, resulting in increased phosphorylation of the JAK target STAT5 that was abrogated by JAK1/2 inhibition. This result was surprising given the dogma in the field that IL7 is required for T-cell development and T-ALL survival. However, further review of the literature indicates that normal human thymocytes show a correlation between the stage of thymic differentiation and IL7 responsiveness, including an IL7-insensitive population between the β-selection and positive selection checkpoints.33,34 Moreover, we did find that some (4 of 11) non-ETP T-ALL samples were hyper-responsive to IL7 stimulation, with subsequent activation of STAT5 after ex vivo exposure to IL7. We hypothesize that the increased dependence on IL7/JAK/STAT signaling in ETP-ALL and a subset of non-ETP T-ALLs reflects the developmental state at which these leukemias arise. Consistent with this notion, IL7 responsiveness corresponds to CD127 expression. We further hypothesize that blasts from this group of non-ETP T-ALL patients will also be sensitive to JAK/STAT inhibition with ruxolitinib, as may other cases of T-ALL, such as the immature T-ALL subtype defined by gene expression profile, which makes up nearly 50% of adult T-ALL.35-39 Future work by our group is dedicated to studying the activity of ruxolitinib in non-ETP T-ALL, as well as the correlation between ex vivo IL7 response, surface CD127 expression, and sensitivity to JAK/STAT inhibition.
Recurrent alterations in JAK1, JAK3, and IL7R, as well as aberrant signaling through the JAK/STAT pathway, led us to hypothesize that the JAK/STAT pathway is a key driver of leukemogenesis and a relevant target in ETP-ALL. Using 6 patient-derived xenograft models of ETP-ALL, we demonstrate the efficacy of the JAK1/2 inhibitor ruxolitinib in all 6 models. The observation that a selective JAK1/2 inhibitor can inhibit leukemia cell growth suggests that JAK3 might be dispensable in IL7 signaling. This raises the possibility that IL7, similar to the γc chain receptor dependent cytokine IL2, might have a distinct preference for JAK1 over JAK3.40 Supporting this notion, JAK1 is required, whereas JAK3 is dispensable, in childhood T-ALL samples expressing a mutant IL-7Rα protein.41
In very recent work, ruxolitinib modestly prolonged survival in a model that phenotypically resembles ETP-ALL, in which IL7R mutants transformed murine thymocytes, inducing a leukemia with stem cell and myeloid features.42 This work provides strong evidence that IL7R mutations are leukemogenic drivers; however, IL7R mutations have been identified in <10% of ETP-ALL cases. As IL7R is known to drive JAK/STAT signaling, it is not surprising that targeting JAK/STAT was effective in that model.12,26 Clinical relevance of the statistically significant but modest effect of ruxolitinib in this model remained unclear. Our study advances these findings by demonstrating robust efficacy of ruxolitinib in patient-derived xenograft models of ETP-ALL. As our studies were designed to identify novel agents with the potential to decrease the high induction failure rate that is a hallmark of ETP-ALL, a 4-week treatment schema with multicompartment analysis of disease burden was used. Therefore, we were not able to assess survival, a potential limitation of these studies.
Importantly and unexpectedly, sensitivity to ruxolitinib was observed regardless of the presence or absence of JAK1 or JAK2 activating mutations. The universal sensitivity to ruxolitinib would not have been anticipated based on known genetic mutations in these samples. Larger studies are needed to determine whether aberrant JAK/STAT signaling and sensitivity to ruxolitinib is a universal finding or present in a subset of ETP-ALL.
The only ETP-ALL sample that did not have JAK/STAT pathway activation was ETP5. ETP5 has a mutation in PTPN11, which activates signaling through MAPK. Interestingly, recent work investigating ALL blasts in patients with Trisomy 21 found that mutations and abnormal activation of MAPK and JAK/STAT are common.43 Moreover, they demonstrated that they are mutually exclusive, eg, a majority of cases of Trisomy 21 ALL have driver mutations in one but not both pathways. We hypothesize a similar phenomenon may exist in ETP-ALL, whereby the majority of cases are dependent on signaling through JAK/STAT, but a subset are driven by abnormal signaling through MAPK. Although ruxolitinib was active in all 6 of our samples, it was the least active in ETP5. We hypothesize this patient sample may be more sensitive to MAPK inhibition and future work will test this hypothesis.
The responses observed in 5 cases, although not complete remissions, are dramatic and notable for single-agent therapy in a disease that relies on combination therapy. Our group and others have treated multiple xenograft models of ALL with dozens of different signal transduction inhibitors.18,21,22,44-46 In the vast majority of cases, active agents have a cytostatic effect when given as monotherapy. Notable exceptions to this include the use of tyrosine kinase inhibitors, including imatinib and dasatinib, in Ph chromosome-positive B-ALL (Ph+ ALL, expressing the BCR-ABL1 translocation) and Ph-like B-ALL with other ABL1 translocations, such as NUP214-ABL1, and the use of ruxolitinib in Ph-like B-ALL with JAK2 translocations.17,18,47,48 In these examples, the leukemias were addicted to the targeted signaling pathway. The degree of response to ruxolitinib in our ETP-ALL xenograft models strongly suggests that ETP-ALL blasts are addicted to signaling through JAK/STAT. To our knowledge, this is the first demonstration of the activity of any agent in a preclinical model of ETP-ALL using patient-derived material. This work clearly demonstrates that ruxolitinib shows promise as an active targeted agent in ETP-ALL.
Despite these impressive results, it is unlikely that monotherapy with ruxolinitib will be curative in patients with ETP-ALL. Unlike patients with chronic myeloid leukemia who can obtain complete and durable remissions with single agent tyrosine kinase inhibitors,49-51 patients with Ph+ ALL have transient responses to these drugs.52,53 Nevertheless, recent studies have shown that a combination of imatinib and conventional cytotoxic agents markedly improves event-free survival and overall survival in children with Ph+ ALL.54,55 We hypothesize that a combination of ruxolitinib or other JAK kinase inhibitors with conventional chemotherapy may have similar effects in ETP-ALL.
Imatinib established the paradigm for targeted therapy by dramatically improving overall survival for Ph+ ALL in combination with standard chemotherapy.54,55 Although case reports show the benefit of kinase inhibitors in select cases with fusions resulting in constitutive kinase activation,56-58 this model has yet to be extended in a broad fashion in non-Ph+ ALL, largely due to the lack of unifying genetic alterations. Our studies suggest that kinase-specific genetic lesions may not be necessary for JAK/STAT pathway hyperactivation or response to JAK inhibition, but rather identification of driver pathways may point to effective targeted therapies. Ruxolitinib was recently evaluated in a phase 1 clinical trial for children with relapsed/refractory malignancies, including ALL, and was well tolerated (#NCT01164163).59 This trial was not focused on ETP-ALL, but future trials using this drug in combination with chemotherapy are in development with ETP-focused strata. Our data suggest that ETP-ALL is sensitive to JAK/STAT inhibition, and a majority may be addicted to the pathway, suggesting a role for kinase inhibitors in this subtype of T-ALL for which current cytotoxic chemotherapy falls short. This provides strong preclinical rationale for this important novel observation to be translated to the clinic quickly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory of the University of Pennsylvania Perelman School of Medicine.
This work was supported by National Institutes of Health, National Cancer Institute grants CA102646 and CA1116660 (to S.A.G.), R56A1099301 (to D.T.T.), and K08CA184418 (to S.K.T.), the Leukemia and Lymphoma Society, Patient Impact Initiative, Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award (to D.T.T.), American Cancer Society Research Scholar grant RSG-14-022-01-CDD (to D.T.T.) and research grant RSG0507101 (to S.A.G.), the Weinberg Fund of the Children’s Hospital of Philadelphia (to S.A.G.), the St. Baldrick’s Foundation (to S.L.M., C.G.M., and M.L.H.), the When Everyone Survives Foundation (to S.L.M.), the Campani Foundation and the Pepp Family Foundation (to M.L.H. and C.D.-M.), the Alex’s Lemonade Stand Foundation and the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania Transdisciplinary Program in Translational Medicine and Therapeutics (to S.K.T.), and grants to the Children’s Oncology Group including U10 CA98543 (Chair's grant and supplement to support the COG High Risk ALL TARGET project), U10 CA98413 (Statistical Center), U24 CA114766 (Specimen Banking), and UL1RR024134 (to S.K.T.). S.D. is supported by a grant from the Australian National Health and Medical Research Council. R.B.L. is supported by a Fellowship from the Australian National Health and Medical Research Council. S.L.M. and C.G.M. are St. Baldrick’s Scholars. S.P.H. is the Ergen Family Chair in Pediatrics. S.K.T. is an Alex’s Lemonade Stand Foundation Scholar in Developmental Therapeutics. Children’s Cancer Institute is affiliated with the University of New South Wales and The Sydney Children’s Hospitals Network.
Authorship
Contribution: S.L.M., S.D., C.D.-M., and T.V. designed the study, analyzed data, and wrote the manuscript; A.R., A.S., T.R., and J.H. contributed to data acquisition and manuscript editing; and A.C.W., S.K.T., S.P.H., M.L.L., C.G.M., B.L.W., M.L.H., S.A.G., R.B.L., and D.T.T. contributed to study design and manuscript editing.
Conflict-of-interest disclosure: S.L.M. and M.L.L. served on an Incyte Corporation Ruxolitinib Advisory Board. The remaining authors declare no competing financial interests.
Correspondence: David T. Teachey, 3008 Colket Translational Research Bldg, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: teacheyd@email.chop.edu.
References
Author notes
S.L.M., S.D., C.D.-M., T.V., R.B.L., and D.T.T. contributed equally to this work.