Key Points
Meis1 is required for the maintenance of MLL-fusion gene leukemia; HLF is a key downstream mediator of Meis1.
Meis1 and HLF restrict oxidative stress; induction of oxidative phosphorylation may be therapeutic in leukemia.
Abstract
Leukemias with MLL translocations are often found in infants and are associated with poor outcomes. The pathogenesis of MLL-fusion leukemias has been linked to upregulation of HOX/MEIS1 genes. The functions of the Hox/Meis1 complex in leukemia, however, remain elusive. Here, we used inducible Meis1-knockout mice coupled with MLL-AF9 knockin mice to decipher the mechanistic role of Meis1 in established MLL leukemia. We demonstrate that Meis1 is essential for maintenance of established leukemia. In addition, in both the murine model and human leukemia cells, we found that Meis1 loss led to increased oxidative stress, oxygen flux, and apoptosis. Gene expression and chromatin immunoprecipitation studies revealed hepatic leukemia factor (HLF) as a target gene of Meis1. Hypoxia or HLF expression reversed the oxidative stress, rescuing leukemia development in Meis1-deficient cells. Thus, the leukemia-promoting properties of Meis1 are at least partly mediated by a low-oxidative state, aided by HLF. These results suggest that stimulants of oxidative metabolism could have therapeutic potential in leukemia treatment.
Introduction
Reciprocal translocations of the 11q23 locus lead to acute leukemias of both myeloid and lymphoid lineages. These leukemias are usually resistant to conventional chemotherapies. The translocation generates an oncogenic fusion protein comprised of an amino terminus derived from MLL (now called KMT2A), the gene at 11q23, fused to a carboxy terminus derived from one of several different genes. Recent studies have revealed that these MLL-fusion proteins then join higher-order protein complexes containing various transcriptional activators and/or elongating factors such as DOT1L, PAFc, and p-TEFB.1,2 Recruitment of these complexes to genomic loci was shown to be associated with chromatin modifications and subsequent changes in gene expression.3 Regardless of the fusion partner involved in the translocation and of the consequential protein complex assembled, the downstream genes activated by the various MLL-fusion proteins are largely identical. Data from patient-derived leukemia cells and from experimental models reveal that MLL-fusion proteins upregulate the expression of posterior HOX-A genes and of the HOX cofactor MEIS1.4-6 Further, retroviral overexpression of a Hox gene (with few exceptions) along with Meis1 in murine hematopoietic cells induces an aggressive leukemia in transplanted mice.7,8 Thus, MLL-fusion protein induced upregulation of HOX and MEIS1 genes is considered central to the pathology of these leukemias.
Studies to test the requirement of HOX-A and MEIS1 genes in MLL-fusion leukemias have yielded mixed results. Although shRNA knockdown of HOXA9 was shown to inhibit leukemia, mice lacking Hoxa9 protein developed leukemia induced by MLL-fusion proteins at the same rate and frequency as mice bearing normal Hoxa9.9,10 These discrepancies could be caused by technical differences in the experimental models. Recent studies using larger patient cohorts, however, show that some MLL-fusion gene leukemias lack expression of all the HOX genes.11,12 On the other hand, MEIS1 is universally expressed in all MLL-fusion leukemias, including those lacking HOX expression. Mice lacking Meis1 are embryonically lethal, and Meis1−/− fetal liver cells were shown to be resistant to transformation by MLL-fusion proteins.13 This suggests that Meis1 is essential for the initiation of leukemia. To be considered a therapeutic target, however, an essential role for MEIS1 in the maintenance of established leukemia needs to be rigorously demonstrated. The mechanisms by which MEIS1 facilitates MLL-fusion–induced leukemia are also unknown. Several previous studies aimed at determining the oncogenic mechanisms of Meis1 have been carried out, using retroviral expression of Hoxa9 and Meis1 or NUP98-HOXD13 and Meis1 in murine hematopoietic cells.7,13-16 The results suggest that the Meis1/Hox complex induced leukemic transformation via intermediates such as Flt3, Trib2, and Ccl3. However, the cellular context in which MEIS1 is found naturally overexpressed (eg, MLL-fusion–induced leukemia) is quite different from that of these retroviral models. We recently reported on the role of Meis1 in normal hematopoietic stem cell function using inducible Meis1-knockout mice.17 In the current study, we used these mice to investigate the role of Meis1 in leukemia. We found that Meis1 was critical for the maintenance of established leukemia and identified HLF as a downstream mediator of Meis1. More importantly, our studies exposed a vulnerability in leukemia to oxidative stress that has the potential for novel therapy.
Materials and methods
Additional methods are included in supplemental Methods, found on the Blood website.
Cell lines
Leukemia cell lines were cultured in Iscove’s modified Dulbecco medium (GE Healthcare) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, and 100 mg/mL streptomycin. For mouse leukemic cells, 10 ng/mL of stem cell factor, IL-3, granulocyte macrophage colony-stimulating factor, and IL-6 were added to the medium (PeproTech). 293T cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% FCS. OP9 stromal cells were maintained in α-Minimum Essential medium supplemented with 20% FCS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (Sigma). For coculture studies, OP9 cells were seeded overnight and puromycin-selected cells were added the next day.
Patient samples
Deidentified patient samples were obtained under an institutional review board–approved protocol at Cincinnati Children’s Hospital Medical Center.
Animal studies
Transgenic Meis1f/f/CreER mice were generated by crossing Meis1f/f mice with Rosa26CreER mice.17 Meis1f/f/CreER mice were crossed with MLL-AF9+/− mice. All mice used in this study were 6 to 8 weeks of age. All animal experiments used were approved by the Institutional Animal Care and Use Committee (IACUC). For transplantation experiments, BoyJ mice were lethally irradiated and injected IV with as much as 106 CD45.2+ leukemic cells mixed with 2.5 × 104 CD45.1+ mononuclear cells. Transplanted mice were treated with tamoxifen (Sigma) (4 mg intraperitoneal [IP]) starting at 2 weeks posttransplantation and repeated at 30-day intervals, to induce Meis1-deletion. For drug studies, 100 mg/kg per day of N-acetyl cysteine amide (NACA) (Sigma) was administered by IP injection 3 times per week, starting at 14 days posttransplantation. All animal studies were approved by the IACUC. The RNA-sequencing data are available in the GEO database under accession #GSE58732.
Results
Meis1 is essential for maintenance of MLL-fusion–induced transformation
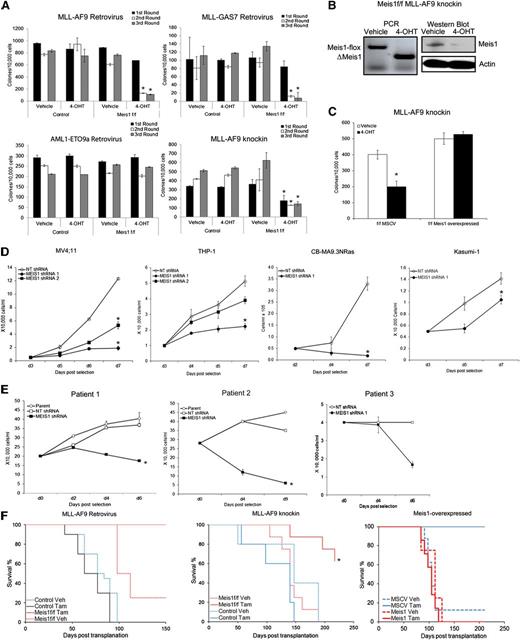
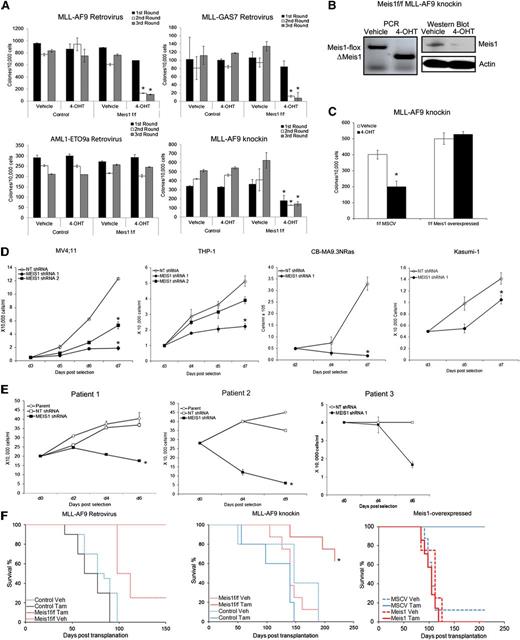
In considering Meis1-targeting as a treatment strategy for leukemia, we first need to explicitly demonstrate a role for Meis1 in established leukemia. We thus studied the role of Meis1 in different experimental models: (1) Retroviral MLL-fusion murine leukemia, (2) MLL-AF9 knockin murine leukemia, (3) retroviral MLL-fusion human leukemia, and (4) primary patient-derived leukemia cells. Our recently described experimental model system allows us to genetically delete Meis1 in leukemic cells.16 We thus transduced lineage-negative (Lin–) bone marrow (BM) cells of inducible knockout (Meis1f/f/CreER) and control (Meis1+/+/CreER) mice with retroviruses expressing an MLL-fusion oncoprotein (MLL-AF9 or MLL-GAS7) or AML1-ETO9a, the latter being an isoform of the AML1-ETO–fusion oncogene. We chose AML1-ETO as a control because leukemias associated with this oncogene exhibit lower Meis1 levels.5 Meis1 expression in MLL-AF9 cells was ∼300-fold higher than that in AML-ETO9a (data not shown). Oncogene-transformed cells were cultured in methylcellulose with either vehicle (ethanol) or 4-OHT, the latter inducing Meis1 deletion (in Meis1f/f/CreER). Serial replating demonstrated that Meis1 deletion significantly reduced MLL-AF9 colonies (Figure 1A). MLL-GAS7 is a relatively weak oncogene compared with MLL-AF9, resulting in fewer colonies.18 Nonetheless, MLL-GAS7 colonies were also significantly reduced upon Meis1 deletion. However, AML1-ETO9a–induced colony replating was unaffected by Meis1 deletion, demonstrating the relative specificity for Meis1 dependence (Figure 1A).
Loss of Meis1 impedes propagation of MLL-fusion gene leukemia. Retroviral and knockin models of leukemia were generated as described. (A) Colony-replating assays were performed in methylcellulose media supplemented with 4-OHT or vehicle. Bar graphs represent colony numbers from 1 representative experiment for each leukemia model. Data represent mean ± standard error of the mean (SEM) of triplicates. *P < .01, Student t test. (B) Genomic PCR and Western blot demonstrating Meis1 depletion by 4-OHT treatment in Meis1f/f MLL-AF9 knockin cells. (C) Meis1f/f MLL-AF9 cells were transduced with control (MSCV) or Meis1 retrovirus. The bar graph represents colony numbers formed by transduced cells cultured in the presence of vehicle or 4-OHT. Data are mean ± SEM for 3 independent experiments. (D) MV4;11, THP-1, CBMA9.3NRas, and Kasumi-1 cells were transduced with lentivirus carrying shRNA against Meis1 or nontargeting (NT) control. Transduced cells were cultured in liquid medium. Cell growth was assessed by counting viable cells, at designated days post-puromycin selection. (E) Leukemic blasts from patients were transduced with MEIS1- or NT-shRNA. Line graphs depict growth curves of 3 different patient samples. (F) Control or Meis1f/f MLL-AF9–transformed cells (retroviral and knockin) were transplanted into irradiated mice. Animals were treated with tamoxifen or vehicle. Graphs depict leukemia-free survival of the various groups in the retroviral (left) and knockin (middle) or Meis1-overexpressed knockin (right) models. n = 5 to 10 mice per group. *P < .01, log-rank test.
Loss of Meis1 impedes propagation of MLL-fusion gene leukemia. Retroviral and knockin models of leukemia were generated as described. (A) Colony-replating assays were performed in methylcellulose media supplemented with 4-OHT or vehicle. Bar graphs represent colony numbers from 1 representative experiment for each leukemia model. Data represent mean ± standard error of the mean (SEM) of triplicates. *P < .01, Student t test. (B) Genomic PCR and Western blot demonstrating Meis1 depletion by 4-OHT treatment in Meis1f/f MLL-AF9 knockin cells. (C) Meis1f/f MLL-AF9 cells were transduced with control (MSCV) or Meis1 retrovirus. The bar graph represents colony numbers formed by transduced cells cultured in the presence of vehicle or 4-OHT. Data are mean ± SEM for 3 independent experiments. (D) MV4;11, THP-1, CBMA9.3NRas, and Kasumi-1 cells were transduced with lentivirus carrying shRNA against Meis1 or nontargeting (NT) control. Transduced cells were cultured in liquid medium. Cell growth was assessed by counting viable cells, at designated days post-puromycin selection. (E) Leukemic blasts from patients were transduced with MEIS1- or NT-shRNA. Line graphs depict growth curves of 3 different patient samples. (F) Control or Meis1f/f MLL-AF9–transformed cells (retroviral and knockin) were transplanted into irradiated mice. Animals were treated with tamoxifen or vehicle. Graphs depict leukemia-free survival of the various groups in the retroviral (left) and knockin (middle) or Meis1-overexpressed knockin (right) models. n = 5 to 10 mice per group. *P < .01, log-rank test.
To ensure that the aforementioned observations were not limited to retroviral transformation, we crossed the Meis1-inducible knockout mice with MLL-AF9 knockin mice to yield MLL-AF9+/−/Meis1f/f/CreER mice. The knockin model has the distinct advantage of containing a single copy of the oncogene in every cell, expressed from the endogenous promoter and thus more physiologic levels, as opposed to the retroviral models, where the oncogene is expressed at much higher levels.19 For the remainder of the text, the MLL-AF9+/−/Meis1f/f/CreER mice will be termed as Meis1f/f, with controls being either MLL-AF9+/−/CreER or MLL-AF9+/− mice. The genotypes of the mice were confirmed by polymerase chain reaction (PCR) analysis of genomic tail DNA. We isolated BM cells from morbid (control and Meis1f/f) mice and analyzed the effects of Meis1 deletion. As shown in Figure 1A, 4-OHT had no effect on serial colony formation of control MLL-AF9 knockin leukemia cells, whereas Meis1f/f cells showed significantly fewer colonies. Meis1 deletion in 4-OHT–treated cells was confirmed by PCR and Western blot (Figure 1B). Retrovirally delivered exogenous Meis1 preserved the colony formation even upon endogenous Meis1 deletion, demonstrating that the aforementioned effects were specifically attributable to Meis1 (Figure 1C). Collectively, these findings demonstrate that Meis1 is required for maintenance of the MLL-AF9–induced transformation.
We next examined MEIS1 dependency in human leukemia cell lines and patient-derived leukemic cells. MEIS1 knockdown by lentiviral-shRNA significantly inhibited the growth of MV4;11 and THP-1 cells (Figure 1D and supplemental Figures 1 and 2). Similarly, leukemia generated by transduction of CD34+ cord blood cells with MLL-AF9 and oncogenic-NRAS (CBMA9.3 NRas) showed significant growth inhibition upon MEIS1 knockdown (Figure 1D). Conversely, the AML1-ETO–bearing cell line Kasumi-1 (with MEIS1 levels ∼50-fold less than those in MV4;11, data not shown) was less affected (Figure 1D). Finally, we evaluated leukemic blasts from 3 patients with acute leukemia bearing MLL translocations (supplemental Table 1). Leukemic blasts transduced with MEIS1-shRNA failed to survive in stromal coculture systems. In contrast, the parent cells or control shRNA transduced blasts expanded nearly twofold (Figure 1E).
We next sought to determine the cellular mechanisms of antileukemic activity upon Meis1 loss. As shown in supplemental Figure 3A, Meis1 depletion in both the retroviral and knockin Meis1f/f-leukemic cells led to a significant increase (six- to sevenfold) in apoptosis, compared with control leukemic cells. Similarly, MEIS1 knockdown induced apoptosis in MV4;11 cells (supplemental Figure 3B). However, Meis1 deletion had no effect on cell cycling (data not shown).
Meis1 is essential for propagation of MLL-fusion leukemia in vivo
To determine the requirement of Meis1 for leukemia propagation in vivo, we transplanted Meis1f/f or control leukemic cells into syngeneic mice. Recipient animals were treated with tamoxifen or vehicle. Mice were killed when displaying signs of distress and classified as leukemic when necropsy revealed splenomegaly, leukocytosis, and infiltration of leukemic cells in organs (ie, liver, spleen, BM). As expected, the majority of the control mice in both the retroviral and knockin models succumbed to leukemia by 100 and 150 days posttransplantation, respectively. On the other hand, Meis1 deletion led to significantly improved survival, with no evidence of leukemia in the retroviral model (Figure 1F, left panel). Few tamoxifen-treated recipients of knockin cells later did develop leukemia, associated with incomplete deletion or reemergence of intact Meis1 (not shown). However, the leukemia-free survival of this group was still significantly prolonged (Figure 1F, middle panel; P < .05). Recipients of Meis1-overexpressing Meis1f/f knockin cells developed leukemia even upon deletion of endogenous Meis1, with a time-course similar to that of controls (Figure 1F, right panel). Sick animals had significantly increased white blood cell counts and donor chimerism (supplemental Figure 4A-C). In contrast, the controls (recipients of murine stem cell virus (MSCV)-transduced cells treated with tamoxifen) displayed normal white blood cell counts and lower donor chimerism at day 100 posttransplantation. Collectively, these data demonstrate that Meis1 is required for the propagation and maintenance of MLL-fusion gene–induced leukemia in vivo.
Meis1 regulates reactive oxygen species and oxygen flux in leukemia
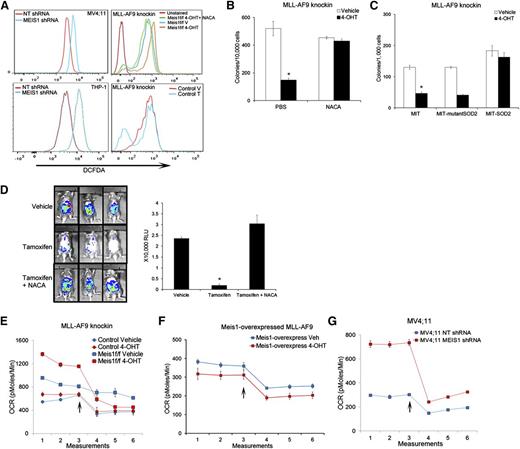
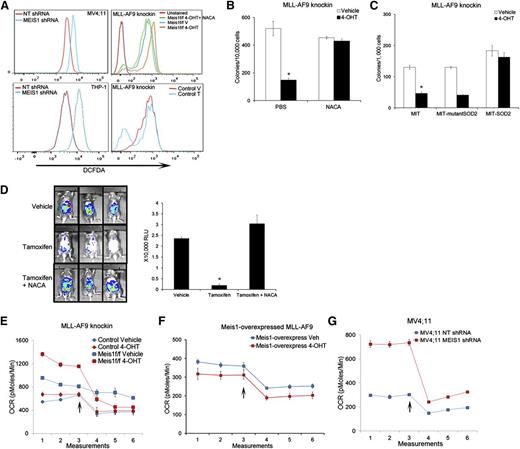
We and others previously reported that Meis1 regulates oxidative stress in hematopoietic stem cells.17,20 As shown in Figure 2A and supplemental Figure 5A, Meis1 loss led to a significant increase in intracellular reactive oxygen species (ROS) in both human and murine leukemia cells. Control MLL-AF9 cells showed no change in ROS levels upon 4-OHT treatment, indicating that this effect was specific to Meis1-loss (Figure 2A). Importantly, treatment with the ROS-quencher NACA reduced ROS levels, and restored the clonogenicity of Meis1-deleted murine cells and, partially, the growth of MV4;11 cells (Figure 2A-B and supplemental Figure 5B). To validate the ROS-restriction role of Meis1 in leukemia, we transduced Meis1f/f cells with SOD2, an enzyme that catalyzes the breakdown of ROS. Meis1 deletion in vector-transduced cells (MIT) generated fewer colonies than controls, whereas SOD2-overexpressing cells maintained colony formation (Figure 2C). An enzymatically silent version of SOD2 (Q143A, MIT-mutant SOD2) failed to rescue the Meis1-deletion phenotype.21 To ascertain that this effect was prevalent in vivo as well, we first generated traceable leukemia cells by transducing knockin MLL-AF9 cells with a retrovirus expressing firefly luciferase. This allows assessment of in vivo leukemia burden in real time by bioluminescent imaging. As shown in Figure 2D, transplant recipients treated with vehicle showed abundant bioluminescent signal, whereas tamoxifen treatment significantly reduced the leukemia burden. Animals treated with NACA and tamoxifen retained leukemia, with the bioluminescence similar to that in control mice (Figure 2D). Collectively, these results demonstrate that Meis1 critically limits oxidative stress in leukemia.
Meis1 regulates oxidative stress and oxygen flux in leukemia. (A) Representative histograms of intracellular ROS (DCFDA) in MV4;11 and THP-1 cells transduced with MEIS1-shRNA (left column). The right panels display ROS levels in Meis1f/f MLL-AF9 knockin or control leukemic cells treated with vehicle (V), 4-OHT, or a combination of 4-OHT and N-acetyl cysteine amide (NACA). (B) Bar graph depicting results of colony assay with Meis1f/f MLL-AF9 cells in the presence of vehicle or 4-OHT, with or without the addition of NACA to the media. Data are mean ± SEM *P < .01, Student t test. (C) Meis1f/f MLL-AF9 cells were transduced with retrovirus carrying SOD2, a nonfunctioning mutant of SOD2, or control retrovirus (MIT). The bar graph depicts colony numbers formed by transduced cells in each condition in the presence of vehicle or 4-OHT. (D) Mice were transplanted with Meis1f/f MLL-AF9 leukemic cells expressing firefly luciferase. Recipients were treated with vehicle, tamoxifen, or tamoxifen + NACA as described. Leukemia burden was analyzed by live-animal imaging. Representative images of mice in each group are shown. The bar graph on the right depicts measured bioluminescence in each group (n = 5 mice per group). (E-G) Oxygen consumption rates (OCR) were measured using the Seahorse XF analyzer. Representative graphs depict OCR measured at 5 minute intervals in control and Meis1f/f MLL-AF9 knockin, Meis1f/f with Meis1-overexpressed, and MV4;11 cells. After 3 measurements at baseline, the ATP-synthase inhibitor oligomycin was added to the wells (arrow) and measurements were repeated. All data points are mean ± SEM of triplicates. n = 3 independent experiments.
Meis1 regulates oxidative stress and oxygen flux in leukemia. (A) Representative histograms of intracellular ROS (DCFDA) in MV4;11 and THP-1 cells transduced with MEIS1-shRNA (left column). The right panels display ROS levels in Meis1f/f MLL-AF9 knockin or control leukemic cells treated with vehicle (V), 4-OHT, or a combination of 4-OHT and N-acetyl cysteine amide (NACA). (B) Bar graph depicting results of colony assay with Meis1f/f MLL-AF9 cells in the presence of vehicle or 4-OHT, with or without the addition of NACA to the media. Data are mean ± SEM *P < .01, Student t test. (C) Meis1f/f MLL-AF9 cells were transduced with retrovirus carrying SOD2, a nonfunctioning mutant of SOD2, or control retrovirus (MIT). The bar graph depicts colony numbers formed by transduced cells in each condition in the presence of vehicle or 4-OHT. (D) Mice were transplanted with Meis1f/f MLL-AF9 leukemic cells expressing firefly luciferase. Recipients were treated with vehicle, tamoxifen, or tamoxifen + NACA as described. Leukemia burden was analyzed by live-animal imaging. Representative images of mice in each group are shown. The bar graph on the right depicts measured bioluminescence in each group (n = 5 mice per group). (E-G) Oxygen consumption rates (OCR) were measured using the Seahorse XF analyzer. Representative graphs depict OCR measured at 5 minute intervals in control and Meis1f/f MLL-AF9 knockin, Meis1f/f with Meis1-overexpressed, and MV4;11 cells. After 3 measurements at baseline, the ATP-synthase inhibitor oligomycin was added to the wells (arrow) and measurements were repeated. All data points are mean ± SEM of triplicates. n = 3 independent experiments.
We next sought to determine the source of increased ROS upon Meis1 depletion. Oxidative phosphorylation (OP) in the mitochondria generates ROS during transfer of electrons along the electron transport chain (ETC). Increased flux through the Krebs’s cycle and subsequent ETC would thus lead to increased oxygen consumption, generating more ROS. As shown in Figure 2E and G, Meis1 loss in murine and human leukemic cells led to a significant increase in oxygen consumption rate (OCR). Importantly, re-expression of Meis1 in the Meis1f/f cells blocked this increase, whereas 4-OHT treatment of control cells had no effect on OCR (Figure 2E-F). Overall, these data suggest that increased oxidative metabolism and subsequent ROS generation are at least partly responsible for the growth inhibition caused by Meis1 loss.
Maintenance of hypoxic-state is one key function of Meis1 in leukemia
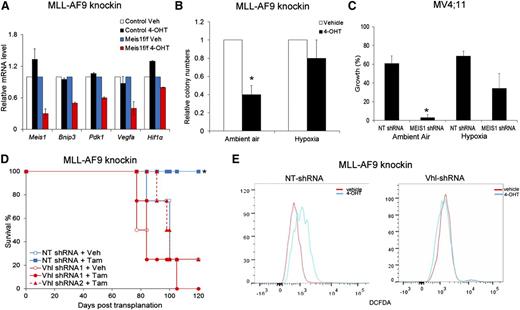
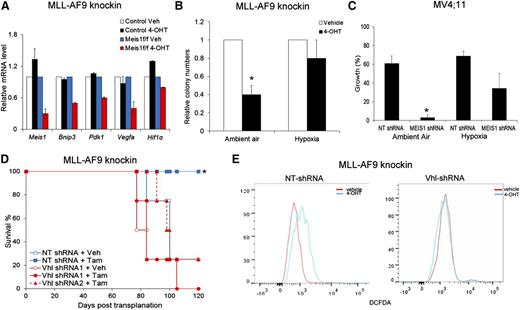
To identify the downstream mediators of Meis1 in leukemia, we compared the gene expression profiles of Meis1-deleted and control cells. Using RNA sequencing, we compared knockin Meis1f/f and control cells, treated with vehicle or 4-OHT. Meis1 depletion resulted in downregulation of ∼500 genes. A comparative analysis of gene sets in the Molecular Signatures Database revealed significant overlap of the Meis1-dependent genes with those associated with hypoxia response (false discovery rate <.05). Quantitative real-time PCR confirmed reduction of Bnip3, Pdk1, and Vegf-a upon Meis1 depletion, whereas these genes were unchanged by 4-OHT treatment of control cells (Figure 3A). MEIS1 knockdown in MV4;11 and THP-1 cells also led to significant downregulation of PDK1 and VEGF-A (data not shown). These genes are targets of Hif-1 and Hif-2, and Meis1 has been suggested as a transcriptional regulator of Hif-1α.20 However, protein/mRNA levels of both Hif-1α and Hif-2α were unchanged upon Meis1 depletion in both the murine or human leukemias (data not shown).
Hypoxic states reverse the effects of Meis1 depletion. (A) Quantitative real-time PCR analysis of a subset of Hif1α target genes in control and Meis1f/f MLL-AF9 cells treated with vehicle (Veh) or 4-OHT. The bar graph displays mean ± SEM. (B) Bar graph depicting colony numbers formed by Meis1f/f MLL-AF9 knockin cells under ambient air or hypoxia, treated with vehicle or 4-OHT. (C) MV4;11 cells transduced with control (NT) or MEIS1-shRNA were cultured in the presence of ambient air or hypoxia (3% oxygen). The bar graph depicts percentage (%) increase in growth over 72 hours for each condition. Data represent mean ± SEM of 2 independent experiments. *P < .01. (D) Meis1f/f MLL-AF9 leukemic cells were transduced with lentivirus-bearing control (NT) or Vhl-shRNA. Transduced cells were transplanted into irradiated mice and recipient animals treated with tamoxifen or vehicle as described. The graph depicts leukemia-free survival of the various groups of mice. *P < .01, log-rank test, n = 5 per group. (E) Fluorescence-activated cell sorting analysis of intracellular ROS (staining) in Meis1f/f MLL-AF9 knockin cells transduced with control (NT) or Vhl-shRNA and treated with vehicle or 4-OHT. Results of 1 representative experiment are shown (n = 3).
Hypoxic states reverse the effects of Meis1 depletion. (A) Quantitative real-time PCR analysis of a subset of Hif1α target genes in control and Meis1f/f MLL-AF9 cells treated with vehicle (Veh) or 4-OHT. The bar graph displays mean ± SEM. (B) Bar graph depicting colony numbers formed by Meis1f/f MLL-AF9 knockin cells under ambient air or hypoxia, treated with vehicle or 4-OHT. (C) MV4;11 cells transduced with control (NT) or MEIS1-shRNA were cultured in the presence of ambient air or hypoxia (3% oxygen). The bar graph depicts percentage (%) increase in growth over 72 hours for each condition. Data represent mean ± SEM of 2 independent experiments. *P < .01. (D) Meis1f/f MLL-AF9 leukemic cells were transduced with lentivirus-bearing control (NT) or Vhl-shRNA. Transduced cells were transplanted into irradiated mice and recipient animals treated with tamoxifen or vehicle as described. The graph depicts leukemia-free survival of the various groups of mice. *P < .01, log-rank test, n = 5 per group. (E) Fluorescence-activated cell sorting analysis of intracellular ROS (staining) in Meis1f/f MLL-AF9 knockin cells transduced with control (NT) or Vhl-shRNA and treated with vehicle or 4-OHT. Results of 1 representative experiment are shown (n = 3).
The concurrent downregulation of hypoxia-response genes, increased oxygen flux, and ROS suggest that these effects might be causally linked. Accordingly, we hypothesized that a forced state of hypoxia would reverse these effects and rescue the leukemic phenotype of Meis1-deleted cells. When cultured in 3% oxygen, MLL-AF9 knockin cells formed the same number of colonies under control and Meis1-depleted states, whereas simultaneous culture of the same cells in ambient air showed fewer colonies upon Meis1 depletion (Figure 3B). Similarly, MV4;11 cells with MEIS1 knockdown showed less growth inhibition when cultured under hypoxia compared with the same cells cultured in ambient air (Figure 3C).
To further investigate a role for the hypoxia-response machinery in Meis1-dependent leukemia, we performed shRNA knockdown of Vhl—the principal degrader of Hif-1 and Hif-2, or overexpressed Hif-1α in the Meis1f/f cells. Both Vhl-knockdown and Hif-1α overexpression prevented colony depletion upon Meis1 deletion (supplemental Figure 6A-C). Further, in vivo leukemogenesis was preserved upon Meis1 deletion in recipients of Vhl-knockdown or Hif-1α overexpressing cells, whereas the controls displayed the expected prolonged survival (Figure 3D and supplemental Figure 6D). Finally, Vhl-knockdown prevented ROS accumulation upon Meis1 deletion, whereas control shRNA–transduced cells showed the expected increase in ROS (Figure 3E). These results demonstrate that an activated hypoxia response can substitute for Meis1 in MLL-AF9 leukemia. Collectively, one critical function of Meis1 in these leukemias is to regulate oxidative processes, thereby preventing oxidative stress.
HLF is a downstream mediator of Meis1 in MLL leukemia
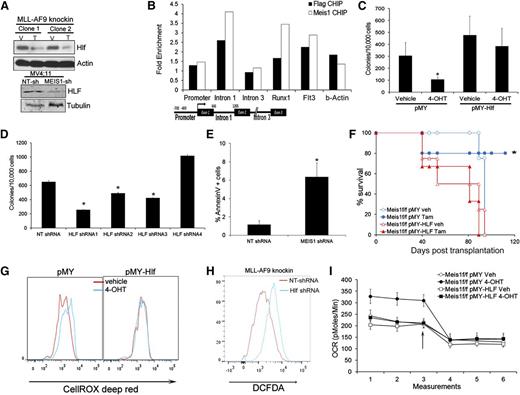
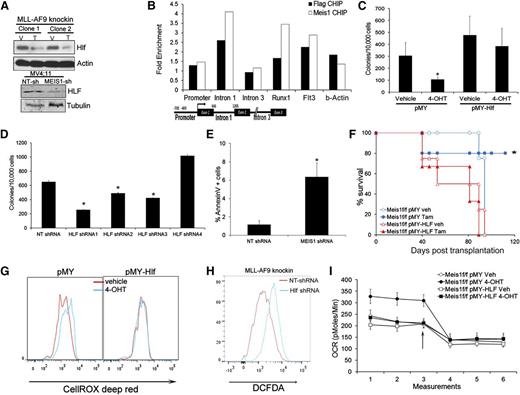
The gene expression data showed that Hlf (hepatic leukemia factor) was downregulated upon Meis1 deletion. This gene is involved in t(17:19) acute lymphoblastic leukemia, a translocation that generates the E2A-HLF fusion.22 Recent studies indicate HLF as a key regulator of hematopoietic stem cell self-renewal.23 Quantitative real-time PCR and Western blots confirmed the dependence of HLF on Meis1 (Figure 4A and supplemental Figure 7A). In addition, MEIS1 knockdown in MV4;11 cells also reduced HLF levels (Figure 4A and supplemental Figure 7A). Previous studies showed upregulation of Hlf in Meis1/NUP98-HOXD13–induced leukemia. To determine whether Hlf is directly regulated by Meis1, we performed chromatin immunoprecipitation (ChIP) in Meisf/f MLL-AF9 knockin cells expressing retroviral Meis1 or FLAG-Meis1. Anti-FLAG or anti-Meis1 ChIP showed enrichment of the first intron of Hlf in the precipitates compared with controls, but not of the promoter or distal intronic regions (Figure 4B). As positive controls, we detected enrichment of the previously reported regulatory regions (Runx1 and Flt3) in both the FLAG and Meis1 ChIPs.24
Meis1 regulates oxygen flux and ROS via HLF. (A) Western blots showing levels of HLF in control and Meis1-depleted MLL-AF9 knockin, and MV4;11 cells. (B) Meis1f/f MLL-AF9 knockin cells were transduced with Meis1 or FLAG-Meis1 and ChIP performed using anti-Meis1 or anti-FLAG antibodies or control IgG. The bar graph depicts fold enrichment of the various regions of the Hlf locus relative to IgG. Previously described genomic regions related to Runx1 and Flt3 were used as positive controls. A schematic of the Hlf locus with location of PCR assays is depicted. (C) Meis1f/f MLL-AF9 knockin cells were transduced with retrovirus-expressing HLF (pMY-HLF) or control (pMY). The bar graph depicts colonies formed by transduced cells cultured in the presence of vehicle or 4-OHT. *P < .01. (D) Bar graph depicting colonies formed by MLL-AF9 knockin cells transduced with NT or 4 different Hlf-shRNAs. Data are mean ± SEM. (E) Bar graph depicting proportions of Annexin V+ cells in MLL-AF9 knockin cells transduced with NT or Hlf-shRNA. (F) Survival curves of mice transplanted with Meis1f/f MLL-AF9 knockin leukemic cells transduced with HLF or control (pMY) retrovirus. Recipients were treated with vehicle or tamoxifen. *P < .01, log-rank test. n = 4 to 5 mice per group. (G) Histograms depicting intracellular ROS in Meis1f/f MLL-AF9 knockin cells transduced with pMY-HLF or control (pMY), treated with vehicle or 4-OHT. (H) Histogram depicting intracellular ROS in MLL-AF9 knockin cells transduced with NT or Hlf-shRNA. (I) Graph depicting OCR in Meis1f/f MLL-AF9 knockin cells transduced with HLF or control retrovirus and treated with vehicle (Veh) or 4-OHT. The arrow indicates the point at which oligomycin was added.
Meis1 regulates oxygen flux and ROS via HLF. (A) Western blots showing levels of HLF in control and Meis1-depleted MLL-AF9 knockin, and MV4;11 cells. (B) Meis1f/f MLL-AF9 knockin cells were transduced with Meis1 or FLAG-Meis1 and ChIP performed using anti-Meis1 or anti-FLAG antibodies or control IgG. The bar graph depicts fold enrichment of the various regions of the Hlf locus relative to IgG. Previously described genomic regions related to Runx1 and Flt3 were used as positive controls. A schematic of the Hlf locus with location of PCR assays is depicted. (C) Meis1f/f MLL-AF9 knockin cells were transduced with retrovirus-expressing HLF (pMY-HLF) or control (pMY). The bar graph depicts colonies formed by transduced cells cultured in the presence of vehicle or 4-OHT. *P < .01. (D) Bar graph depicting colonies formed by MLL-AF9 knockin cells transduced with NT or 4 different Hlf-shRNAs. Data are mean ± SEM. (E) Bar graph depicting proportions of Annexin V+ cells in MLL-AF9 knockin cells transduced with NT or Hlf-shRNA. (F) Survival curves of mice transplanted with Meis1f/f MLL-AF9 knockin leukemic cells transduced with HLF or control (pMY) retrovirus. Recipients were treated with vehicle or tamoxifen. *P < .01, log-rank test. n = 4 to 5 mice per group. (G) Histograms depicting intracellular ROS in Meis1f/f MLL-AF9 knockin cells transduced with pMY-HLF or control (pMY), treated with vehicle or 4-OHT. (H) Histogram depicting intracellular ROS in MLL-AF9 knockin cells transduced with NT or Hlf-shRNA. (I) Graph depicting OCR in Meis1f/f MLL-AF9 knockin cells transduced with HLF or control retrovirus and treated with vehicle (Veh) or 4-OHT. The arrow indicates the point at which oligomycin was added.
To determine the functional significance of the Meis1-Hlf axis, we transduced Meis1f/f cells with retrovirus carrying Hlf. As shown in Figure 4C, Hlf overexpression rescued colony formation upon Meis1 depletion, in contrast to vector (pMY)-transduced cells. Further, Hlf-knockdown in leukemic cells significantly reduced colony numbers, inhibited growth in liquid culture, and induced apoptosis (Figure 4D-E and supplemental Figure 7B-D). Finally, in vivo leukemia establishment was sustained by HLF overexpression in Meis1-deleted cells, whereas control vector-transduced cells failed to induce leukemia (Figure 4F).
Given that Hlf is a downstream mediator of Meis1 oncogenesis, we tested the relationship between Hlf and Meis1 dependent oxidative processes. In Meis1f/f cells, HLF overexpression prevented ROS accumulation upon Meis1 deletion, whereas the controls displayed moderate increase in ROS as expected (Figure 4G). Conversely, Hlf knockdown resulted in significant ROS accumulation in these cells (Figure 4H). Finally, HLF-overexpression prevented the increase in OCR upon Meis1 deletion, whereas the controls (pMY-transduced cells) displayed the expected increase in basal OCR (Figure 4I and supplemental Figure 7E). Collectively, these results reveal HLF as a downstream mediator of Meis1 in leukemia.
Leukemia cells are selectively sensitive to induction of OP
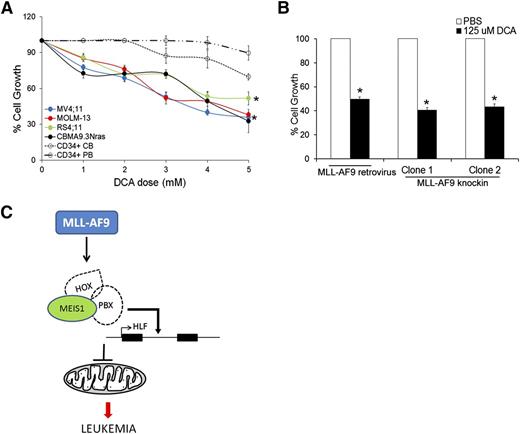
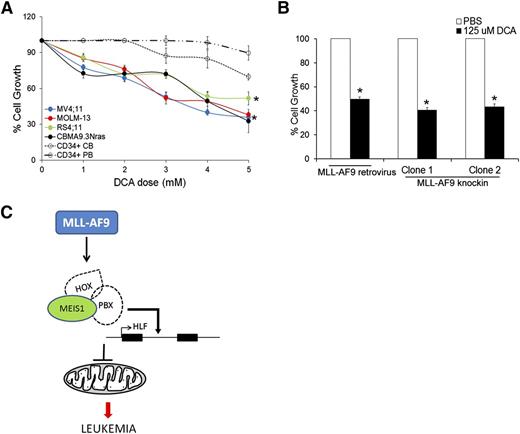
Our data so far indicate that Meis1 depletion in leukemia led to increased oxygen flux and increased ROS and apoptosis. We thus sought to replicate this phenomenon as a potential therapeutic strategy against leukemia. Dichloroacetate (DCA), an inhibitor of mitochondrial pyruvate dehydrogenase kinase (PDK), induces oxidative breakdown of glucose, generating ROS from the ETC.25 In published studies, DCA was shown to induce ROS and apoptosis in a variety of solid cancers.26-30 We tested the ROS-generating potential and cytotoxicity of DCA in murine and human leukemias. Treatment of various human leukemia cells with DCA led to increased intracellular ROS and significant inhibition of cell growth (Figure 5A, supplemental Figure 7F, and supplemental Table 2). In contrast, normal CD34+ cells, derived from cord blood or peripheral blood were relatively resistant to DCA treatment (Figure 5A and supplemental Table 2). A similar antiproliferative effect of DCA was observed in murine MLL-AF9 retroviral and knockin leukemic cells (Figure 5B). These data provide proof of principle that induction of OP and the consequent ROS accumulation reduces growth of leukemic cells to a greater extent than that of normal hematopoietic stem/progenitor cells (HSPCs), suggesting that this approach should be further developed as a therapeutic strategy.
Induction of OP inhibits growth of mouse and human leukemic cells. (A) Human leukemic cell lines and normal CD34+ cells isolated from cord blood (CB) or peripheral blood (PB) were treated with different concentrations of DCA and cell growth measured by MTT assay. Line graph represents % cell growth of each cell line compared with corresponding untreated control cells with 5 days of DCA treatment. Data represent mean ± SEM. *P < .01. (B) Bar graph depicting % cell growth of murine MLL-AF9 leukemic cells (retroviral and knockin) treated with DCA compared with respective phosphate-buffered saline (PBS)-treated control cells at day 4 posttreatment. (C) Graphic summary of the results depicting regulation of HLF and mitochondrial respiration by Meis1 in MLL-fusion gene leukemia.
Induction of OP inhibits growth of mouse and human leukemic cells. (A) Human leukemic cell lines and normal CD34+ cells isolated from cord blood (CB) or peripheral blood (PB) were treated with different concentrations of DCA and cell growth measured by MTT assay. Line graph represents % cell growth of each cell line compared with corresponding untreated control cells with 5 days of DCA treatment. Data represent mean ± SEM. *P < .01. (B) Bar graph depicting % cell growth of murine MLL-AF9 leukemic cells (retroviral and knockin) treated with DCA compared with respective phosphate-buffered saline (PBS)-treated control cells at day 4 posttreatment. (C) Graphic summary of the results depicting regulation of HLF and mitochondrial respiration by Meis1 in MLL-fusion gene leukemia.
Discussion
Leukemias with MLL-fusion proteins remain resistant to current treatments, and there is an urgent need for effective, specific therapies. Overexpression of HOXA9 and MEIS1 are also found in several other acute leukemias such as those associated with NUP98 fusions and NPM1 mutations.31 Prompted by these observations, we pursued the current studies of interrogating the oncogenic mechanism of MEIS1 in leukemia, with the goal of identifying novel targets for therapy. The experimental model combining MLL-AF9 knockin and inducible Meis1 knockout allowed us to explicitly test the continued requirement of Meis1 in the maintenance of leukemia in the context of a physiologically relevant disease model. Being a transcription factor, Meis1 is currently not amenable to inhibition by conventional approaches. We thus sought to determine the molecular mechanisms of Meis1-mediated oncogenesis. Here we demonstrate that Meis1 is essential for the continued propagation of established MLL-fusion leukemia. We identified HLF as a key downstream mediator of Meis1. Also, Meis1 loss led to increased intracellular ROS and apoptosis of leukemia cells. Reducing ROS levels by either the scavenger NACA or by SOD2 overexpression reversed the growth-inhibitory effects of Meis1 loss. We also found increased oxygen flux through the mitochondria upon Meis1 depletion, suggesting OP as a source of ROS. Thus, induction of OP may be a potentially useful therapy against leukemia.
In normal hematopoiesis, we previously reported that Meis1 deletion led to loss of quiescence in hematopoietic stem cells (HSCs) and progenitor cells.17 In our current studies, leukemic cells displayed apoptosis upon Meis1 deletion, with no discernible effect on cell cycling. Our approach of silencing Meis1 in established leukemias is reciprocal and complementary to the experimental design of overexpression of Hoxa9+ Meis1 used by others.32,33 The one advantage of the model used in our studies is the background of MLL-fusion leukemia, in which the MLL-fusion oncoprotein is expressed at physiologic levels. This major technical difference precludes a direct comparison of these results with those from previous studies. However, we did compare the list of Meis1-dependent genes from our study with the Hoxa9-dependent genes identified by Huang et al in their Hoxa9 + Meis1 retroviral leukemia model. A simple gene list overlap resulted in a relatively small number of genes in common. However, when we compared the top 50 gene sets (MSigDB) represented in each of the Meis1-dependent and Hoxa9-dependent gene lists, we found 11 gene sets in common (P = 1.6 × e−12, hypergeometric distribution), including hypoxia-regulated genes. Further, both gene lists included Hlf, which we have identified as a key downstream mediator of Meis1 in our study. Consistent with the results reported by Huang et al, our ChIP studies demonstrated Meis1 binding in a proximal intron of Hlf but not at the promoter. The fact that HLF lies downstream of the Hox/Meis1 complex likely explains the neutrality of the E2A-HLF leukemic oncogene toward Hoxa9/Meis1.13,34 The expression pattern of Hlf in normal hematopoiesis is similar to that of Hox genes and Meis1, with expression being highest in HSCs and progenitor cells and declining with differentiation.23,35 In addition, gene expression studies show that leukemias with higher HLF mRNA levels also display high MEIS1.36 Further, overexpression of HLF expands HSCs.23,37,38 Recent studies also show that HLF is 1 of 6 factors that, upon transient overexpression, can reprogram mature blood cells to generate HSCs.38 Interestingly, addition of Meis1 significantly improved the efficiency of this reprogramming, suggesting an additional posttranscriptional interaction between Meis1 and HLF. Although our results suggest that MEIS1 regulates HLF transcription, the relationship between these factors is likely far more complex. Collectively, all of these data suggest that activation of the MEIS1-HLF axis is likely a key contributor to the aberrant increased self-renewal of MLL-fusion leukemic cells.
Our studies show that MEIS1 loss led to increased oxygen flux and lethal oxidative stress in both the murine and human leukemia models. However, we did not detect significant changes in the levels of Hif-1α or Hif-2α (mRNA or protein) upon Meis1 depletion. Further, in reporter assays using the Hif-sensitive vascular endothelial growth factor promoter, addition of Meis1 or Hoxa9 + Meis1 did not augment the transcriptional activity of Hif-1α (data not shown). Thus, in leukemia, the levels or activities of Hif-1α and Hif-2α are not regulated by Meis1. The reversal of Meis1-deletion phenotype by Hif-1α stabilization (Vhl-knockdown included) could be caused by blockade of oxidative metabolism and thus reduced ROS generation, or by shared downstream pathways. Also, recent reports show that MLL-fusion genes can transform hematopoietic cells even in the absence of Hif-1α.39 On the other hand, we found that HLF blocked the increased oxygen flux and ROS production in Meis1-depleted leukemia cells. Previous studies have also suggested a role for HLF and other circadian rhythm proteins in regulating oxidative stress, although the mechanisms of this effect are not well known.40 More recent studies show regulation of mitochondrial respiration and oxygen consumption by circadian rhythm proteins.41 Thus, it is possible that Meis1 and HLF promote self-renewal by regulating mitochondrial respiration, which in turn prevents lethal oxidative stress. The phenomenon of increase in oxidative stress upon loss of a critical factor is not unique to Meis1.42 The mechanisms by which these various factors limit ROS in normal and leukemic stem cells, however, might differ. Our results indicate that apoptosis could be induced in leukemia by upregulation of OP, which would lead to increased ROS. Although the data are preliminary and need validation in vivo, we found that MLL-fusion leukemia cells are more sensitive to DCA than normal HSPCs. We used DCA as a model-compound because it has shown therapeutic benefit in small studies for other cancers.43 The short half-life and high concentrations required to achieve an effect preclude the use of DCA in its current form for in vivo studies. Other inhibitors of PDK or novel formulations of DCA, such as addition of lipophilic cation linkers that enhance its stability and mitochondrial targeting, might be more suitable for therapeutic use.44 In conclusion, our data demonstrate an obligatory role for Meis1 in the propagation of MLL-fusion gene leukemia and suggest that therapeutic induction of oxidative processes could be a novel treatment strategy for these leukemias and other cancers that are sensitive to oxidative stress.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jay Hess for providing the FLAG-tagged Meis1 plasmid and ChIP primer sequences for Flt3 and Runx1, Dr David Hildeman for the Sod2 plasmids, Dr James Bridges for assistance with the Hif-1α experiments, and Dr David Plas for helpful discussions. CB samples were received through the Normal Donor Repository at the Translational Core Laboratory, Cincinnati Children’s Hospital Medical Center. The flow cytometry core, comprehensive mouse and cancer core, DNA sequencing core, and veterinary services facilities provided invaluable support.
This research was supported by grants from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Centers of Excellence in Experimental Hematology (P30DK090971), the NIH National Heart, Lung, and Blood Institute (R01-HL111192), and CancerFree KIDS (A.R.K.).
Authorship
Contribution: J.R. and G.G.-M. performed majority of the experiments; J.P.C. managed the mouse breeding and mouse leukemia studies; Z.U. assisted in retroviral experiments; M.W. and K.A.L. assisted in patient-derived samples and AML1-ETO9a studies; N.D. and B.A. analyzed RNA-seq data; G.H. and J.C.M. provided reagents and intellectual support; and A.R.K. supervised all the studies and coauthored the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Z.U. is University of Rochester Medical Center, Rochester, NY.
Correspondence: Ashish Kumar, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: ashish.kumar@cchmc.org.